Abstract
A 56-year-old patient was admitted to our hospital, presenting with dysphagia as the only symptom. He was very concerned about this difficulty in swallowing. Diseases of the upper digestive tract were suspected, but further investigations revealed a neurological disorder. He had suffered a brain stem stroke and, as a consequence, developed an aspiration pneumonia. What seemed a digestive disorder was indeed a brain stem stroke, therefore we had to deal with the diverse impacts of this condition.
Background
Dysphagia is the symptom of difficulty in swallowing. It is a sensation that suggests difficulty in the passage of solids or liquids from the mouth to the stomach, and can be a sign of either a digestive or a neurological disorder. When dysphagia goes undiagnosed or untreated, patients are at high risk of pulmonary aspiration and subsequent aspiration pneumonia. Our patient had developed dysphagia and aspiration pneumonia due to a lateral medullary infarction, which went unnoticed until our patient presented with fever and dyspnoea.
Case presentation
A 56-year-old man was admitted to the hospital presenting difficulty in swallowing. He had type 2 diabetes and hypertension. He suddenly felt unwell and subsequently noticed he could swallow solids, but not liquids, so he came to the hospital for evaluation within the first 24 h hours after the onset of symptoms. On examination, he was alert and oriented, but he looked anxious and was very concerned about his pharyngeal discomfort. The patient had no dysarthria and no abnormal gait. His voice was normal. Tone and strength of the limbs were normal. The tests of coordination were normal, as was the Romberg’s test. Sensory and deep tendon reflex examination was normal. No dysphonia, ptosis or facial muscle weakness was found. Although cranial nerve examination was performed and was normal, pharyngeal reflex was not examined.
The initial differential diagnoses considered were motor or mechanical disorders of the oesophagus, rather than a neurological condition. Therefore, the patient was allowed to eat, and a gastroscopy was ordered, but before it could be performed, on day 3 after postadmission, he presented with fever and dyspnoea, and chest X-ray evaluation revealed right basal pneumonia.
Investigations
Routine blood tests, neck and thorax CT scan, and gastroscopy, were normal. Neuroimaging revealed an acute ischaemic lesion in the brain stem (right medulla oblongata) (figures 1 and 2). Diffusion-weighted MRI scanner and apparent diffusion coefficient confirmed the lesion caused by the occlusion of the right posterior inferior cerebellar artery.
Figure 1.
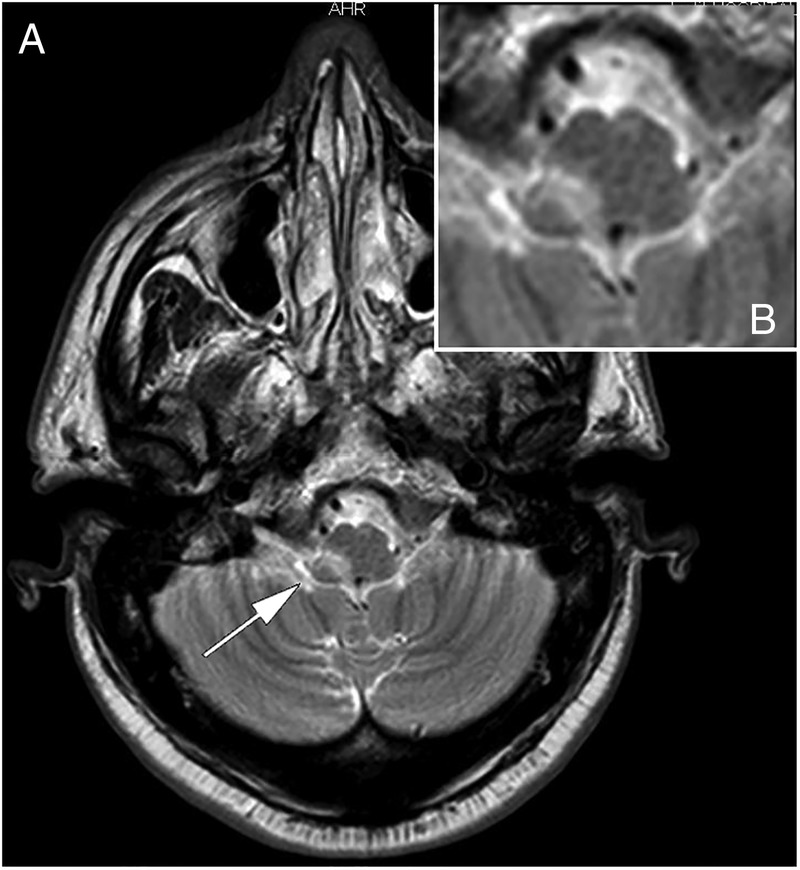
MRI scan showing right lateral brain stem infarction.
Figure 2.
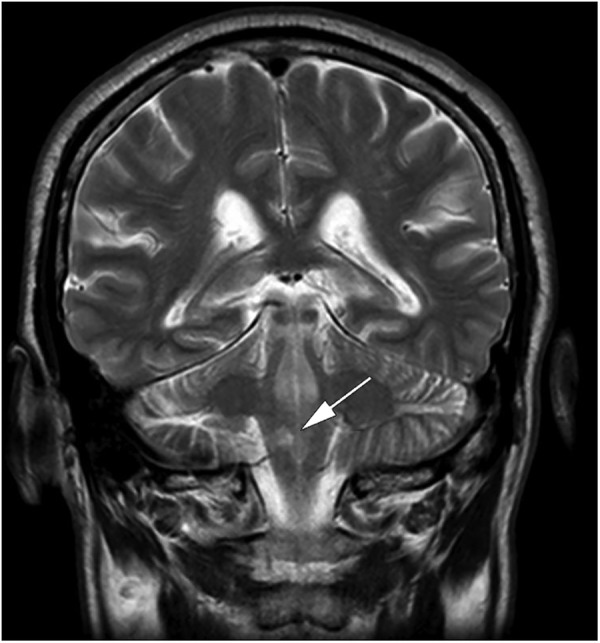
Diffusion-weighted MRI showing the acute right-sided dorsal lateral medullary infarction.
Since the patient was considered to be at high risk of aspiration, we used videofluoroscopy to evaluate him. This test showed the patient had aspiration. On a new examination, we confirmed the impaired, absent pharyngeal reflex.
Carotid ultrasound, EKG and echocardiography tests were normal.
Differential diagnosis
Difficulty to initiate a swallow, or swallowing that leads to choking, nasal regurgitation, or cough, is most likely due to an oropharyngeal disorder. Swallowing that results in the sensation of food sticking in the chest (oesophagus) is most likely due to an oesophageal dysphagia.
Dysphagia for solids alone suggests a mechanical obstruction to the passage of food, whereas dysphagia for solid and liquid food is more likely to be caused by motor disorder of the oesophagus. Intermittent symptoms are more likely to be caused by diseases of smooth muscle (oesophageal motility disorder) than by obstruction of the oesophagus. Progressive symptoms suggest scleroderma or achalasia. Dysphagia that progresses rapidly over weeks or a few months might suggest a malignant tumour, oesophageal ring, eosinophilic oesophagitis or peptic disease.1 2
Assessment of dysphagia should include radiological oesophagogram, videofluoroscopy, upper endoscopic examination, manometry, electromyography and 24 h pH monitoring.3 4 Fibreoptic endoscopic evaluation of swallowing can also be a good method to obtain information on pharynx and larynx anatomy and physiology, and cough and gag reflexes,5 but it cannot assess the oral stage of swallowing disorders nor can it determine bolus movement at the point of swallowing. In contrast, videofluoroscopy provides a dynamic assessment of the oral, pharyngeal and upper oesophageal phases.6 7
Regarding neuromuscular disorders, we must rule out: stroke, brain tumours, brain injury, bulbar and pseudobulbar paralysis, neurodegenerative diseases, dyskinesis (Parkinson’s and Huntington’s disease), myasthaenic syndromes, myopathies and peripheral neuropathies.
We eventually established the diagnosis: lateral medullary infarction due to the occlusion of direct penetrators from the vertebral artery. We were tempted to call it Wallenberg syndrome or PICA syndrome, our patient, however, was not presenting any other symptom related to lateral medullary syndrome but only dysphagia (no sensory deficits affecting the trunk, limbs or the face or cranial nerves, no loss of pain, no temperature sensation and no Horner’s syndrome).
The frequency of dysphagia in patients with lateral medullary infarction is high, and associated with aspiration pneumonia. However, dysphagia as the only presentation of medullary infarction has rarely been reported.8 Dysphagia is not the most common symptom at onset of a lateral medullary infarction, which is why we consider this case an atypical presentation of this disease. Lateral medullary infarction can be associated with vertigo, ataxia or dysphonia (wet voice), but these were not present on patient admission.
Treatment, outcome and follow-up
Immediately after developing fever and dyspnoea, antibiotic treatment was started and a nasogastric tube for feeding was placed. Aspirin 300 mg daily was started. Our patient rapidly recovered from the pneumonia, but he could not resume oral intake. He underwent percutaneous endoscopic gastrostomy (PEG) tube placement and was referred for rehabilitation. Gag reflex was assessed several times, but was absent at discharge (he presented with dysphagia for both solids and liquids), so he was not allowed to resume oral intake at discharge, a month and a half after admission.
Although the lesion was unilateral, the effect on oropharyngeal swallowing was bilateral (the absent pharyngeal reflex was bilateral). However, the contralateral centre in the medulla oblongata may eventually begin to operate and overcome the severity and long-term persistence of dysphagia. Follow-up was carried out and after 8 months, the patient could swallow properly and the PEG tube could be removed.
Discussion
Neurogenic dysphagia results from impairment of the oropharyngeal phases of swallowing, due to a neurological disorder,9 10 for instance, a stroke. Patients may have difficulty swallowing due to absent or delayed swallowing reflex. Symptoms may initially go unnoticed, and the incidence of aspiration within the first 5 days can be up to 42%.10 It is important to assess patients for their swallowing function prior to administering oral medications or food.11 Therefore, a careful evaluation of diseases associated with dysphagia should be performed, such as lateral medullary infarction.
Central control of swallowing is regulated by a central pattern generator positioned dorsally in the solitary tract nucleus and neighbouring medullary reticular formation, which activates the cranial nerve motor neurons, including the nucleus ambiguus and vagal dorsal motor nucleus, which then innervate the muscles of degluition.12 Acute occlusion of the posterior inferior cerebellar artery can lead to the lateral medullary syndrome. We think it rather likely the nucleus ambiguus was involved in the brain stem infarction we report, instead of other nuclei or neural tracts, as published by Kwon et al.13 As reported by Zand et al,8 a small infarction involving the rostral oesophagomotor formation of the nucleus ambiguus can lead to having dysphagia as the only symptom.
Dysphagia seen on lateral medullary infarction can often be accompanied by dysarthria and dysphonia, but our patient only had difficulty in swallowing, which helped to confuse us. That is, the absence of a lateral medullary syndrome led to confusion.
Unlike most cases of stroke, in which the symptoms affect only one side of the body, a stroke affecting the brainstem can produce difficult to recognise symptoms, as in this case. Gag reflex was not tested, thus neurogenic disorders were not included in the differential diagnosis.
Learning points.
Dysphagia is a subjective sensation of difficulty in swallowing. Oropharyngeal dysphagia is characterised by difficulty initiating a swallow, frequently caused by an acute stroke.
A lack of the gag reflex (pharyngeal reflex) can be a good predictor for dysphagia. Acute-stroke patients have more problems swallowing liquids than solids or semisolids.
Acute-onset dysphagia after stroke is frequently associated with increased risk of aspiration pneumonia, and it is compulsory to assess the swallow function prior to initiating oral intake.
Early recognition of stroke is deemed important as this can expedite diagnostic tests and treatments. Therefore, any suspected swallow dysfunction should have speech and language assessment prior to eating assessment, because missing this important step could put the patient in danger and subject him or her to avoidable harm.
In this case report the absence of a lateral medullary syndrome is important, that could be the origin of our misleading diagnosis, and the lack of adequate assessment of a swallowing and feeding disorder, which led to respiratory complications.
Acknowledgments
The authors would like to thank Dr Emilia Perez Moro for supporting us in the creation of this manuscript and for encouraging us at every stage of its preparation.
Footnotes
Twitter: Follow Rafael García Carretero at @rafa_linux
Contributors: RGC drafted the first version of the manuscript. MRB, NR-A and JR-M made suggestions and amendments in order to improve the final paper.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Horner J, Buoyer FG, Alberts MJ et al. Dysphagia following brain-stem stroke: clinical correlates and outcome. Arch Neurol 1991;48:1170–3. 10.1001/archneur.1991.00530230078026 [DOI] [PubMed] [Google Scholar]
- 2.Olszewski J. [Causes, diagnosis and treatment of neurogenic dysphagia as an interdisciplinary clinical problem]. Otolaryngol Pol 2005;60:491–500. [PubMed] [Google Scholar]
- 3.Meng NH, Wang TG, Lien IN. Dysphagia in patients with brainstem stroke: incidence and outcome. Am J Phys Med Rehabil 2000;79:170–5. 10.1097/00002060-200003000-00010 [DOI] [PubMed] [Google Scholar]
- 4.Daniels SK. Neurological disorders affecting oral, pharyngeal swallowing. GI Motil online. Nature Publishing Group, 2006. [Google Scholar]
- 5.Nacci A, Ursino F, La Vela R et al. Fiberoptic endoscopic evaluation of swallowing (FEES): proposal for informed consent. Acta Otorhinolaryngol Ital 2008;28:206–11. [PMC free article] [PubMed] [Google Scholar]
- 6.Rofes L, Arreola V, Mukherjee R et al. Sensitivity and specificity of the eating assessment tool and the volume-viscosity swallow test for clinical evaluation of oropharyngeal dysphagia. Neurogastroenterol Motil 2014;26:1256–65. 10.1111/nmo.12382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaffer NM, Ng E, Au FW et al. Fluoroscopic evaluation of oropharyngeal dysphagia: anatomic, technical, and common etiologic factor. AJR Am J Roentgenol 2015;204:49–58. 10.2214/AJR.13.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zand R, Ganta K, Afshani M. Isolated dysphagia after a small posterolateral medullary infarct: a case report. Can J Neurol Sci 2012;39:398–9. 10.1017/S0317167100013603 [DOI] [PubMed] [Google Scholar]
- 9.Buchholz DW. Neurogenic dysphagia: what is the cause when the cause is not obvious? Dysphagia 1994;9:245–55. 10.1007/BF00301918 [DOI] [PubMed] [Google Scholar]
- 10.Trapl M, Enderle P, Nowotny M et al. Dysphagia bedside screening for acute-stroke patients: the Gugging Swallowing Screen. Stroke 2007;38:2948–52. 10.1161/STROKEAHA.107.483933 [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Chung CS, Lee KH et al. Aspiration subsequent to a pure medullary infarction: lesion sites, clinical variables, and outcome. Arch Neurol 2000;57:478–83. 10.1001/archneur.57.4.478 [DOI] [PubMed] [Google Scholar]
- 12.Martino R, Terrault N, Ezerzer F et al. Dysphagia in a patient with lateral medullary syndrome: insight into the central control of swallowing. Gastroenterology 2001;121:420–6. 10.1053/gast.2001.26291 [DOI] [PubMed] [Google Scholar]
- 13.Kwon M, Lee JH, Kim JS. Dysphagia in unilateral medullary infarction: lateral vs medial lesions. Neurology 2005;65:714–18. 10.1212/01.wnl.0000174441.39903.d8 [DOI] [PubMed] [Google Scholar]


