Abstract
Primary focal segmental glomerular sclerosis (FSGS), one of the major causes of nephrotic syndrome, eventually results in end-stage renal disease. Currently, FSGS is treated with immunosuppressive therapies, which include calcinuerin inhibitors (cyclosporine), glucocorticoids, B-cell depleting agents (rituximab) and, recently, a T-cell co-stimulatory inhibitor (abatacept). Until recently, there had been no cases reporting resistance to all current therapies. We report a case of a 62-year-old Caucasian man with biopsy-proven FSGS, who responded well to oral prednisolone therapy. However, 2 years later, he had a relapse and failed to respond to prednisolone. Subsequent treatments then included cyclosporine, rituximab and cyclophosphamide, which were not successful. The patient was then administered abatacept, a novel T-cell co-stimulatory inhibitor—though he did not experience any side effects, there was no change in proteinuria nor in creatinine.
Background
Focal segmental glomerular sclerosis (FSGS) is currently the leading cause of nephrotic syndrome and leads to end-stage renal disease (ESRD) in millions of people worldwide.1 2 Primary FSGS leads to massive loss of protein in the urine due to disruption of podocyte foot process and the interposed slit diaphragm. Treatment-resistant idiopathic FSGS is one of the most common morbid conditions faced by nephrologist around the world.
In the majority of cases this was thought secondary to unknown circulating factors. However, currently, the supportive clinical data are inconsistent.3 Treatment is primarily with immunosuppressive agents, where primary FSGS appears to respond well to glucocorticoids.4 Calcinuerin inhibitors (CNIs), such as cyclosporine and tacrolimus, as well as a B-cell depleting agent (rituximab), have been shown to have varying degrees of success with steroid dependant, resistant and relapsing FSGS.
Mycophenolate has not been shown to be effective for this condition.5 Finally, plasmapheresis has been shown to be beneficial in some patients with refractory primary FSGS.6 Recently, a novel therapy using a T-cell co-stimulatory signal inhibitor, abatacept, has been shown to be beneficial in patients with recurrent FSGS.7
Unremitting nephrotic syndrome confers high risk of vascular thrombosis, acute renal failure, malnutrition as well as progressive kidney failure. Risk of accelerated cardiovascular disease and recurrence of FSGS post-transplantation exist. The benefits of achieving remission are large, but need to be weighed against the toxicity and cost of the therapy. We report a case of a patient with steroid-resistant primary FSGS whose treatment was unsuccessful on cyclosporine, rituximab and the T-cell co stimulatory inhibitor, abatacept.
Case presentation
A 62-year-old Caucasian man presented with nephrotic range proteinuria, oedema and hypoalbuminaemia. Secondary causes of nephrotic syndrome were excluded and a renal biopsy was performed, which confirmed diagnosis of FSGS. Subsequently, the patient was started on oral prednisolone therapy (1 mg/kg) along with ACE inhibitor, 3-hydroxy-3-methylglutaryl-coenzyme (HMG-CoA) reductase inhibitor and diuretic therapy. He had significant improvement of the proteinuria within weeks of starting therapy, resulting in a gradual tapering of the steroids over 18 months. He achieved complete remission with steroid therapy and sustained it for 2 years. He was noted to have worsening proteinuria 2 years postcessation of steroid therapy. A second course of prednisolone was started at 1 mg/kg dosage with an aim to achieve remission of the disease. Unfortunately, the patient's proteinuria continued to worsen over the next few weeks. In view of his poor response to therapy, he was started on CNI therapy cyclosporine, which he took for 2 months, achieving a therapeutic serum drug level (target trough level 100–150 ng/mL). Despite introducing the CNI, the patient's creatinine continued to deteriorate with worsening proteinuria. A follow-up renal biopsy at this stage was consistent with persisting FSGS changes with 41% of glomeruli showing focal segmental sclerosis and additional mild chronic interstitial inflammation was noted; this was attributed to possible CNI toxicity.
Following this, immunosuppressive therapy was changed to high-dose alternate day prednisolone and cyclophosphamide. The patient continued on cyclophosphamide for a total of 6 months. Though he tolerated it well, he had fluctuating renal function with minimal improvement in proteinuria. Owing to poor response and gradually declining renal function, this therapy was stopped at the 6-month mark.
Following CNI and cyclophosphamide therapy, the patient was considered for trial on the B-cell depleting agent, rituximab. His serum albumin and proteinuria continued to deteriorate following four doses of rituximab (375 mg/m2). Lacking any improvement with rituximab, and after careful risk benefit analysis, our patient was considered for a trial of the T-cell co-stimulatory inhibitor, abatacept. A repeat biopsy was recommended at this stage, which the patient refused to undergo. He received four doses of abatacept (750 mg intravenously) over a month, at weekly intervals. Though he tolerated this regimen well, without any adverse effects, he had no noticeable improvement in proteinuria nor in serum creatinine (Table 1 below). This regimen was subsequently ceased.
Table 1.
The table depicts patient demographics and laboratory parameters before and after abatacept therapy
| Baseline | Before abatacept | 8 Weeks post-abatacept | 50 Weeks post-abatacept | |
|---|---|---|---|---|
| Age (years) | 62 | 62 | NA | 63 |
| Sex | Male | Male | NA | NA |
| Racial origin | Caucasian | Caucasian | NA | NA |
| Creatinine (µmol/L) | 113 | 169 | 164 | 190 |
| Urine PCR (mg/mmol) | 460 | 1010 | 1050 | 1210 |
| Serum albumin (g/L) | 24 | 16 | 16 | 17 |
PCR, protein creatinine ratio; NA, not applicable.
Investigations
Initial blood tests revealed creatinine of 160 µmol/L, serum albumin at 26 g/L and urine protein creatinine ratio of 460 mg/mmol. Hepatitis b and c virus, human immune deficiency virus, Ebstein-Barr virus and cytomegalovirus serology were negative, and serum protein electrophoresis was normal. Initial renal biopsy revealed 5/16 glomeruli with focal segmental sclerosis and 1/16 glomeruli with global sclerosis with less than 10% interstitial fibrosis. No evidence of interstitial inflammation was noted and no immunofluorescence activity was detected. The laboratory value trend is shown in figure 1.
Figure 1.
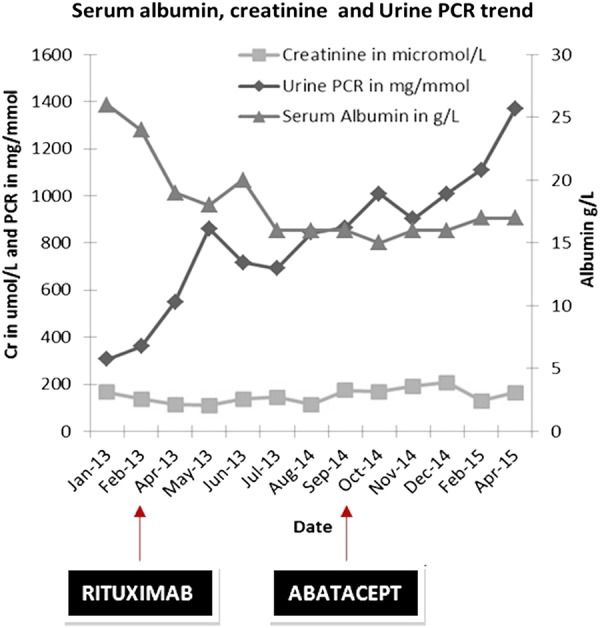
The graph depicts plasma creatinine, urine protein–creatinine ratio (PCR) and serum albumin concentration trends during the treatment phase with rituximab and abatacept. Date scale: January 2013 to April 2015.
Differential diagnosis
The differential diagnoses for nephrotic syndrome include minimal change disease, focal segmental glomerulosclerosis (primary or secondary) and membranous nephropathy (primary or secondary). Rare causes include IgA nephropathy and other nephritic/nephrotic syndromes.
Treatment
Immunosuppression is the mainstay of treatment for primary focal segmental glomerulosclerosis. Our patient was initially managed with high-dose oral prednisolone therapy and achieved remission. However, with subsequent relapse and failure of response to repeat high-dose steroid treatment, other potent immunosuppressants such as cyclosporine, rituximab and cyclophosphamide, were tried, with no benefit. Finally, the novel T-cell co-stimulatory inhibitor, abatacept, was administered, but this therapy did not achieve remission in our patient. However, no adverse side effects were noted due to this therapy.
Outcome and follow-up
In this patient with relapsed FSGS that had been resistant to all of the potent immunosuppressive agents, we report no benefit with the novel T lymphocyte co-simulatory inhibitor, abatacept. Currently, he remains only on ACE inhibitor, HMG-CoA reductase inhibitor and diuretic therapy, for management of FSGS, and his proteinuria remains at 8–10 g/24 h with gradually declining renal function.
Discussion
This case demonstrates the difficulty in managing patients with steroid and rituximab-resistant FSGS. Although rituximab has been successfully used in patients with steroid dependant and relapsing FSGS, the benefit in achieving remission seems to be limited in steroid-resistant cases.8 9 Our patient was trialled on rituximab on this basis but, unfortunately, failed to respond. Following reports of successful use of T-cell co-stimulatory inhibitors in patients with transplant with recurrent FSGS, this therapy was considered for managing our patient as a fall back. Unfortunately, frozen sections were not available to add B7-1 immunostaining and our patient refused to have a repeat renal biopsy.
Podocyte injury has been well associated with nephrotic syndrome. It is well known that various mutations of podocin can lead to steroid-resistant FSGS. B7-1 expression and upregulation in podocytes have been attributed to the pathogenesis of proteinuria, and this has been proven by in vitro studies. Abatacept (CTLA-4-Ig) is an inhibitor of the T-cell co-stimulatory molecule B7-1 (CD 80). Yu et al7 showed that abatacept reversed nephrotic range proteinuria in four patients with transplant with recurrent FSGS in the renal allograft, and in one patient with primary FSGS in the native kidney. In our patient, abatacept induced neither partial nor complete remission.
This case demonstrates that abatacept may not achieve remission in all patients with glucocorticoid and rituximab-resistant FSGS, but may be helpful in very selective populations with resistant disease. Though B7-1 immunostaining has been shown to be a predictive factor for identifying patients who might respond to abatacept,7 the role of CD80 immunostaining is not certain in patients with idiopathic or recurrent FSGS in transplant grafts.10 Further, Delville et al11 reported nil effect of both abatacept as well as belatacept in achieving remission in nine cases with recurrent FSGS in renal allografts. Larger trials are necessary to clarify the role of this novel agent in treating patients with steroid-resistant FSGS. Cost factors should be considered along with risk benefit analysis prior to instituting a new and novel therapy such as abatacept, as clinicians are compelled to use such therapies due to overwhelming information available in the World Wide Web and in the era of information abundance.
Learning points.
We report failure of abatacept in achieving remission in steroid-resistant case of focal segmental glomerular sclerosis (FSGS).
The value of B7-1 immunostaining in FSGS is dubious.
Cost of medication should be considered along with risk benefit analysis prior to instituting such therapy with little evidence.
Footnotes
Twitter: Follow Vinothkumar Kavarthapol Jayaraman at @kj_vino
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Dragovic D, Rosenstock JL, Wahl SJ et al. Increasing incidence of focal segmental glomerulosclerosis and an examination of demographic patterns. Clin Nephrol 2005;63:1–7. 10.5414/CNP63001 [DOI] [PubMed] [Google Scholar]
- 2.Haas M, Meehan SM, Karrison TG et al. Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976–1979 and 1995–1997. Am J Kidney Dis 1997;30:621–31. 10.1016/S0272-6386(97)90485-6 [DOI] [PubMed] [Google Scholar]
- 3.D'Agati VD, Kaskel FJ, Falk RJ. Focal segmental glomerulosclerosis. N Engl J Med 2011;365:2398–411. 10.1056/NEJMra1106556 [DOI] [PubMed] [Google Scholar]
- 4.Banfi G, Moriggi M, Sabadini E et al. The impact of prolonged immunosuppression on the outcome of idiopathic focal-segmental glomerulosclerosis with nephrotic syndrome in adults. A collaborative retrospective study. Clin Nephrol 1991;36:53–9. [PubMed] [Google Scholar]
- 5.Canetta PA, Radhakrishnan J. Impact of The National Institutes of Health Focal Segmental Glomerulosclerosis (NIH FSGS) clinical trial on the treatment of steroid-resistant FSGS. Nephrol Dial Transplant 2013;28:527–34. 10.1093/ndt/gfs563 [DOI] [PubMed] [Google Scholar]
- 6.Mitwalli AH. Adding plasmapheresis to corticosteroids and alkylating agents: does it benefit patients with focal segmental glomerulosclerosis? Nephrol Dial Transplant 1998;13:1524–8. 10.1093/ndt/13.6.1524 [DOI] [PubMed] [Google Scholar]
- 7.Yu CC, Fornoni A, Weins A et al. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 2013;369:2416–23. 10.1056/NEJMoa1304572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bagga A, Sinha A, Moudgil A. Rituximab in patients with the steroid-resistant nephrotic syndrome. N Engl J Med 2007;356:2751–2. 10.1056/NEJMc063706 [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Fresnedo G, Segarra A, González E et al. Rituximab treatment of adult patients with steroid-resistant focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2009;4:1317–23. 10.2215/CJN.00570109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novelli R, Gagliardini E, Ruggiero B, et al. Any value of podocyte B7-1 as a biomarker in human MCD and FSGS? Am J Physiol Renal Physiol. 2015 doi: 10.1152/ajprenal.00510.2015. [DOI] [PubMed] [Google Scholar]
- 11.Delville M, Baye E, Durrbach A et al. B7-1 blockade does not improve post-transplant nephrotic syndrome caused by recurrent FSGS. J Am Soc Nephrol 2015:Dec 23. pii: ASN.2015091002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


