Abstract
The bacterial Mfd protein is a transcription-repair coupling factor that performs two key functions during transcription-coupled DNA repair. The first is to remove RNA polymerase (RNAP) complexes that have been stalled by a DNA lesion from the site of damage, and the second is to mediate the recruitment of DNA repair proteins. Mfd also displaces transcription complexes that have been stalled by protein roadblocks, and catalyses the reactivation of transcription complexes that have become ‘backtracked’. We have identified amino acid substitutions in the β subunit of Escherichia coli RNAP that disrupt a direct interaction between Mfd and RNAP. These substitutions prevent Mfd displacing stalled RNAP from DNA in vivo and in vitro. They define a highly conserved surface-exposed patch on the β1 domain of RNAP that is required by Mfd for the initial step of transcription-coupled repair, the enhancement of roadblock repression and the reactivation of backtracked transcription complexes.
INTRODUCTION
Many different proteins interact with RNA polymerase (RNAP) to control its activity during transcription initiation, elongation and termination. Various regions of RNAP function as targets for these regulatory proteins: bacterial RNAP comprises six subunits (α2ββ′σω) and all but the ω subunit have been shown to interact with transcription factors (1). High-resolution crystal structures of several multi-subunit RNAPs are available [reviewed in (2–5)], and much is known about the properties and functions of each RNAP subunit. Identification of a transcription factor's target in RNAP therefore allows the location of that factor in the transcription complex to be predicted, and provides insights into its likely mode of action. In this work, we have identified residues in Escherichia coli RNAP that are contacted by the Mfd protein, a transcription factor that exerts its effects on RNAP during transcription elongation.
The E.coli Mfd protein is a 130 kDa monomeric protein that contains superfamily 2 helicase motifs and that is able to bind to RNAP, the DNA repair protein UvrA and DNA (6–8). Similar proteins are encoded by most bacterial genomes that have been sequenced to date. Mfd was originally identified as a transcription-repair coupling factor responsible for ensuring the preferential repair of certain types of DNA damage in the transcribed strand of active genes (8). This process, termed transcription-coupled repair, occurs when RNAP encounters a DNA lesion that prevents further transcription. The Mfd protein plays two roles in transcription-coupled repair: it removes RNAP from DNA and it recruits the nucleotide excision repair apparatus to correct the damage. The displacement of RNAP is an ATP-dependent process in which Mfd binds to RNAP and to the DNA immediately upstream of the stalled transcription complex (9). Displacement is dependent on the integrity of the Mfd helicase domains and an adjacent region called the TRG (Translocation in RecG) motif (6,10).
In addition to its function in transcription-coupled DNA repair, Mfd plays a role in other cellular processes that require removal of stalled transcription complexes. It increases the efficiency of transcriptional regulation by ‘roadblock repression’ in both E.coli and Bacillus subtilis (10–12). Proteins bound to DNA can block the progress of a transcription elongation complex and thus repress transcription of the DNA sequences that lie downstream (13). In the absence of Mfd, RNAP stalled by a protein roadblock remains bound to the DNA and may be able to resume transcription when the repressor protein dissociates from its binding site. Mfd causes RNAP stalled by a roadblock to terminate transcription (7,14), and thus ensures that the downstream sequences are not transcribed. Mfd is also involved in transcription termination by the bacteriophage HK022 Nun protein. Nun is a transcription factor that binds to specific sequences in the nascent RNA transcript and causes RNAP to stall (15). Mfd completes the transcription termination process by removing the stalled transcription complexes from the DNA (16).
Our understanding of the mechanism by which Mfd displaces RNAP from DNA was greatly advanced by the observation that Mfd causes forward translocation of transcription complexes that have become ‘backtracked’ (9). Paused transcription elongation complexes are able to slide along the DNA template in the opposite direction to transcription; during this process the RNA:DNA hybrid remains in register and so as RNAP moves backwards the 3′ end of the nascent transcript is extruded from the active site (17). Such backtracked complexes are catalytically inactive, but can be reactivated by transcript cleavage factors such as GreA and GreB, which cause cleavage of the nascent RNA to generate a new 3′ end within the active site of RNAP (18). Mfd can also reactivate backtracked transcription complexes, in vitro at least, but it does so without causing cleavage of the extruded RNA (9). The helicase/TRG domains of Mfd are highly similar to those of RecG, a bacterial protein that is able to catalyse the movement of Holliday junction-like DNA structures (19). By analogy to RecG, Mfd is likely to be capable of translocating double-stranded DNA. The model proposed for the reactivation of backtracked complexes by Mfd is, therefore, that Mfd binds to RNAP and to the DNA upstream of RNAP, and as the helicase/TRG domains move along the DNA in an ATP-dependent fashion RNAP is pushed forward until the 3′ end of the transcript is returned to the active site. Transcription can then resume. The removal of RNAP from templates blocked by DNA damage, protein roadblocks or other impediments to transcription is presumed to be a consequence of the same translocation mechanism. It is predicted that if RNAP is unable to resume transcription, the translocation activity of Mfd will destabilize and eventually dissociate the transcription complex, probably by partially rewinding the transcription bubble and shortening the RNA:DNA hybrid (9,20).
In each of its roles, Mfd is thought to be recruited to stalled transcription complexes via a direct interaction with RNAP, although a requirement for an Mfd:RNAP interaction has only been demonstrated experimentally for the displacement of complexes stalled by nucleotide starvation (6). The initial evidence that Mfd could bind to RNAP came from ‘pull-down’ assays, in which purified RNAP core or holoenzyme was found to bind to a maltose binding protein (MBP)–Mfd fusion protein immobilized on amylose resin (6). Experiments with deletion mutants of Mfd suggested that residues 379–571 of Mfd were involved in this interaction (6). The interacting regions were subsequently defined more precisely using yeast two-hybrid assays. In a global yeast two-hybrid analysis of protein–protein interactions in Helicobacter pylori, a fragment of Mfd was found to interact with the single large subunit of RNAP (the β and β′ chains are fused in H.pylori) (21). The equivalent region of E.coli Mfd, termed the RNAP interaction domain (RID), comprises residues 472–603 (9). Further yeast two-hybrid experiments, using proteins from E.coli, demonstrated that the binding site of the Mfd RID lies within the first 142 amino acids of the β subunit of RNAP (9). This region of the β subunit is part of the β1 domain, which forms the tip of one of the ‘claws’ of the RNAP crab-claw structure (22). In this study, we have identified the residues within the N-terminal region of the β subunit of RNAP that are required for interaction with Mfd. We demonstrate that substitution of these residues interferes with the ability of Mfd to displace stalled transcription elongation complexes, affect the efficiency of transcription repression by a protein roadblock and reactivate backtracked elongation complexes.
METHODS
Plasmid construction
Plasmid pETLRpoB carries the E.coli rpoB gene under the control of a non-repressible lacUV5 promoter, and also carries the lacIQ gene. It was constructed in several stages as follows. The E.coli rpoB gene was amplified by PCR from the plasmid pMKSe2 (23) and was cloned into pET21term (10) at NdeI/HindIII sites to create pETRpoB. A double-stranded oligonucleotide corresponding to residues −36 to +5 of the lacUV5 promoter was then inserted into BglII/XbaI sites upstream of rpoB in pETRpoB to create pETLRpoB. The T7 promoter present in pET21term was removed during this procedure. Derivatives of pETLRpoB encoding tryptophan or alanine substitutions in the β subunit were generated using site-directed mutagenesis (24).
Plasmid pETLRpoBHis encodes His-tagged β subunits that contain the sequence [Gly]2-[His]6 at the C-terminus. It was constructed in several stages as follows. Plasmid pETLRpoB-lacI, a derivative of pETLRpoB that does not contain the lacIQ gene, was created by cleaving pETLRpoB with BtgI and religating the vector after the removal of the BtgI–BtgI fragment carrying lacIQ. Codons 1231–1342 of rpoB were amplified by PCR using a mutagenic downstream primer that inserted a linker encoding [Gly]2-[His]6 between the last wild-type rpoB codon and the stop codon. The PCR product was cloned into pETLRpoB-lacI at BsrGI/HindIII sites to create pETLRpoBHis. Derivatives of pETLRpoBHis encoding His-tagged β subunits with alanine substitutions were constructed by transferring NdeI/KpnI fragments from the appropriate pETLRpoB derivative to pETLRpoBHis. All constructs were confirmed by sequencing, and the nucleotide sequences of primers used in plasmid construction are available on request.
CAT assays
Chloramphenicol acetyl transferase (CAT) activity was assayed in E.coli K-12 strain AB1157 (mfd+), which was obtained from the E.coli Genetic Stock Centre, Yale University. AB1157 cells were transformed with the roadblock repression reporter plasmid pRCB-CAT1 (10) and with pETLRpoB expression plasmids. Cultures were grown at 37°C in M9 medium containing 80 μg/ml ampicillin and 20 μg/ml tetracycline. At OD600 ∼0.5, 1.5 ml of culture were harvested by centrifugation, and cells were lysed with 150 μl Bugbuster (Novagen) according to the manufacturer's protocol. Cell extracts were stored at −80°C, and the Quant T CAT assay kit (Amersham) was used according to the manufacturer's protocol in order to quantify CAT activity. Protein concentrations were determined using the Bradford assay (BioRad).
Proteins
His-tagged wild-type and mutant RNAP core and holoenzymes were purified from the rapA− strain DJ473 (25) transformed with plasmid pETLRpoBHis or derivatives, following the procedure of Niu et al. (26). The activity of each RNAP preparation was tested in multi-round in vitro transcription reactions as described previously (27). Mfd was purified as described previously (10).
RNAP displacement assays
Electrophoretic mobility shift assays (EMSAs) were used to analyse the displacement of transcription elongation complexes stalled by nucleotide starvation in vitro. Transcripts initiating at the T7 A1 promoter require UTP to be incorporated at +2 and +21, and transcription complexes stalled at +20 can be generated by using the dinucleotide ApU to initiate transcription and omitting UTP from the reaction mixture (28). A 529 bp RsaI/SmaI fragment of plasmid pAR1707 (28), which contains the T7 A1 promoter, was end-labelled using T4 polynucleotide kinase and [γ-32P]ATP. Transcription initiation complexes were formed by mixing 0.4 nM labelled fragment with 10 nM RNAP holoenzyme in repair assay buffer [40 mM HEPES pH 8.0, 100 mM KCl, 8 mM MgCl2, 4% glycerol (v/v), 5 mM DTT and 100 μg/ml BSA]. The reaction mixtures were incubated at 37°C for 15 min and then heparin was added at a final concentration of 10 μg/ml. Transcription was initiated by adding ApU, ATP, GTP and CTP at final concentrations of 80 μM, 1.7 mM, 8 μM and 8 μM, respectively, and the reaction mixtures were incubated at 37°C for 15 min to allow stalled elongation complexes to form. Mfd was added at the concentrations indicated and the reactions were incubated at 37°C. At timed intervals, aliquots were removed and loaded onto a 4.5% acrylamide/1× TBE gel. Gels were run at 4°C. Radiolabelled bands were detected using a phosphor screen and quantified using ImageQuant software (Molecular Dynamics).
In vitro transcription assays
The template used for immobilized in vitro transcription assays was equivalent to the RsaI/SmaI fragment of pAR1707 used in displacement assays, but it was generated by PCR using a biotinylated primer so that biotin was attached to the 5′ end of the non-transcribed strand. Biotinylated template DNA was bound to streptavidin paramagnetic beads (Promega) by incubating 20 nM DNA with 1 mg/ml beads at room temperature for 10 min. Immobilized transcription initiation complexes were formed in 20 μl reactions that contained 20 nM His-tagged RNAP, 10 nM template DNA and 0.5 mg/ml beads in repair assay buffer. The reactions were incubated at 37°C for 15 min, heparin was added at a final concentration of 10 μg/ml and the reactions were incubated at 37°C for a further 10 min.
To generate immobilized transcription elongation complexes stalled at +20 and +26, 30 μl of a mixture of nucleotides in repair assay buffer was added to the reaction containing transcription initiation complexes. The concentration of nucleotides in the resulting 50 μl reactions was 56 μM ApU, 5.6 μM ATP, 5.6 μM CTP and 1.1 μM GTP. Each reaction also contained 5 μCi [α-32P]GTP. The reactions were incubated at 37°C for 15 min. Unincorporated nucleotides were removed by pelleting the streptavidin paramagnetic beads with a magnetic field and washing three times with 50 μl of repair assay buffer. To generate complexes stalled at +27, 50 μl of 10 μM UTP in repair assay buffer was added to the pellet of streptavidin beads carrying +20/+26 complexes, and the reaction was incubated at 37°C for 15 min. Complexes were washed three times and incubated in repair assay buffer at 37°C for 10 min to induce backtracking (29). Run-off reactions were performed by adding 50 μl of 50 μM CTP, UTP and GTP and 2 mM ATP in repair assay buffer to the pellet of streptavidin beads carrying +27 complexes. Where indicated, 250 nM Mfd was also added. The reactions were incubated at 37°C for 15 min and then RNA transcripts were purified by phenol/chloroform extraction of the reaction mixture and ethanol precipitation. RNA was resolved on a 7 M urea/10% acrylamide denaturing gel.
Yeast two-hybrid assay
Fragments encoding residues 1–142 of wild-type and mutant β subunits were amplified by PCR and cloned into NcoI/BamHI sites of the plasmid pACT2 (Clontech) to create an in-frame fusion to the C-terminus of the Gal4 transcriptional activation domain. A fragment encoding the RID of Mfd (amino acids 472–603) was amplified by PCR and cloned into NcoI/BamHI sites of the plasmid pAS2.1 (Clontech) to create an in-frame fusion to the C-terminus of the Gal4 DNA-binding domain. Plasmids were transformed into the yeast strain Y190 (30) using lithium acetate (31). Transformants were grown overnight in selective medium and cells were lysed by freeze-thawing. β-galactosidase assays were carried out as described previously (32).
‘Pull-down’ assays
‘Pull-down’ assays were carried out essentially as described by Selby and Sancar (6), with some modifications. Cell-free extract was made from 100 ml of XL1-Blue cells (Stratagene) transformed with pMALMfd (6), which encodes MBP–Mfd fusion protein. MBP–Mfd was bound to amylose resin (NEB) and 100 μl of the amylose resin was used in each pull-down reaction such that each reaction contained ∼200 pmol of MBP–Mfd. As a negative control MBP alone was bound to amylose resin. Binding of RNAP, washing to remove unbound protein, and elution of bound RNAP were carried out as described previously (6). Each reaction contained 1 μg His-tagged RNAP. To detect bound RNAP, 18% of the final elution volume was resolved on a 10% SDS–polyacrylamide gel and protein was transferred onto an immobilonP PVDF membrane (Millipore). Membranes were probed with polyclonal anti-β or anti-σ70 antibodies (a gift from A. Ishihama) using standard western blotting techniques. Detection was carried out using the POD chemiluminescence system (Roche).
RESULTS
Identification of substitutions in the β subunit of RNAP that affect Mfd function in vivo
The Mfd RID (amino acids 472–603) binds to the first 142 amino acids of the β subunit of RNAP in yeast two-hybrid experiments (9). In order to identify the residues in the N-terminal region of the RNAP β subunit that are involved in this interaction, we carried out site-directed mutagenesis of the rpoB gene of E.coli and assessed the effect of the mutants in an in vivo assay that is sensitive to Mfd activity (10).
The crystal structure of Thermus aquaticus core RNAP (33,34) and a sequence alignment of the rpoB gene from various bacterial species were used to identify conserved residues in the first 142 amino acids of the E.coli RNAP β subunit that are likely to be surface-exposed. Mfd from B.subtilis can displace E.coli RNAP (35), and so it is likely that the residues involved in the Mfd:RNAP interaction are conserved between these two species. In selecting targets for mutation, particular consideration was therefore given to amino acid residues that were common to E.coli and B.subtilis. Five residues distributed across the surface of the β1 domain were chosen for substitution, and plasmids encoding E.coli RNAP β subunits with tryptophan substitutions of residues Q36, E77, E84, K118 and D132 were constructed by site-directed mutagenesis (Figure 1A).
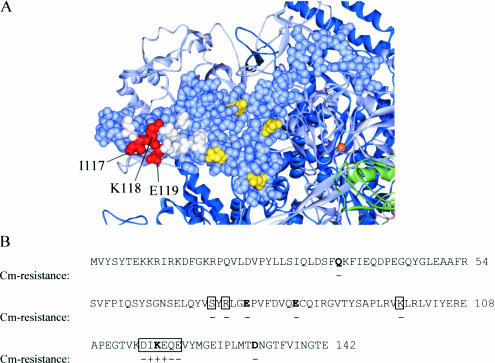
Figure 1.
An in vivo screen for amino acid substitutions in the β subunit of RNAP that affect Mfd function. (A) Model showing the distribution of the substituted residues in the β subunit of RNAP. The Figure shows part of the crystal structure of core T.aquaticus RNAP (34); PDB entry 1HQM.pdb. The region of the β1 domain known to be contacted by Mfd is shown in space-filling representation [residues 2–133 of the β subunit of T.aquaticus RNAP, equivalent to residues 1–142 of the β subunit of E.coli RNAP (9)] and other regions of RNAP are shown in ribbon form. Residues equivalent to those that were substituted in E.coli RNAP are highlighted; positions at which alanine or tryptophan substitutions conferred an Mfd− phenotype in the in vivo screen are coloured red, positions at which tryptophan substitution did not affect the Mfd phenotype are coloured yellow, and positions at which alanine substitution did not affect the Mfd phenotype are coloured white. Labels refer to residue numbers in E.coli RNAP. (B) Sequence of amino acids 1–142 of the β subunit of E.coli RNAP. Residues that were substituted with tryptophan are indicated by bold text and residues that were substituted with alanine are boxed. AB1157 cells were transformed with the reporter plasmid pRCB-CAT1 and derivatives of the rpoB expression plasmid pETLRpoB. The ability of transformants to grow on M9 agar plates containing 10 μg/ml chloramphenicol (Cm) is indicated below the sequence (+ indicates a Cm-resistant phenotype).
To determine whether any of these substitutions affected Mfd:RNAP interactions, we used a reporter system that detects the effect of Mfd on roadblock repression in vivo (10). The reporter plasmid pRCB-CAT1 carries a cat gene that is transcribed from a constitutive promoter. CAT expression is repressed by the Lac repressor protein, which binds to an operator located between the promoter and the cat gene and causes transcription elongation complexes to stall upstream of the cat coding region. Mfd increases the efficiency with which the Lac repressor roadblock represses transcription of the cat gene (10), presumably because Mfd removes the stalled RNAP from the DNA and so prevents RNAP from continuing transcription when the repressor protein transiently releases the operator. Strains in which Mfd is unable to remove RNAP from the DNA therefore express a higher level of CAT than strains with wild-type Mfd activity. Mutations that disrupt the ability of Mfd to displace RNAP can be detected by growing cells in the presence of chloramphenicol: at certain concentrations of chloramphenicol mutants with disrupted Mfd function (and elevated CAT expression) will grow, but cells with a wild-type Mfd phenotype will not (10).
AB1157 cells (mfd+) were transformed with the roadblock repression reporter plasmid pRCB-CAT1 and derivatives of plasmid pETLRpoB encoding wild-type β subunits or β subunits with tryptophan substitutions of residues Q36, E77, E84, K118 or D132. In these assays, the mutant β subunits were produced in-trans to wild-type β subunits expressed from the chromosomal rpoB allele. Any changes in the reporter expression observed would therefore be trans-dominant phenotypes: the mutant β subunits had to be incorporated into RNAP holoenzyme and initiate transcription effectively in competition with wild-type RNAP in order for their effect on roadblock repression to be detected. The effect of each β subunit on CAT expression was examined by incubating the transformants on M9 agar plates containing 10 μg/ml chloramphenicol (Figure 1B). Transformants expressing wild-type β subunits were unable to grow under these conditions, but transformants expressing β subunits with a KW118 substitution formed colonies. No other substitution tested had an effect on the chloramphenicol-resistance phenotype of the reporter strain.
The elevated CAT expression observed in cells expressing the βKW118 subunit suggested that this substitution decreased the efficiency of roadblock repression in the reporter strain, and that Mfd might contact the surface of the β subunit close to residue K118. To determine which side-chains within this region were important for Mfd activity, surface-exposed amino acids adjacent to K118 were individually substituted with alanine (Figure 1A). Plasmids encoding E.coli RNAP β subunits with alanine substitutions of residues S72, R74, K99, D116, I117, K118, E119, Q120 and E121 were constructed by site-directed mutagenesis. AB1157 cells were transformed with pRCB-CAT1 and derivatives of plasmid pETLRpoB encoding wild-type β subunits or β subunits with individual alanine substitutions, and the chloramphenicol-resistance phenotypes of the transformants were analysed as described above (Figure 1B). The only transformants that were able to grow in the presence of 10 μg/ml chloramphenicol were those expressing β subunits with IA117, KA118 or EA119 substitutions.
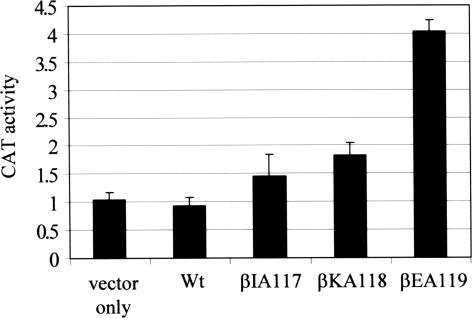
The effect of these alanine substitutions on roadblock repression was assayed quantitatively. AB1157 cells were transformed with pRCB-CAT1 and derivatives of plasmid pETLRpoB encoding βIA117, βKA118, βEA119 or wild-type β subunits. The transformants were grown to mid-exponential phase in M9 medium and then cells were harvested and CAT activity was determined (Figure 2). The CAT activity in cells expressing βEA119 subunits was 4-fold higher than the activity in cells expressing only wild-type β subunits. Cells expressing βKA118 subunits showed a small but reproducible increase in CAT activity, and CAT activity in cells expressing βIA117 subunits was not significantly different from wild-type. These results indicate that the βEA119 substitution has a greater effect on roadblock repression in vivo than the other substitutions tested, but they do not exclude a role for residues I118 and K118 in Mfd:RNAP interactions. The effects of mutant β subunits in this assay were limited by the presence of wild-type β subunits expressed from the chromosomal rpoB allele. Furthermore, we have previously found that the chloramphenicol-resistance phenotype on agar plates is a more sensitive assay of Mfd function than the measurement of CAT activity in liquid cultures: Mfd derivatives with only moderately impaired function conferred a chloramphenicol-resistant phenotype but did not significantly increase CAT activity in liquid culture (10).
Figure 2.
The effects of amino acid substitutions in the β subunit of RNAP on roadblock repression in vivo. AB1157 cells were transformed with the reporter plasmid pRCB-CAT1 and plasmid pETLRpoB encoding wild-type (Wt) β subunits or derivatives as indicated. In control experiments, cells were transformed with the plasmid pET21term (‘vector only’). Cultures were grown to OD600 ∼0.5 in M9 media containing appropriate antibiotics. CAT activities are expressed in nmol chloramphenicol acetylated/min/mg of protein and are shown as the average of three independent experiments (with SD).
Substitutions in the β subunit of RNAP affect dissociation of RNAP by Mfd in vitro
One explanation for the effect of the βIA117, βKA118 and βEA119 subunits on roadblock repression in vivo is that these substitutions interfere with the ability of Mfd to dissociate stalled RNAP from DNA. To test this hypothesis, we determined the effect of these substitutions on the displacement of purified transcription elongation complexes by Mfd in vitro.
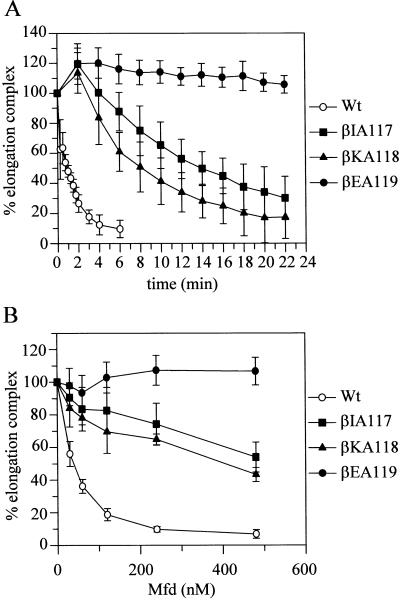
Wild-type and mutant RNAP holoenzymes were purified using a His6 tag at the C-terminus of the β subunit. Stalled elongation complexes were formed by incubating each RNAP with a radiolabelled DNA fragment that contained the T7 A1 promoter, and a mixture of nucleotides that lacked UTP. Under these conditions, RNAP will transcribe as far as +20 and then stall because of the absence of UTP (28). Mfd was added to the stalled elongation complexes and samples were removed at timed intervals. The samples were analysed by EMSA, and the proportion of transcription elongation complex remaining in each sample was determined (Figure 3A). As we have shown previously (10), ∼90% of wild-type elongation complexes were displaced after 5 min incubation with 250 nM Mfd. However, Mfd was unable to displace stalled elongation complexes formed with RNAP βEA119. Elongation complexes formed with RNAP βIA117 and RNAP βKA118 were displaced by Mfd, but more slowly than wild-type elongation complexes. These results confirm that the βIA117, βKA118 and βEA119 substitutions affect the ability of Mfd to displace stalled RNAP, which explains the effects of these substitutions on roadblock repression in vivo.
Figure 3.
The effects of alanine substitutions in the β subunit on the displacement of RNAP by Mfd in vitro. Displacement of stalled transcription complexes from radiolabelled DNA was monitored by EMSA, and quantified using a phosphorimager and ImageQuant software. Data points represent the amount of intact elongation complex present in each sample, expressed as a percentage of the amount present prior to the addition of Mfd (t = 0). Displacement assays were carried out on wild-type RNAP (open circles), RNAP βIA117 (filled squares), RNAP βKA118 (filled triangles) and RNAP βEA119 (filled circles). Values are the average of at least three independent experiments (with SD). (A) Time course of displacement of stalled RNAP at 37°C by 250 nM Mfd. (B) Effect of Mfd concentration on displacement of stalled RNAP. Samples were incubated with Mfd for 5 min at 37°C.
If the substitutions in the patch defined by β residues 117–119 decrease the affinity of Mfd for RNAP, the effect of the substitutions should be overcome if the concentration of one or both of the interacting partners is increased sufficiently. We therefore examined the effect of Mfd concentration on the displacement of wild-type and mutant elongation complexes. Stalled transcription complexes were incubated with Mfd at a range of concentrations, and after 5 min samples were analysed by EMSA (Figure 3B). Transcription elongation complexes formed with RNAP βIA117 and RNAP βKA118 were displaced more rapidly by 480 nM Mfd than by 240 nM Mfd, but Mfd did not displace transcription elongation complexes formed with RNAP βEA119 at any concentration tested. These results suggest that the defect caused by the βIA117 and βKA118 substitutions can be overcome by increasing Mfd concentration, which is consistent with a model in which they affect the affinity of Mfd for RNAP. The effect of the βEA119 substitution could not be overcome at experimentally accessible Mfd concentrations, suggesting that it either decreases the affinity of Mfd for RNAP to a much greater extent than the other two substitutions or it affects a later step in the displacement process that is not sensitive to the concentration of Mfd.
Substitutions in the β subunit of RNAP affect Mfd-catalysed reactivation of backtracked transcription complexes
The ability of Mfd to reactivate backtracked transcription elongation complexes is presumed to depend on an Mfd:RNAP interaction, but reactivation does not require Mfd to displace RNAP from the DNA template. To determine whether the βEA119 substitution specifically prevented removal of RNAP from DNA, or whether it caused a more general defect in Mfd function, we examined the ability of Mfd to reactivate backtracked complexes that contained RNAP βEA119.
To generate backtracked complexes, transcription complexes were formed on a biotinylated T7 A1 DNA template that was bound to streptavidin paramagnetic beads. Transcriptional arrest occurs more frequently at some positions on a DNA template than at others, and complexes stalled at position +27 downstream of the T7 A1 promoter readily become backtracked and arrested (17). The transcription complexes immobilized on streptavidin beads were ‘walked’ to defined positions on the template by sequential addition of different combinations of ribonucleotides (NTPs), interspersed with wash steps to remove unincorporated nucleotides (36). At each step, the progress of the transcription reaction was monitored by analysing the radiolabelled transcript on a denaturing acrylamide gel (data not shown). An elongation complex stalled at +26 was formed by the addition of ApU, ATP, GTP and CTP to an open complex formed on the T7 A1 promoter. Under these conditions, the transcription complex should stall at +20 (the position preceding the first U in the transcript), but, as reported previously (28), we observed significant readthrough to +26 (the position preceding the second U in the transcript). Readthrough of +20 is enhanced at elevated NTP concentrations (28) and probably results from the presence of low levels of UTP generated by deamination of CTP. The +26 complexes were walked forward to +27 by the addition of UTP.
To determine whether the complexes stalled at +27 were backtracked, a mixture of all four NTPs was added: non-backtracked complexes should transcribe to the end of the DNA template, but backtracked complexes should be unable to extend their transcripts despite the presence of NTPs. In parallel experiments, the ability of Mfd to reactivate backtracked complexes was determined by adding Mfd with the mixture of NTPs. The transcripts were analysed by denaturing gel electrophoresis (Figure 4).
Figure 4.
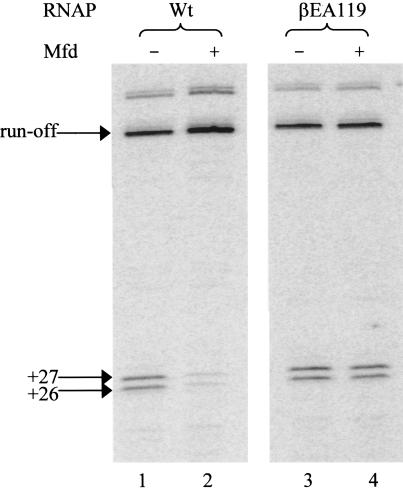
Reactivation of arrested transcription complexes in vitro. Immobilized transcription elongation complexes were incubated with 50 μM CTP, GTP and UTP and 2 mM ATP in the presence and absence of Mfd. The transcripts produced were analysed by denaturing acrylamide gel electrophoresis. Arrows indicate the position of transcripts 26 and 27 nt long, and transcripts that terminate at the end of the linear DNA template (‘run-off’).
In the absence of Mfd, the results obtained with wild-type RNAP and RNAP βEA119 were essentially identical. In both cases, the ‘run-off’ transcript was predominant, indicating that many of the stalled transcription complexes had been able to resume transcription when NTPs were added. In addition to the ‘run-off’ transcript that terminated at the end of the linear template, a longer transcript was evident: this is a common observation in transcription reactions that use a linear template and arises from end-to-end transfer of RNAP between DNA molecules (37). The presence of a transcript 27 nt long indicates that, as expected, a proportion of the complexes stalled at +27 were arrested. The presence of a transcript 26 nt long indicates that, under the conditions used in these experiments, some complexes stalled at +26 were also arrested.
In the presence of Mfd virtually all of the transcripts formed by wild-type RNAP were full-length ‘run-off’ transcripts, and the intensities of the bands corresponding to complexes stalled at +26 or +27 were greatly reduced (Figure 4, compare lanes 1 and 2). This suggests that Mfd enabled most of the arrested complexes formed by wild-type RNAP to resume transcription, presumably by causing RNAP to translocate forward until the 3′ end of the transcript was returned to the active site as described previously (9). In contrast, Mfd had no effect on the pattern of transcripts observed in reactions containing RNAP βEA119 (Figure 4, compare lanes 3 and 4). This indicates that Mfd is not able to reactivate arrested transcription complexes formed by RNAP βEA119, and demonstrates that the effect of the βEA119 substitution is not restricted to processes that involve the removal of RNAP from DNA.
The βEA119 substitution disrupts a direct interaction between the β subunit of RNAP and Mfd
Direct interactions between Mfd and RNAP have been detected in ‘pull-down’ assays (6) and in yeast two-hybrid experiments (9,21). To determine whether the βEA119 substitution disrupts a direct interaction between RNAP and Mfd, we analysed its effect in both of these systems.
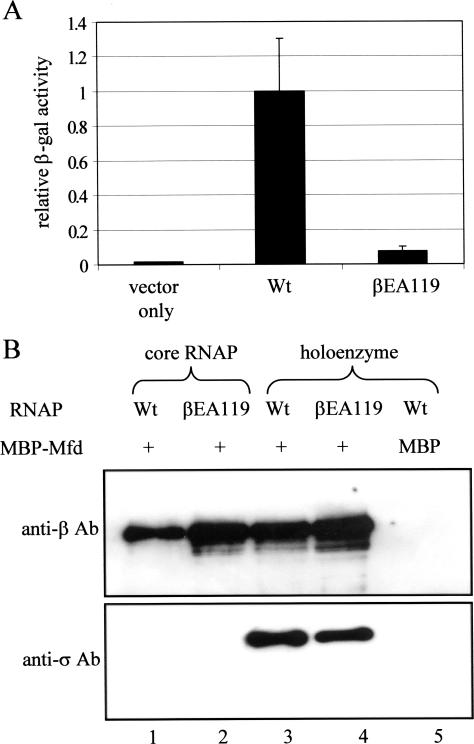
We used yeast two-hybrid assays to examine the effect of the βEA119 substitution on the interaction of the Mfd RID and the N-terminal region of the RNAP β subunit. We constructed plasmids encoding residues 1–142 of wild-type or EA119 RNAP β subunits fused to the Gal4 activation domain, and residues 472–603 of Mfd fused to the Gal4 DNA-binding domain [replicating the system used in (9)]. Yeast reporter strain Y190 was transformed with combinations of the plasmids, and the level of β-galactosidase expressed from a Gal4-dependent reporter gene was determined (Figure 5A). In accordance with the previously published work (9), the interaction between the wild-type β subunit fragment and the Mfd RID resulted in an elevated level of β-galactosidase expression. The level of activation was greatly reduced when the β subunit fusion protein contained the EA119 substitution, indicating that the substitution disrupts this protein–protein interaction.
Figure 5.
Analysis of Mfd:RNAP interactions. (A) Yeast two-hybrid analysis. Yeast strain Y190 was transformed with a plasmid encoding a Gal4DBD–Mfd (472–603) fusion protein, and with a plasmid encoding either Gal4AD (‘vector only’) or a Gal4AD fusion protein to residues 1–142 of the wild-type (Wt) β subunit, or a Gal4AD fusion protein to residues 1–142 of βEA119. β-galactosidase activities shown are the average of three independent assays (with SD). (B) ‘Pull-down’ analysis. Wild-type or βEA119 RNAP was incubated with MBP–Mfd linked to amylose resin, and protein that remained bound after washing was detected by western blotting using polyclonal antibodies against the β and σ70 subunits (lanes 1–4). The specificity of the interaction was confirmed by measuring the ability of MBP bound to amylose resin to retain RNAP (lane 5).
We then used ‘pull-down’ assays to examine the effect of the βEA119 substitution on the binding of full-length Mfd to RNAP core and holoenzyme. An MBP–Mfd fusion protein was immobilized on amylose resin and incubated with wild-type RNAP or RNAP βEA119. The resin was washed, and RNAP that remained bound was detected by western blotting using antibodies against the β subunit and the σ70 subunit (Figure 5B). Wild-type RNAP core and holoenzyme both bound to MBP–Mfd in this assay, and RNAP did not bind to MBP in control experiments. Both β and σ70 were detected in the samples of bound holoenzyme, in accordance with previous reports that Mfd and the σ subunit can bind to RNAP simultaneously (6). In contrast to the result of the yeast two-hybrid experiments, the βEA119 substitution had no effect on the ability of RNAP to bind to MBP-Mfd in ‘pull-down’ assays. RNAP core and holoenzyme containing βEA119 subunits both bound to Mfd in the ‘pull-down’ assay, (Figure 5B) and we could detect no difference in the amount of RNAP bound or the salt-sensitivity of the Mfd:RNAP complexes in experiments with wild-type or mutant enzymes (data not shown). One explanation for this result is that interactions between the Mfd RID and residues I117 and K118 of the β subunit were sufficient for Mfd and RNAP to bind one another under the conditions of this assay, even in the absence of the E119 side chain. To test this theory, we purified core RNAP in which the β subunit contained simultaneous alanine substitutions of I117, K118 and E119. In ‘pull-down’ assays, this triple-mutant RNAP bound to MBP–Mfd as effectively as wild-type RNAP (data not shown).
The results of the two-hybrid assays indicate that a single alanine substitution at E119 can disrupt the interaction between the Mfd RID and the N-terminus of the β subunit, but alanine substitution of β residues I117, K118 and E119 was not sufficient to abolish interactions between Mfd and RNAP in ‘pull-down’ experiments. As the interaction between the Mfd RID and the N-terminus of the β subunit is the only known contact between the proteins, these results most likely reflect the different sensitivities of the two assays to changes in binding affinity. However, as the pull-down experiments involved full-length proteins and the yeast two-hybrid experiments involved protein fragments, it is not possible to exclude the existence of a second contact between some other regions of Mfd and RNAP. If such a contact exists, it was not detected in the global analysis of protein–protein interactions in H.pylori that identified the Mfd RID (21).
DISCUSSION
Shortly after Mfd was identified as the transcription-repair coupling factor, it was predicted that mutations in the mfd gene, the uvrA gene and in one or more of the rpo genes that encode subunits of RNAP should give rise to the Mfd− phenotype (38). However, all reported mutants with an Mfd− phenotype have mutations in the mfd gene (6,8,10,39). In this work, we have isolated rpoB mutants that cause defects in Mfd function in vivo and in vitro and that show no apparent defects in transcription. To the best of our knowledge, these are the first RNAP mutants shown to be specifically defective in a step of transcription-coupled repair, or any other Mfd-dependent process. The mutants contain amino acid substitutions that define a surface-exposed patch on the surface of the β subunit of E.coli RNAP that is essential for the action of Mfd on transcription complexes. The key residue within this patch is E119: an alanine substitution at this position interfered with the ability of Mfd to affect roadblock repression in vivo and abolished the ability of Mfd to dissociate stalled transcription complexes or reactivate backtracked transcription complexes in vitro. Alanine substitutions of I117 and K118 had similar, but less pronounced, effects on the function of Mfd.
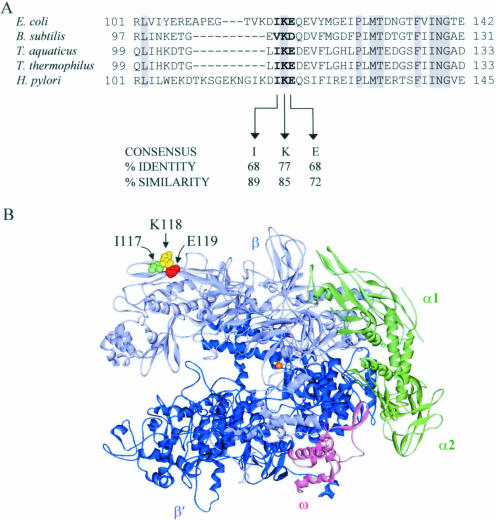
Residues I117, K118 and E119 are highly conserved in β subunits from different bacterial species (Figure 6A). Interestingly, given that B.subtilis Mfd can displace E.coli RNAP, the Mfd-interaction surface of RNAP from the two species is not identical: the IKE sequence of E.coli is replaced with VKD in B.subtilis. The Mfd interaction ‘motif’ appears to be a hydrophobic residue followed by a positively charged residue and then by a negatively charged residue. The crystal structure of prokaryotic core RNAP has a shape like a crab claw, with one arm of the claw formed mainly by the β subunit, and the other arm formed mainly by the β′ subunit (Figure 6B) (33,34). The RNAP residues that interact with Mfd form a small surface-exposed patch on the surface of the β1 domain (β residues 22–130 and 336–392 in T.aquaticus), close to the tip of the β arm of the claw. The β1 domain is one of four mobile modules within RNAP holoenzyme that are able to move relative to the catalytic core module and to each other (22). In RNAP holoenzyme, it makes extensive interactions with region 3 of the σ subunit, and it has been suggested that binding of the σ subunit to core RNAP triggers movement of the β1 domain (22). Region 3 of the σ subunit appears to ‘slide’ over the surface of the β1 domain, as the relative position of the two domains differs between RNAP holoenzyme crystal structures (22,40–42). The residues of RNAP that interact with Mfd are close to, but separate from, the residues contacted by region 3 of σ in all of the available RNAP holoenzyme crystal structures (22,40–42). The observation that the binding sites for Mfd and σ70 do not overlap is consistent with the ability of Mfd to bind to RNAP holoenzyme as well as core [this work and (6)].
Figure 6.
Conservation of residues involved in Mfd function. (A) ClustalW (43) alignment of part of the RNAP β subunit sequence from bacterial species for which an Mfd:RNAP interaction has been demonstrated (E.coli, B.subtilis and H.pylori), or for which the structure of RNAP is known (T.aquaticus and T.thermophilus). Residues shown to be important for Mfd function are indicated by bold text. Positions at which residues are identical in all five species are highlighted in grey. A consensus sequence for the three residues required by Mfd was derived by alignment of RNAP β subunit sequences from 47 eubacterial strains using the Conserved Domain Database (44). Similarity to the consensus was defined using the ClustalW groupings of conserved substitutions (43): I, A, V, F, P, M, L or W at position 1; K, R or H at position 2; E or D at position 3. (B) Crystal structure of T.aquaticus core RNAP (34) showing the position of residues found to be important for Mfd function. The residues highlighted are T.aquaticus residues I108 (green), K109 (yellow) and E110 (red). The labels indicate the identity of the equivalent residues in E.coli RNAP (I117, K118 and E119). The Mg2+ ion at the active site is shown as an orange sphere.
Which step in the displacement of RNAP by Mfd is affected by substitution of E119 and neighbouring residues? In order to displace a stalled transcription complex, the Mfd protein must first be recruited, and must then destabilize the elongation complex by the ATP-dependent action of the helicase/TRG domains on upstream DNA. In some circumstances, additional ATP-dependent translocation steps are required to return the 3′ end of the transcript to the active site prior to displacement (9). Interactions between Mfd and RNAP may be necessary for the recruitment of Mfd, for the maintenance of Mfd:RNAP contacts during translocation, for the destabilization of the transcription complex or for a combination of all three. Several lines of evidence suggest that the side chains of β subunit residues 117–119 are involved in a direct interaction between Mfd and RNAP, and that this interaction is required prior to the destabilization and displacement step. First, the defects caused by substitution of these residues appear to be restricted to Mfd-dependent processes; we did not detect any effect of the substitutions on the ability of RNAP to initiate and terminate transcription in vitro in the absence of Mfd (data not shown). Second, the defects caused by βIA117 and βKA118 substitutions in in vitro RNAP-displacement assays could be overcome in part by increasing the concentration of Mfd, suggesting that the substitutions had decreased the affinity of RNAP for Mfd. Third, backtracked elongation complexes formed using RNAP βEA119 could not be reactivated by Mfd, indicating that the effect of the βEA119 substitution on Mfd function is not restricted to processes that require RNAP to be removed from DNA. Finally, the βEA119 substitution abolished the interaction between the N-terminal region of the RNAP β subunit and the RID of Mfd in a yeast two-hybrid experiment, demonstrating that a direct interaction between the two proteins is dependent on E119.
These observations indicate that the interaction between the Mfd RID and the β1 domain of RNAP occurs early on in the displacement process; most likely at the time that Mfd is recruited to stalled RNAP. However, the results do not preclude the possibility that the same interaction also plays a role in a later stage of RNAP displacement. The mechanism by which Mfd removes RNAP from DNA is not well understood, and Mfd-induced conformational changes in RNAP may be important for destabilization of the elongation complex. Movement of the β1 domain is one of the conformational changes associated with the opening and closing of the main channel of RNAP: it is suggested that the ‘open’ state is important during transcription initiation and the ‘closed’ state is associated with the stably bound elongation complex (4). It is possible that the interaction of Mfd with the β1 domain does more than simply position the protein in a convenient location to interact with the upstream DNA, and that movement of the β1 domain triggered by ATP-driven translocation of Mfd plays a part in the destabilization of the transcription complex during Mfd-dependent transcription termination.
Acknowledgments
We are grateful to Professor Akira Ishihama for antibodies against the β and σ70 subunits of RNAP, to Dr Chris Selby for plasmid pMALMFD, to Dr Ding Jun Jin for E.coli strain DJ473, and to Anna Chambers and members of the University of Bristol DNA–protein interactions group for many helpful discussions. This work was supported by research grant G15249 from the BBSRC (UK). Funding to pay the Open Access publication charges for this article was provided by the JISC.
REFERENCES
- 1.Severinov K. RNA polymerase structure-function: insights into points of transcriptional regulation. Curr. Opin. Microbiol. 2000;3:118–125. doi: 10.1016/s1369-5274(00)00062-x. [DOI] [PubMed] [Google Scholar]
- 2.Borukhov S., Nudler E. RNA polymerase holoenzyme: structure, function and biological implications. Curr. Opin. Microbiol. 2003;6:93–100. doi: 10.1016/s1369-5274(03)00036-5. [DOI] [PubMed] [Google Scholar]
- 3.Ebright R.H. RNA polymerase: structural similarities between bacterial RNA polymerase and eukaryotic RNA polymerase II. J. Mol. Biol. 2000;304:687–698. doi: 10.1006/jmbi.2000.4309. [DOI] [PubMed] [Google Scholar]
- 4.Murakami K.S., Darst S.A. Bacterial RNA polymerases: the wholo story. Curr. Opin. Struct. Biol. 2003;13:31–39. doi: 10.1016/s0959-440x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 5.Hahn S. Structure and mechanism of the RNA polymerase II transcription machinery. Nature Struct. Mol. Biol. 2004;11:394–403. doi: 10.1038/nsmb763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selby C.P., Sancar A. Structure and function of transcription-repair coupling factor. I. Structural domains and binding properties. J. Biol. Chem. 1995;270:4882–4889. doi: 10.1074/jbc.270.9.4882. [DOI] [PubMed] [Google Scholar]
- 7.Selby C.P., Sancar A. Structure and function of transcription-repair coupling factor. II. Catalytic properties. J. Biol. Chem. 1995;270:4890–4895. doi: 10.1074/jbc.270.9.4890. [DOI] [PubMed] [Google Scholar]
- 8.Selby C.P., Sancar A. Molecular mechanism of transcription-repair coupling. Science. 1993;260:53–58. doi: 10.1126/science.8465200. [DOI] [PubMed] [Google Scholar]
- 9.Park J.S., Marr M.T., Roberts J.W. E.coli transcription repair coupling factor (Mfd protein) rescues arrested complexes by promoting forward translocation. Cell. 2002;109:757–767. doi: 10.1016/s0092-8674(02)00769-9. [DOI] [PubMed] [Google Scholar]
- 10.Chambers A.L., Smith A.J., Savery N.J. A DNA translocation motif in the bacterial transcription-repair coupling factor, Mfd. Nucleic Acids Res. 2003;31:6409–6418. doi: 10.1093/nar/gkg868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng X., Galinier A., Saxild H.H. Catabolite repression of dra-nupC-pdp operon expression in Bacillus subtilis. Microbiology. 2000;146:2901–2908. doi: 10.1099/00221287-146-11-2901. [DOI] [PubMed] [Google Scholar]
- 12.Zalieckas J.M., Wray L.V., Jr, Ferson A.E., Fisher S.H. Transcription-repair coupling factor is involved in carbon catabolite repression of the Bacillus subtilis hut and gnt operons. Mol. Microbiol. 1998;27:1031–1038. doi: 10.1046/j.1365-2958.1998.00751.x. [DOI] [PubMed] [Google Scholar]
- 13.Deuschle U., Gentz R., Bujard H. lac Repressor blocks transcribing RNA polymerase and terminates transcription. Proc. Natl Acad. Sci. USA. 1986;83:4134–4137. doi: 10.1073/pnas.83.12.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selby C.P., Sancar A. Transcription-repair coupling and mutation frequency decline. J. Bacteriol. 1993;175:7509–7514. doi: 10.1128/jb.175.23.7509-7514.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung S.C., Gottesman M.E. Phage HK022 Nun protein arrests transcription on phage lambda DNA in vitro and competes with the phage lambda N antitermination protein. J. Mol. Biol. 1995;247:428–442. doi: 10.1006/jmbi.1994.0151. [DOI] [PubMed] [Google Scholar]
- 16.Washburn R.S., Wang Y., Gottesman M.E. Role of E.coli Transcription-repair coupling factor Mfd in Nun-mediated transcription termination. J. Mol. Biol. 2003;329:655–662. doi: 10.1016/s0022-2836(03)00465-0. [DOI] [PubMed] [Google Scholar]
- 17.Komissarova N., Kashlev M. Transcriptional arrest: Escherichia coli RNA polymerase translocates backward, leaving the 3′ end of the RNA intact and extruded. Proc. Natl Acad. Sci. USA. 1997;94:1755–1760. doi: 10.1073/pnas.94.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borukhov S., Sagitov V., Goldfarb A. Transcript cleavage factors from E.coli. Cell. 1993;72:459–466. doi: 10.1016/0092-8674(93)90121-6. [DOI] [PubMed] [Google Scholar]
- 19.Singleton M.R., Scaife S., Wigley D.B. Structural analysis of DNA replication fork reversal by RecG. Cell. 2001;107:79–89. doi: 10.1016/s0092-8674(01)00501-3. [DOI] [PubMed] [Google Scholar]
- 20.Roberts J., Park J.S. Mfd, the bacterial transcription repair coupling factor: translocation, repair and termination. Curr. Opin. Microbiol. 2004;7:120–125. doi: 10.1016/j.mib.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Rain J.C., Selig L., De Reuse H., Battaglia V., Reverdy C., Simon S., Lenzen G., Petel F., Wojcik J., Schachter V., et al. The protein–protein interaction map of Helicobacter pylori. Nature. 2001;409:211–215. doi: 10.1038/35051615. [DOI] [PubMed] [Google Scholar]
- 22.Murakami K.S., Masuda S., Darst S.A. Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 Å resolution. Science. 2002;296:1280–1284. doi: 10.1126/science.1069594. [DOI] [PubMed] [Google Scholar]
- 23.Severinov K., Soushko M., Goldfarb A., Nikiforov V. Rifampicin region revisited. New rifampicin-resistant and streptolydigin-resistant mutants in the beta subunit of Escherichia coli RNA polymerase. J. Biol. Chem. 1993;268:14820–14825. [PubMed] [Google Scholar]
- 24.Sambrook J., Russel D.W. Molecular Cloning: A Laboratory Manual 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 25.Sukhodolets M.V., Jin D.J. Interaction between RNA polymerase and RapA, a bacterial homolog of the SWI/SNF protein family. J. Biol. Chem. 2000;275:22090–22097. doi: 10.1074/jbc.M000056200. [DOI] [PubMed] [Google Scholar]
- 26.Niu W., Kim Y., Tau G., Heyduk T., Ebright R.H. Transcription activation at class II CAP-dependent promoters: two interactions between CAP and RNA polymerase. Cell. 1996;87:1123–1134. doi: 10.1016/s0092-8674(00)81806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Savery N.J., Lloyd G.S., Kainz M., Gaal T., Ross W., Ebright R.H., Gourse R.L., Busby S.J. Transcription activation at Class II CRP-dependent promoters: identification of determinants in the C-terminal domain of the RNA polymerase alpha subunit. EMBO J. 1998;17:3439–3447. doi: 10.1093/emboj/17.12.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin J.R., Krummel B., Chamberlin M.J. Isolation and properties of transcribing ternary complexes of Escherichia coli RNA polymerase positioned at a single template base. J. Mol. Biol. 1987;196:85–100. doi: 10.1016/0022-2836(87)90512-2. [DOI] [PubMed] [Google Scholar]
- 29.Adelman K., Yuzenkova J., La Porta A., Zenkin N., Lee J., Lis J.T., Borukhov S., Wang M.D., Severinov K. Molecular mechanism of transcription inhibition by Peptide antibiotic microcin j25. Mol. Cell. 2004;14:753–762. doi: 10.1016/j.molcel.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Harper J.W., Adami G.R., Wei N., Keyomarsi K., Elledge S.J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 31.Gietz D., St Jean A., Woods R.A., Schiestl R.H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rupp S. LacZ assays in yeast. Methods Enzymol. 2002;350:112–131. doi: 10.1016/s0076-6879(02)50959-9. [DOI] [PubMed] [Google Scholar]
- 33.Zhang G., Campbell E.A., Minakhin L., Richter C., Severinov K., Darst S.A. Crystal structure of Thermus aquaticus core RNA polymerase at 3.3 Å resolution. Cell. 1999;98:811–824. doi: 10.1016/s0092-8674(00)81515-9. [DOI] [PubMed] [Google Scholar]
- 34.Minakhin L., Bhagat S., Brunning A., Campbell E.A., Darst S.A., Ebright R.H., Severinov K. Bacterial RNA polymerase subunit omega and eukaryotic RNA polymerase subunit RPB6 are sequence, structural, and functional homologs and promote RNA polymerase assembly. Proc. Natl Acad. Sci. USA. 2001;98:892–897. doi: 10.1073/pnas.98.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayora S., Rojo F., Ogasawara N., Nakai S., Alonso J.C. The Mfd protein of Bacillus subtilis 168 is involved in both transcription-coupled DNA repair and DNA recombination. J. Mol. Biol. 1996;256:301–318. doi: 10.1006/jmbi.1996.0087. [DOI] [PubMed] [Google Scholar]
- 36.Nudler E., Gusarov I., Bar-Nahum G. Methods of walking with the RNA polymerase. Methods Enzymol. 2003;371:160–169. doi: 10.1016/S0076-6879(03)71011-8. [DOI] [PubMed] [Google Scholar]
- 37.Nudler E., Avetissova E., Markovtsov V., Goldfarb A. Transcription processivity: protein–DNA interactions holding together the elongation complex. Science. 1996;273:211–217. doi: 10.1126/science.273.5272.211. [DOI] [PubMed] [Google Scholar]
- 38.Selby C.P., Sancar A. Mechanisms of transcription-repair coupling and mutation frequency decline. Microbiol. Rev. 1994;58:317–329. doi: 10.1128/mr.58.3.317-329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witkin E.M. Radiation-induced mutations and their repair. Science. 1966;152:1345–1353. doi: 10.1126/science.152.3727.1345. [DOI] [PubMed] [Google Scholar]
- 40.Murakami K.S., Masuda S., Campbell E.A., Muzzin O., Darst S.A. Structural basis of transcription initiation: an RNA polymerase holoenzyme–DNA complex. Science. 2002;296:1285–1290. doi: 10.1126/science.1069595. [DOI] [PubMed] [Google Scholar]
- 41.Vassylyev D.G., Sekine S., Laptenko O., Lee J., Vassylyeva M.N., Borukhov S., Yokoyama S. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature. 2002;417:712–719. doi: 10.1038/nature752. [DOI] [PubMed] [Google Scholar]
- 42.Artsimovitch I., Patlan V., Sekine S., Vassylyeva M.N., Hosaka T., Ochi K., Yokoyama S., Vassylyev D.G. Structural basis for transcription regulation by alarmone ppGpp. Cell. 2004;117:299–310. doi: 10.1016/s0092-8674(04)00401-5. [DOI] [PubMed] [Google Scholar]
- 43.Chenna R., Sugawara H., Koike T., Lopez R., Gibson T.J., Higgins D.G., Thompson J.D. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marchler-Bauer A., Anderson J.B., DeWeese-Scott C., Fedorova N.D., Geer L.Y., He S., Hurwitz D.I., Jackson J.D., Jacobs A.R., Lanczycki C.J., et al. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 2003;31:383–387. doi: 10.1093/nar/gkg087. [DOI] [PMC free article] [PubMed] [Google Scholar]