Abstract
A lymphocoele or cystic hygroma is a benign lymphatic malformation that usually presents as a congenital mass in infancy. These masses are most common in the cervicofacial region and more rarely occur elsewhere in the body. Spontaneous, atraumatic presentation in adulthood is extremely rare. We present a case of a 59-year-old woman who presented with a fluctuant mass in the axilla, which was diagnosed as a lymphocoele. Initial management by ultrasound-guided aspiration of the cyst proved unsuccessful due to recurrence. Surgical excision of the cyst was then successfully performed and histological analysis proved the diagnosis. The incidence of adult-onset lymphocoele without a history of prior trauma or operation is rare. This case report adds to the literature and reviews the various management strategies that have been successfully employed.
Background
A lymphocoele, also referred to as a cystic lymphangioma or a cystic hygroma, is a collection of lymphatic fluid without an epithelial lining that develops in anatomical compartments, often after surgery or trauma.1 However, it can also, rarely, be spontaneous in its development.1–11
Lymphocoeles are single/multiple cystic masses with little or no communication with normal lymph channels. Smith et al classified lymphatic malformations into microcystic (<2 cm) and macrocystic (>2 cm).12 These are also termed cystic lymphangiomas and cystic hygromas, respectively. An acquired lymphangioma (lymphangiectasia) can occur after surgery or radiotherapy, because of damage to the draining lymphatic channels. These may be small, and, if so, are reabsorbed.
Approximately 90% of cystic hygromas are identified before the age of 2 years.13 Indeed, adult onset cystic hygromas are exceedingly rare and <150 have been described in the literature.1 The majority of cases occur in the cervicofacial region (75%), with 20% presenting as axillary masses and 5% developing elsewhere.14
The cause of cystic hygromas is unknown. Those occurring in children are likely congenital in nature, and may be due to miscommunication between the lymphatic and venous pathways.13 Moreover, aneuploidy has been demonstrated to increase the risk of cystic hygromas.13 Those presenting later in life may be congenital in origin, and have suddenly enlarged in response to trauma, repetitive stress or infection. Lymphatic fluid lacks platelet or clotting factors and so, on trauma, is unable to clot off. While smaller collections often are asymptomatic and reabsorb spontaneously, larger collections may cause local pressure symptoms and may become infected.
Case presentation
A 59-year-old woman presented with a lump in her right axilla. She was otherwise completely asymptomatic. She had a history of cervical radiculopathy, 17 years previously, treated with neck traction. She had also, in the past, suffered from carpal tunnel syndrome, osteoarthritis, hypertension and idiopathic alopecia.
On examination, there was a fluctuant swelling in the axilla (figure 1). Breast examination revealed no masses and no other lymphadenopathy. Mammography demonstrated a large opacity in the right axilla, fatty stroma with some intermammary lymph nodes and minor residual glandular tissue. Chest radiography was unremarkable. Ultrasound showed a sonolucent structure in the low right axilla. A CT scan showed a well defined 91×62 mm collection in the right axilla, adjacent to a few enlarged lymph nodes (figure 2A). No evidence of calcifications or internal septations was noted. There was no axillary, supraclavicular, mediastinal or hilar lymphadenopathy. No other significant abnormality was detected. Fine needle aspiration was carried out and 750 mL of straw-coloured translucent fluid aspirated. The cyst aspirate fluid was assessed by Gram staining and Ziehl–Neelsen staining. Results, alongside microscopy and culture for both routine organisms and tuberculosis bacilli, showed no abnormality.
Figure 1.
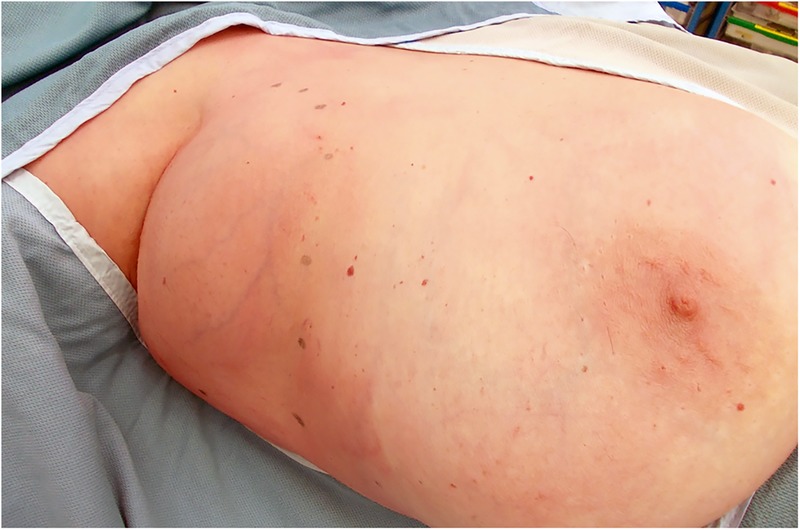
Photograph showing mass in right axilla.
Figure 2.
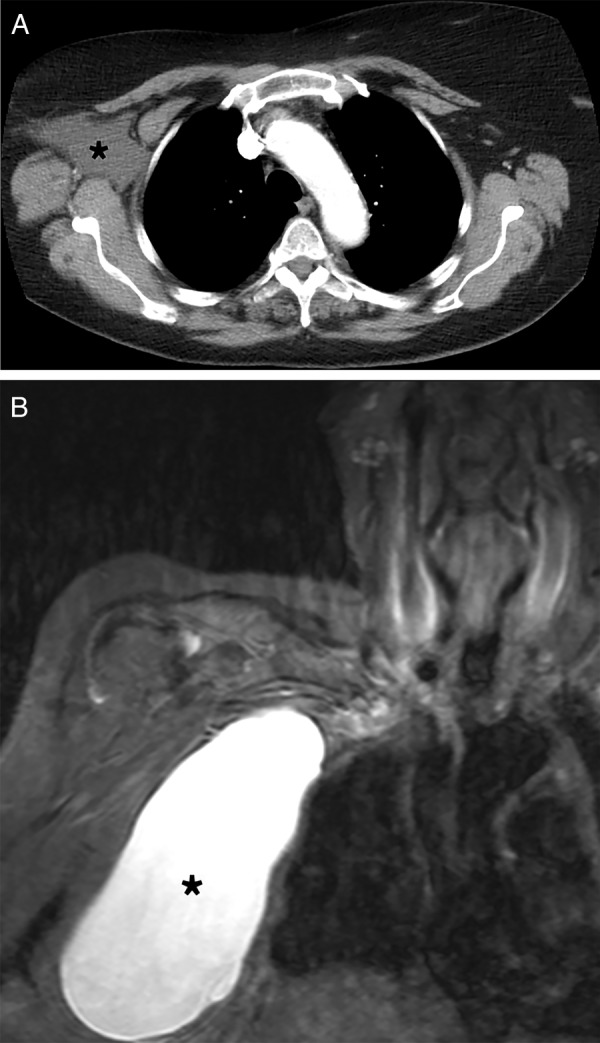
(A) CT scan, axial view; and (B) MRI scan, coronal view, showing cystic mass in right axilla (*).
The cyst enlarged in size over the following 4 weeks. In light of this reaccumulation of cystic fluid, an MRI scan of the axilla was carried out and found a well-defined cyst measuring 15 cm, with evidence of neither internal septations nor of mural nodules (figure 2B). The lesion was deep to muscles of the chest wall. No evidence of axillary lymphadenopathy was demonstrated. Thus a further attempt at aspiration was carried out, and 676 mL removed.
In spite of this, the cyst returned over the ensuing weeks and the decision was taken to carry out a right axillary cyst excision. Through a transverse incision, the cyst was dissected away from major axillary vessels and from the underlying latissimus dorsi muscle posterolaterally, and the pectoralis major and serratus anterior muscle anteriorly (figure 3A). The cyst wall was punctured and fluid inside aspirated. The posterior attachment of the wall to the underlying tissue was dissected away (figure 3B). A small portion of the cyst wall extending underneath the chest wall muscles passed close to the vertebral column and, due to inaccessibility, was not excised (figure 3C). The wound was closed primarily.
Figure 3.
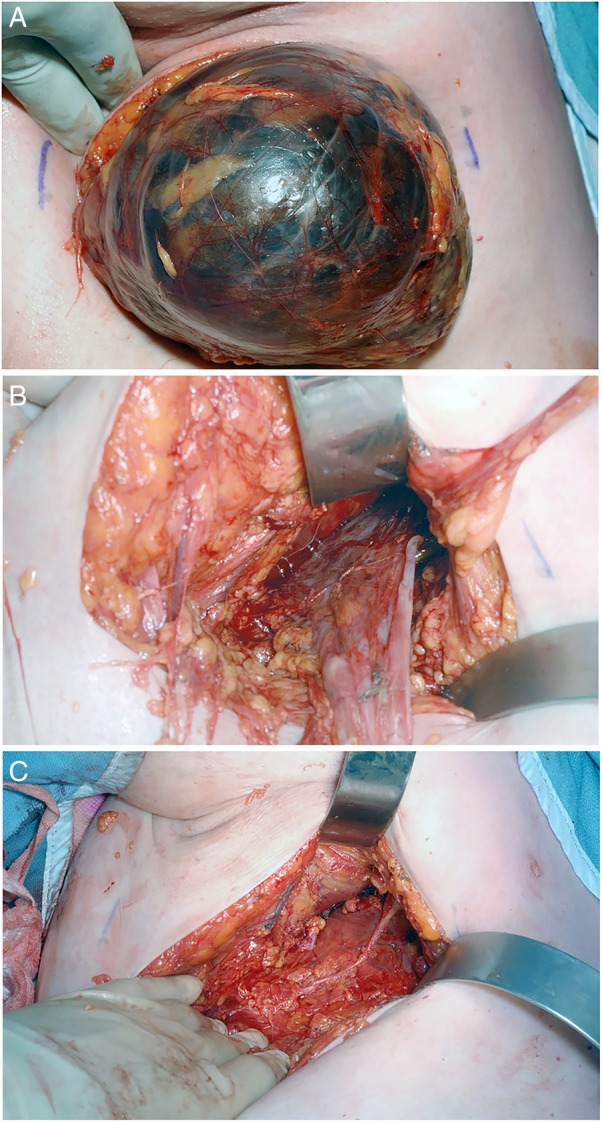
Intraoperative photography showing: (A) Mobilisation of the cyst into wound. (B) Posterior attachment of cyst wall. (C) Detachment of cyst wall posteriorly.
Histology of the operative specimen weighed 65.1 g and, when spread out like a disc, measured 100×115×10 mm. Wall thickness varied from less than 1 mm to fatty areas up to 6 mm. No focal lesions were seen. There were some areas of haemorrhage on the surface and within the centre of the cyst, in keeping with postoperative changes. On microscopy, sections showed fibroadipose tissue including the cyst wall, which was composed of collagen lined by simple epithelium. Local fibroplasia was present with granulation tissue. There were no signs of granulomatous infection. A lymph node was also present with the sample, and showed focal sclerosis.
Investigations
Initial imaging of suspected lymphocoeles is by ultrasound, which demonstrates a thin walled cyst that is hypoechoic and well-circumscribed.15 Septae may also be visualised. CT and MRI scanning show the smooth mass well as a non-enhancing, homogenous lesion.15 However, due to the risk of radiation, some experts advise MRI scan as standard protocol when suspecting a lymphocoele.15
Differential diagnosis
Patients with lymphocoele often present with complaints of cosmesis; however, the pressure effect from an enlarging cyst can cause a number of symptoms due to compression of neighbouring structures, depending on anatomical location.1
The differential of an axillary mass includes haematoma, pseudoaneurysm, abscess and postoperative seromas. Thus, in the work up of these patients, it is important to gather a good history to rule out any trauma to the axilla and to assess inflammatory markers on blood tests to rule out infective causes. Cystic hygromas present as non-tender masses with smooth edges, without skin warmth. They are fluctuant and translucent in nature. There should be no associated lymphadenopathy in this benign disease.
Treatment
Management of lymphocoeles in the axilla depends on the symptomatology and to what extent the lesion causes cosmetic disturbance to the patient. Observation alone may be all that is necessary if the lesion is small and not causing any cosmetic effect.16 If intervention is warranted, aspiration of the cyst under ultrasound guidance is the initial treatment modality used. However, recurrence rates of up to 80% have been reported, with repeated aspiration being a risk factor for the development of infection.16 One author has advised, in light of this, that aspiration should be attempted once and, if unsuccessful, another treatment modality should be sought.16
Percutaneous catheter drainage, a relatively simple procedure, has also been attempted. The drain is left in situ to prevent reaccumulation of fluid and, once there is a reduction in drain output, it is removed. Studies in which drains were left in situ for a mean of 14.5–22 days, reported success rates of 87–97%; despite this, recurrence rates of 13–63% are also described.17–19 Moreover, success is defined by some authors as reduction in cyst size with no subsequent enlargement, rather than complete resolution.11 Drains provide a potential route for infection and may also cause discomfort to the patient.
Instillation of sclerosants, using agents such as OK-432, bleomycin, doxycycline, acetic acid and alcohol, has also been used in tackling cystic hygromas.20 21 It is especially effective in cysts that may be difficult to excise surgically due to anatomical location. Sclerotherapy may be ineffective in multiloculated lesions and may cause systemic effects. Indeed, some sclerosants can cause anaphylactic reactions.22 Ethanol is the most commonly used agent as it is inexpensive, and its systemic side effect profile has been well-studied.23 Again, success is described by some as reduction in size rather than resolution, so comparable efficacy with excision is difficult.11 However, success rates as high as 94–100% have been reported.20 24
Surgical excision is the definitive therapeutic option when all other options have failed, and can achieve total excision and prevention of recurrence, as the cyst wall is removed. It does, however, carry the added risk of anaesthesia and surgical complications such as infection, bleeding and seroma formation. Depending on the anatomical location, further morbidity may be associated with damage to local structures. A 15% recurrence rate is cited if the cyst wall is not fully excised.25
Outcome and follow-up
Postoperative recovery was uncomplicated and cosmetic appearance satisfactory. The patient showed no signs of recurrence on clinical examination in routine outpatient follow-up and has reported no recurrence of symptoms over the 7-month follow-up period.
Discussion
In conclusion, this case was unusual in that a large axillary lymphocoele presented de-novo in an adult with no history of trauma. It adds to the small body of literature of spontaneous lymphocoeles in this anatomical region.1–11 Excision was successful after two failed attempts of cyst aspiration. Comparison of treatment modalities is difficult due to the heterogeneity of lymphatic malformations and the differences in reporting success rates; however, surgery is the most effective strategy if the anatomical location permits it.
Patient's perspective.
The initial discovery of the cyst was a huge shock, it may have developed slowly but my perception was that it had appeared very suddenly at around the size of a tennis ball. The first aspiration gave me relief from the uncomfortable feeling of having an internal, ever-expanding cushion in my armpit. However, the subsequent rate at which it refilled and grew again made me think that aspiration was unlikely to provide a solution.
I was unsettled by the uncertainty over the diagnosis and did worry that cancer might be detected at some stage right up to the postoperative all clear.
Once the decision to operate was made, I looked forward to its removal. Understanding the huge number of lymph nodes in that area, as indicated by Mr Mirza during the preoperative consultation, I consider myself extremely fortunate that it was so cleanly removed and I had absolutely no oedema or any other aftereffects post surgery. My sincere thanks to all involved.
Learning points.
Lymphocoeles are unusual causes of spontaneous masses in the axilla.
Sclerotherapy may be effective in cysts that are difficult to excise surgically, but evidence regarding the efficacy of this treatment modality may be overestimated.
Surgical excision is the definitive therapeutic option and can achieve total excision and prevention of recurrence, provided the cyst wall is entirely removed.
Acknowledgments
The authors would like to thank Mark Moughton, clinical photographer at Hinchingbrooke Hospital, for providing the intraoperative photography for this article.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Góes Junior AM, Jeha SA. Idiopathic lymphocele: a possible diagnosis for infraclavicular masses. Case Rep Surg 2012;2012:593028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCaffrey F, Taddeo J. Surgical management of adult-onset cystic hygroma in the axilla. Int J Surg Case Rep 2015;7C:29–31. 10.1016/j.ijscr.2014.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith RC, Sherk HH, Kollmer C et al. Cystic lymphangioma in the adult: an unusual axillary mass. Magn Reson Imaging 1989;7:561–3. 10.1016/0730-725X(89)90411-6 [DOI] [PubMed] [Google Scholar]
- 4.Philippakis GE, Manoloudakis N, Marinakis A. A rare case of a giant cavernous lymphangioma of the chest wall and axilla in an adult patient. Int J Surg Case Rep 2013;4:164–6. 10.1016/j.ijscr.2012.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krainick-Strobel U, Krämer B, Walz-mattmüller R et al. Massive cavernous lymphangioma of the breast and thoracic wall: case report and literature review. Lymphology 2006;39:147–51. [PubMed] [Google Scholar]
- 6.Michail O, Michail P, Kyriaki D et al. Rapid development of an axillary mass in an adult: a case of cystic hygroma. South Med J 2007;100:845–9. 10.1097/SMJ.0b013e3180f60e09 [DOI] [PubMed] [Google Scholar]
- 7.Stoss S, Kalbermatten DF, Robertson A et al. Large cystic tumour at the chest wall mimicking an echinococcosis: a case report. J Plast Reconstr Aesthet Surg 2008;61:13–16. 10.1016/j.bjps.2007.10.040 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen K, Karsif K, Lee S et al. Lymphangioma in an elderly patient: an unusual cause of axillary mass. Breast 2011;17:416–26. 10.1111/j.1524-4741.2011.01103.x [DOI] [PubMed] [Google Scholar]
- 9.Gebrim LH, de Lima GR, Tanaka CI. Axillary cystic lymphangioma in pregnancy. Int J Gynaecol Obstet 1995;48:327–8. 10.1016/0020-7292(94)02315-P [DOI] [PubMed] [Google Scholar]
- 10.Quack Loetscher KC, Jandali AR, Garzoli E et al. Axillary cavernous lymphangioma in pregnancy and puerperium. Gynecol Obstet Invest 2005;60:108–11. 10.1159/000085584 [DOI] [PubMed] [Google Scholar]
- 11.Gow L, Gulati R, Khan A et al. Adult-onset cystic hygroma: a case report and review of management. Grand Rounds 2011;11:5–11. 10.1102/1470-5206.2011.0002 [DOI] [Google Scholar]
- 12.Giguere CM, Bauman NM, Smith RJ. New treatment options for lymphangioma in infants and children. Ann Otol Rhinol Laryngol 2002;111(12 Pt 1):1066–75. 10.1177/000348940211101202 [DOI] [PubMed] [Google Scholar]
- 13.Bloom DC, Perkins JA, Manning SC. Management of lymphatic malformations. Curr Opin Otolaryngol Head Neck Surg 2004;12:500–4. 10.1097/01.moo.0000143971.19992.2d [DOI] [PubMed] [Google Scholar]
- 14.Sarin YK. Cystic hygroma. Indian Pediatr 2000;37:1139–40. [PubMed] [Google Scholar]
- 15.Romeo V, Maurea S, Mainenti PP et al. Correlative imaging of cystic lymphangiomas: ultrasound, CT and MRI comparison. Acta Radiol Open 2015;4:2047981614564911 10.1177/2047981614564911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metcalf KS, Peel KR. Lymphocele. Ann R Coll Surg Engl 1993;75:387–92. [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JK, Jeong YY, Kim YH et al. Postoperative pelvic lymphocele: treatment with simple percutaneous catheter drainage. Radiology 1999;212:390–4. 10.1148/radiology.212.2.r99au12390 [DOI] [PubMed] [Google Scholar]
- 18.Alago W Jr, Deodhar A, Michell H et al. Management of postoperative lymphoceles after lymphadenectomy: percutaneous catheter drainage with and without povidone-iodine sclerotherapy. Cardiovasc Intervent Radiol 2013;36:466–71. 10.1007/s00270-012-0375-3 [DOI] [PubMed] [Google Scholar]
- 19.Zargar-Shoshtari MA, Soleimani M, Salimi H et al. Symptomatic lymphocele after kidney transplantation: a single-center experience. Urol J 2008. Winter;5:34–6. [PubMed] [Google Scholar]
- 20.Zuckerman DA, Yeager TD. Percutaneous ethanol sclerotherapy of postoperative lymphoceles. AJR Am J Roentgenol 1997;169:433–7. 10.2214/ajr.169.2.9242748 [DOI] [PubMed] [Google Scholar]
- 21.Impellizzeri P, Romeo C, Borruto FA et al. Sclerotherapy for cervical cystic lymphatic malformations in children. Our experience with computed tomography-guided 98% sterile ethanol insertion and a review of the literature. J Pediatr Surg 2010;45:2473–8. 10.1016/j.jpedsurg.2010.07.023 [DOI] [PubMed] [Google Scholar]
- 22.Perkins JA, Manning SC, Tempero RM et al. Lymphatic malformations: review of current treatment. Otolaryngol Head Neck Surg 2010;142:795–803, 803.e1. 10.1016/j.otohns.2010.02.026 [DOI] [PubMed] [Google Scholar]
- 23.Cheng D, Amin P, Ha TV. Percutaneous sclerotherapy of cystic lesions. Semin Intervent Radiol 2012;29:295–300. 10.1055/s-0032-1330063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawhney R, D'Agostino HB, Zinck S et al. Treatment of postoperative lymphoceles with percutaneous drainage and alcohol sclerotherapy. J Vasc Interv Radiol 1996;7:241–5. 10.1016/S1051-0443(96)70769-8 [DOI] [PubMed] [Google Scholar]
- 25.Morley S, Ramesar K, Macleod D. Cystic hygroma in an adult: a case report. J R Coll Surg Edinb 1999;44:57–8. [PubMed] [Google Scholar]


