Abstract
IMPORTANCE
The burden and determinants of complications and comorbidities in contemporary youth-onset diabetes are unknown.
OBJECTIVE
To determine the prevalence of and risk factors for complications related to type 1 diabetes vs type 2 diabetes among teenagers and young adults who had been diagnosed with diabetes during childhood and adolescence.
DESIGN, SETTING, AND PARTICIPANTS
Observational study from 2002 to 2015 in 5 US locations, including 2018 participants with type 1 and type 2 diabetes diagnosed at younger than 20 years, with single outcome measures between 2011 and 2015.
EXPOSURES
Type 1 and type 2 diabetes and established risk factors (hemoglobin A1c level, body mass index, waist-height ratio, and mean arterial blood pressure).
MAIN OUTCOMES AND MEASURES
Diabetic kidney disease, retinopathy, peripheral neuropathy, cardiovascular autonomic neuropathy, arterial stiffness, and hypertension.
RESULTS
Of 2018 participants, 1746 had type 1 diabetes (mean age, 17.9 years [SD 4.1]; 1327 non-Hispanic white [76.0%]; 867 female patients [49.7%]), and 272 had type 2 (mean age, 22.1 years [SD 3.5]; 72 non-Hispanic white [26.5%]; 181 female patients [66.5%]). Mean diabetes duration was 7.9 years (both groups). Patients with type 2 diabetes vs those with type 1 had higher age-adjusted prevalence of all measured complications except cardiovascular autonomic neuropathy. After adjustment for established risk factors measured over time, participants with type 2 diabetes vs those with type 1 had significantly higher odds of diabetic kidney disease, retinopathy, and peripheral neuropathy but no significant difference in the odds of arterial stiffness and hypertension.
| Complication | Age-Adjusted Prevalence, % | Absolute Difference, % (95% CI) | P Value | Adjusted Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|---|---|
|
| ||||||
| Type 2 Diabetes | Type 1 Diabetes | |||||
| Diabetic kidney disease | 19.9 | 5.8 | 14.0 (9.1 to 19.9) | <.001 | 2.58 (1.39–4.81) | .003 |
| Retinopathy | 9.1 | 5.6 | 3.5 (0.4 to 7.7) | .02 | 2.24 (1.11–4.50) | .02 |
| Peripheral neuropathy | 17.7 | 8.5 | 9.2 (4.8 to 14.4) | <.001 | 2.52 (1.43–4.43) | .001 |
| Cardiovascular autonomic neuropathy | 15.7 | 14.4 | 1.2 (−3.1 to 6.5) | .62 | 0.98 (0.57–1.67) | .93 |
| Arterial stiffness | 47.4 | 11.6 | 35.9 (29.0 to 42.9) | <.001 | 1.07 (0.63–1.84) | .80 |
| Hypertension | 21.6 | 10.1 | 11.5 (6.8 to 16.9) | <.001 | 0.85 (0.50–1.45) | .55 |
CONCLUSIONS AND RELEVANCE
Among teenagers and young adults who had been diagnosed with diabetes during childhood or adolescence, the prevalence of complications and comorbidities was higher among those with type 2 diabetes compared with type 1, but frequent in both groups. These findings support early monitoring of youth with diabetes for development of complications.
The increased prevalence of type 2 diabetes among children and adolescents has been relatively recent in most populations,1–3 beginning in the early to mid-1990s. Additionally, a long-term increase in type 1 diabetes has been observed both worldwide4 and in the United States.3 These recent epidemiologic trends in type 1 and type 2 diabetes diagnosed in young individuals raise the question of whether the pattern of complications differs by diabetes type at similar ages and diabetes duration. Recent studies have reported higher prevalence of some5–7 but not all8 complications in children and adolescents with type 2 diabetes compared with those with type 1. Reasons for discrepancies across previous studies include population differences, relatively small sample sizes, variable length of duration of diabetes at outcome assessment, variable ages, and reliance on routine clinical or administrative records to document outcomes. Preliminary findings have suggested that there is a higher prevalence of selected complications and risk factors among adolescents and young adults with type 2 diabetes compared with type 1.9
Given those findings, this study was designed as an outcomes evaluation to comprehensively estimate the prevalence of multiple diabetes-related complications (retinopathy, neuropathy, and nephropathy) and comorbidities (hypertension and arterial stiffness) by type of diabetes. The a priori hypothesis was that the prevalence of complications and comorbidities would be higher in adolescents and young adults with type 2 diabetes compared with those with type 1. The secondary hypothesis was that the increased prevalence of complications would be reduced, at least in part, by adjustment for longitudinally measured established risk factors, including glycemia (hemoglobin A1c level), obesity (body mass index [BMI]; waist-height ratio), and blood pressure (mean arterial pressure).
Methods
Study Population
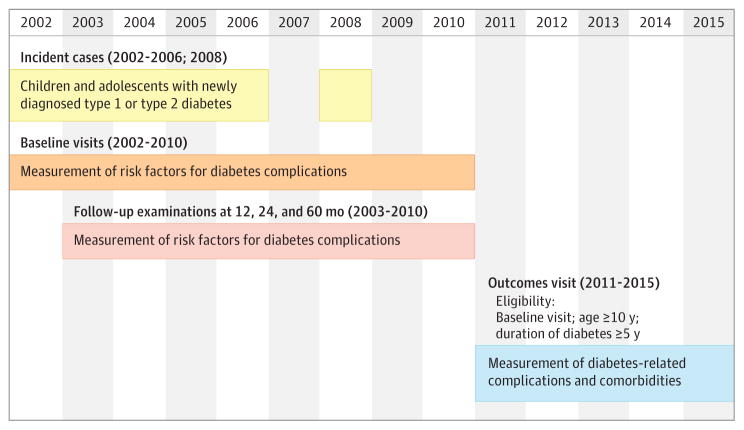
Children and adolescents with diabetes diagnosed at younger than 20 years were identified from a population-based incidence registry network at 5 US sites (South Carolina; Cincinnati, Ohio, and surrounding counties; Colorado with southwestern Native American sites; Seattle, Washington, and surrounding counties; and Kaiser Permanente, Southern California) by the SEARCH for Diabetes in Youth registry study.9 Patients received a new diagnosis of type 1 or type 2 diabetes in 2002–2006 or 2008 and were identified from ongoing surveillance of networks of hospitals and other clinical sites. Patients who could be contacted were recruited for a baseline visit (mean of 9.3 months [SD, 6.4] from diagnosis) and, if they completed it, were asked to return for visits at 12, 24, and 60 months to measure risk factors for diabetes complications (Figure 1).
Figure 1. Design of the SEARCH for Diabetes in Youth Cohort Study.
Risk factors measured at baseline, follow-up, and outcome visits included glycemia (measured as hemoglobin A1c), obesity (body mass index and waist-height ratio), and blood pressure levels. Outcomes measured at the outcome visits included diabetic retinopathy, neuropathy, nephropathy, hypertension, and arterial stiffness.
A subset of participants aged 10 years or older who had at least 5 years of diabetes duration (to increase the likelihood of detection of complications) were recruited for an outcome visit between 2011 and 2015 (mean of 7.9 years [SD, 1.9] from diagnosis), for whom a single prevalent measurement of diabetes-related complications and comorbidities was completed. A flowchart depicting included and excluded participants is shown in Figure 2. The study was approved by institutional review boards with jurisdiction, and for all participants, the parent, adolescent or young adult, or both provided consent or assent.
Figure 2. Flow of Participants in the SEARCH for Diabetes in Youth Cohort Study.
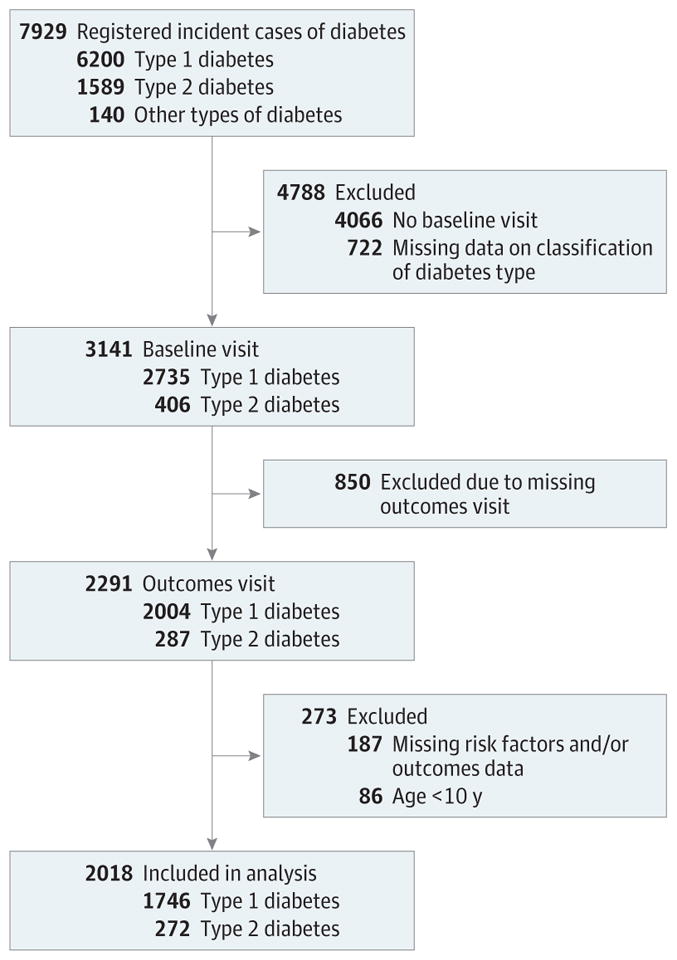
“Other” types included unknown, hybrid, and missing diabetes type.
Research Visits
Trained personnel administered questionnaires, made measurements, and obtained blood samples. Because race and ethnicity are often related to differing disease outcomes, US census methods10 were used that provided a series of fixed race and ethnicity categories, as well as an “other” option for the self-report by parent or participant, depending on age. These were further categorized into non-Hispanic white and minority racial/ethnic groups, including Hispanic (regardless of race), non-Hispanic black, American Indian, Asian/Pacific Islander, and other or multiple races/ethnicities. Education and income were self-reported. Body mass index was calculated as weight in kilograms divided by height in meters squared and converted to a z score.11 Waist circumference was measured with the natural waist location and was used to calculate the ratio of waist to height. The mean of 3 systolic and diastolic blood pressure levels was obtained with an aneroid manometer after at least 5 minutes of rest. A blood draw occurred after an 8-hour overnight fast, and medications, including short-acting insulin, were withheld the morning of the visit.
Laboratory Measures
Specimens were analyzed for glutamic acid decarboxylase–65 antibodies and insulinoma-associated-2 antibodies at the central laboratory12 (Northwest Lipid Metabolism and Diabetes Research). Zinc-T8 autoantibody was analyzed at the Eisenbarth Laboratory (University of Colorado).13 Levels of fasting C-peptide, hemoglobin A1c, glucose, lipids, creatinine, cystatin C, and urine albumin and creatinine were also measured.
Type of Diabetes
Diabetes type was defined with an etiologic classification14,15 based on 1 or more positive diabetes autoantibody results and estimated insulin sensitivity score (validated equation including waist circumference and hemoglobin A1c and triglyceride levels) at the baseline visit. Type 1 diabetes was defined as at least 1 positive antibody result, regardless of insulin sensitivity, or no positive antibody results and insulin sensitivity (score ≥8.15). Type 2 diabetes was defined as negative antibody results and insulin sensitivity (score <8.15).
Outcome Measures
Diabetic Kidney Disease
A first-morning urine void at home was brought to the outcome visit by 92% of participants, and if not available (in 8%), a spot sample was used. Diabetic kidney disease was defined as the presence of albuminuria (≥30μg/mg of creatinine) or estimated glomerular filtration rate less than or equal to 60 mL/min/1.73 m2 with the CKD-Epi equations with serum creatinine and cystatin C.16 Only 1 participant was classified as having diabetic kidney disease according to low estimated glomerular filtration rate.
Diabetic retinopathy was determined by grading 45° color digital fundus images centered on the disc and macula of both eyes, taken with a nonmydriatic camera (Visucam Pro N; Carl Zeiss Meditech). The Wisconsin Ocular Epidemiology Reading Center graded photos masked to all clinical characteristics. Retinopathy severity was based on the worse eye and categorized as none, minimal nonproliferative, mild to moderate nonproliferative, or proliferative.17 Diabetic retinopathy was defined as presence of mild, moderate, or proliferative retinal changes.
Peripheral neuropathy was assessed with the Michigan Neuropathy Screening Instrument examination and was defined as a score greater than 2.18,19
Cardiovascular Autonomic Neuropathy
Assessment of heart rate variability used the SphygmoCor-Vx device (AtCor Medical).20 Indices of heart rate variability were derived from the electrocardiographic R-R intervals and included standard deviation of the intervals, root mean square differences of successive intervals, normalized high frequency power, normalized low-frequency power, and the low-frequency to high-frequency ratio. Cardiovascular autonomic neuropathy was defined as at least 3 of 5 abnormalities based on fifth or lower percentiles or 95th or greater percentiles of data observed in age- and sex-matched control participants in the SEARCH Cardiovascular Disease ancillary study.20
Arterial Stiffness
Pulse wave velocity was measured in the carotid–femoral arterial segment with the SphygmoCor-Vx device. A pulse wave velocity of 90th centile or greater of controls from the SEARCH Cardiovascular Disease study defined arterial stiffness.21
Hypertension
Hypertension was defined as blood pressure levels 95th or greater centile for age (<18 years),22 140/90 mm Hg or higher (≥18 years), or relevant medical therapy.23
Statistical Methods
Descriptive analyses calculated the mean (SD) or median (interquartile range [when data were not normally distributed]) for continuous variables and the number and percentage for categorical variables. Variables labeled “current” were from the outcomes visit and those labeled “mean over time” were the mean of all available visits from baseline to the outcomes visit (maximum possible = 5 visits). Hemoglobin A1c levels (glucose control), BMI levels (overall obesity), and waist height ratio (central obesity) from all visits were averaged. Mean arterial pressure was calculated as ([2 × diastolic] + systolic)/3 at each visit and averaged.
Prevalence of Outcomes
The prevalence of each outcome was estimated at age 21 years by diabetes type, as well as by type and race/ethnicity, with logistic regression. Absolute differences and 95% CIs were calculated from the age-adjusted prevalence estimates.
Risk Factors and Prevalence Differences
To understand which risk factors were associated with prevalence differences, odds ratios (ORs) and 95% CIs for the associations between diabetes type (type 2 vs 1) and each outcome were computed in sequentially adjusted models. A base model adjusted for age, sex, duration of diabetes, and clinical site. Additional models explored whether adjustment for individual covariates reduced the strength of the associations: race/ethnicity, hemoglobin A1c level, BMI, waist-height ratio, and mean arterial pressure averaged over time (except when hypertension was the outcome, in which case mean arterial pressure was not included). A final model explored the changes in the ORs, with adjustment for all risk factors (except BMI, which was highly correlated with waist-height ratio). Model fit was determined with the Hosmer-Lemeshow goodness-of-fit test. Analyses used SASversion9.4. All analyses used 2-sided P = .05 as statistically significant and were not adjusted for multiple comparisons. No formal tests for interaction were conducted.
Results
There were 2018 adolescents and young adults for this analysis. Table 1 shows that participants with type 1 diabetes and those with type 2 had a distribution of characteristics similar to those of the registry population from which they came on demographic factors (average diabetes onset age, sex, and race/distribution) and similar to those of the participants with a baseline visit on important clinical variables (hemoglobin A1c level, BMI converted to a z score, waist circumference, waist height ratio, and fasting C-peptide and blood pressure levels) and socioeconomic factors (insurance, household income, and parental education).
Table 1.
Characteristics of SEARCH Registered Cases and Those of Participants With an Outcomes Visit Included in This Analysis
| Variable | Type 1 Diabetes | Type 2 Diabetes | ||
|---|---|---|---|---|
|
|
|
|||
| Registered | Outcome Participants | Registered | Outcome Participants | |
| Registered (2002–2006, 2008)a | ||||
|
| ||||
| No. | 6200a | 1746b | 1589a | 272b |
|
| ||||
| Diabetes onset age, mean (SD), y | 9.9 (4.6) | 10.0 (3.9) | 14.7 (2.8) | 14.2 (2.6) |
|
| ||||
| Male patients, No. (%) | 3242 (52.3) | 879 (50.3) | 637 (40.1) | 91 (33.5) |
|
| ||||
| Race/ethnicity, No. (%) | ||||
|
| ||||
| Non-Hispanic white | 4262 (70.7) | 1327 (76.0) | 357 (23.9) | 72 (26.5) |
|
| ||||
| Minority | 1768 (29.3) | 419 (24.0) | 1139 (76.1) | 200 (73.5) |
|
| ||||
| At Baseline Examinationb | ||||
|
| ||||
| No. | 2735b | 1746b | 406b | 272b |
|
| ||||
| HbA1c, mean (SD), % | 7.7 (1.6) | 7.6 (1.5) | 7.5 (2.3) | 7.4 (2.3) |
|
| ||||
| BMI z score, mean (SD) | 0.53 (1.06) | 0.51 (1.04) | 2.12 (0.65) | 2.10 (0.65) |
|
| ||||
| Fasting C-peptide, median (IQR), ng/mL | 0.5 (0.2, 1.1) | 0.6 (0.2, 1.1) | 3.6 (2.4, 5.2) | 3.6 (2.5, 5.1) |
|
| ||||
| Waist circumference, mean (SD), cm | 66.2 (12.3) | 65.7 (11.7) | 102.3 (17.3) | 101.9 (17.4) |
|
| ||||
| Waist-height ratio, mean (SD) | 0.45 (0.06) | 0.45 (0.05) | 0.62 (0.10) | 0.62 (0.10) |
|
| ||||
| Systolic blood pressure, mean (SD), mm Hg | 100.1 (11.9) | 99.6 (11.7) | 116.8 (12.0) | 115.7 (11.7) |
|
| ||||
| Diastolic blood pressure, mean (SD), mm Hg | 63.1 (10.1) | 62.6 (10.1) | 71.8 (10.0) | 71.0 (9.2) |
|
| ||||
| Mean arterial pressure, mean (SD), mm Hg | 75.4 (9.7) | 74.9 (9.6) | 86.8 (9.1) | 85.9 (8.5) |
|
| ||||
| Urine albumin, median (IQR), g/dL | 0.0006 (0.4–1.2) | 0.0006 (0.4–1.2) | 0.001 (0.5–2.6) | 0.0009 (0.5–2.3) |
|
| ||||
| Insurance, No. (%) | ||||
|
| ||||
| Private | 2089 (77.2) | 1379 (79.8) | 184 (46.1) | 127 (46.9) |
|
| ||||
| Medicaid/Medicare | 496 (18.3) | 283 (16.4) | 182 (45.6) | 123 (45.4) |
|
| ||||
| Other | 71 (2.6) | 42 (2.4) | 17 (4.3) | 11 (4.1) |
|
| ||||
| None | 49 (1.8) | 25 (1.4) | 16 (4.0) | 10 (3.7) |
|
| ||||
| Household income, No. (%), $ | ||||
|
| ||||
| <25 000 | 380 (14.1) | 223 (12.9) | 176 (43.7) | 120 (44.4) |
|
| ||||
| 25 000–49 999 | 579 (21.5) | 346 (20.1) | 97 (24.1) | 66 (24.4) |
|
| ||||
| 50 000–74 999 | 512 (19.0) | 336 (19.5) | 39 (9.7) | 29 (10.7) |
|
| ||||
| ≥75 000 | 1044 (38.8) | 703 (40.8) | 33 (8.2) | 22 (8.1) |
|
| ||||
| Do not know/refused to answer | 176 (6.5) | 116 (6.7) | 58 (14.4) | 33 (12.2) |
|
| ||||
| Parental education, No. (%) | ||||
|
| ||||
| <High school graduate | 122 (4.5) | 71 (4.1) | 69 (17.6) | 43 (16.1) |
|
| ||||
| High school graduate or higher | 2579 (95.5) | 1655 (95.9) | 324 (82.4) | 224 (83.9) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HbA1c, hemoglobin A1c; IQR, interquartile range.
Outcome participants are included in the registered cases totals. For registered cases, type of diabetes was as assigned by the investigator.
For cases with an examination, type of diabetes was assigned with the SEARCH classification (see Methods).
Table 2 shows demographic and clinical characteristics by type of diabetes. Compared with patients with type 1 diabetes, those with type 2 were older at diagnosis (mean age, 14.2 vs 10.0 years at diagnosis) and at the outcome visit (mean age, 22.1 vs 17.9 years), with a higher proportion of female participants (66.5% vs 49.7%) and minority participants (non-Hispanic black, Hispanic, American Indian, and other) (73.5% vs 24.0%). Duration of diabetes at the outcome visit was 7.9 years for both groups. Neither current nor mean hemoglobin A1c levels were significantly different for participants with type 1 and type 2 diabetes. Measures of obesity and mean arterial pressure (current and mean over time) were significantly higher in type 2 diabetes than in type 1. A total of 72 type 2 diabetes participants (27%)did not report treatment with diabetes medications at the outcomes study visit.
Table 2.
Characteristics of Study Participants With Type 1 and Type 2 Diabetes (N = 2018)
| Characteristic | Type 1 (n = 1746) | Type 2 (n = 272) | P Valuea |
|---|---|---|---|
| Current age, mean (SD), y | 17.9 (4.1) | 22.1 (3.5) | <.001 |
| Age at diagnosis, mean (SD), y | 10.0 (3.9) | 14.2 (2.6) | <.001 |
| Male sex, No. (%) | 879 (50.3) | 91 (33.5) | <.001 |
| Race/ethnicity, No. (%) | |||
| Non-Hispanic white | 1327 (76.0) | 72 (26.5) | <.001 |
| Non-Hispanic black | 136 (7.8) | 116 (42.6) | |
| Hispanic | 211 (12.1) | 56 (20.6) | |
| American Indian | 5 (0.3) | 19 (7.0) | |
| Other, including multiple | 67 (3.8) | 9 (3.3) | |
| No. of visits, mean (SD) | 3.2 (1.2) | 3.0 (1.1) | .01 |
| Diabetes duration at outcome visit, mean (SD), y | 7.9 (1.9) | 7.9 (2.0) | .66 |
| HbA1c | |||
| Current, mean (SD), % | 9.2 (1.8) | 9.1 (3.0) | .82 |
| Current, mean (SD), mmol/mol | 76.7 (20.1) | 76.2 (32.5) | .82 |
| Current glycemic control | |||
| Good (<7.5%) | 274 (15.9) | 103 (38.1) | <.001 |
| Intermediate (7.5% to <9.0%) | 611 (35.5) | 31 (11.5) | |
| Poor (≥9.0%) | 834 (48.5) | 136 (50.4) | |
| Mean over time, mean (SD), %b | 8.4 (1.3) | 8.3 (2.2) | .32 |
| Mean over time, mean (SD), mmol/molb | 68.8 (13.9) | 67.3 (24.6) | .32 |
| Weight | |||
| Current BMI z score, mean (SD) | 0.60 (0.95) | 1.79 (0.79) | <.001 |
| Current BMI category | |||
| Normal (<85th percentile or <25) | 1027 (59.0) | 28 (10.3) | <.001 |
| Overweight (85th to <95th percentile or 25 to <30) | 465 (26.7) | 48 (17.6) | |
| Obese (≥95th percentile or ≥30) | 249 (14.3) | 196 (72.1) | |
| BMI z score mean over time, mean (SD)b | 0.55 (0.89) | 1.96 (0.67) | <.001 |
| Current waist-height ratio, mean (SD) | 0.47 (0.07) | 0.63 (0.12) | <.001 |
| Current waist-height ratio ≥0.5, mean (SD) | 422 (24.3) | 238 (87.5) | <.001 |
| Waist-height ratio mean over time, mean (SD)b | 0.46 (0.05) | 0.62(0.10) | <.001 |
| Current fasting C-peptide (median [range]), ng/mL | 0.05 (0.02–4.46) | 2.7 (0.02–10.97) | <.001 |
| Current fasting C-peptide category, No. (%) | |||
| <0.8, absent | 1650 (98.7) | 34 (13.1) | <.001 |
| ≥0.8, preserved | 22 (1.3) | 226 (86.9) | |
| Current systolic blood pressure, mean (SD), mm Hg | 106.3 (10.8) | 118.2 (13.4) | <.001 |
| Current diastolic blood pressure, mean (SD), mm Hg | 68.8 (8.8) | 76.0 (10.2) | <.001 |
| Current mean arterial pressure, mm Hg | 81.3 (8.6) | 90.1 (10.4) | <.001 |
| Mean arterial pressure over time, mean (SD), mm Hgb | 78.1 (7.2) | 87.8 (7.4) | <.001 |
| Medication use | |||
| Current insulin, No. (%) | 1746 (100) | 135 (49.8) | <.001 |
| Current insulin regimen, No. (%) | |||
| Insulin pump | 984 (56.4) | 7 (5.4) | <.001 |
| Basal/bolus injections | 323 (18.5) | 13 (10.1) | |
| Other insulin regimens | 438 (25.1) | 109 (84.5) | |
| Current metformin, No. (%) | 55 (3.2) | 101 (37.3) | <.001 |
| Current antihypertensive, No. (%) | 114 (6.5) | 48 (17.7) | <.001 |
| Current dyslipidemic, No. (%) | 58 (3.3) | 23 (8.5) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HbA1c, hemoglobin A1c.
SI conversion factors: To convert fasting C-peptide to nmol/L, multiply by 0.331.
P values from t test or Wilcoxon test (continuous) or χ2 test (categorical).
Data reported as “mean over time” are for the 7.9 years of average duration of diabetes.
Table 3 shows the number of outcomes observed and the age-adjusted prevalence for each outcome at aged 21 years by type of diabetes and by both type and race/ethnicity, along with absolute differences. A higher prevalence of complications and comorbidities was observed overall among adolescents and young adults with type 2 diabetes compared with type 1, with the exception of cardiovascular autonomic neuropathy.
Table 3.
Outcomes According to Diabetes Type Overall and by Diabetes Type and Race/Ethnicity
| Complication and Race/Ethnicity Category | Type 1 | Type 2 | Difference (Type 2 – Type 1), % (95% CI) | P Valueb | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| No. | Denominator | Age-Adjusted Prevalence, % (95% CI)a | No. | Denominator | Age-Adjusted Prevalence, % (95% CI)a | |||
| Diabetic kidney disease | 89 | 1541 | 5.8 (4.6 to 7.4) | 44 | 221 | 19.9 (15.1 to 25.7) | 14.0 (9.1 to 19.9) | <.001 |
|
| ||||||||
| Non-Hispanic white | 59 | 1177 | 5.2 (3.9 to 7.0) | 6 | 64 | 9.3 (4.2 to 19.2) | 4.0 (−1.1 to 13.7) | .18 |
|
| ||||||||
| Minority | 30 | 364 | 8.0 (5.3 to 11.9) | 38 | 157 | 24.4 (18.2 to 31.9) | 16.4 (9.5 to 24.1) | <.001 |
|
| ||||||||
| Retinopathy | 71 | 1710 | 5.6 (4.4 to 7.0) | 36 | 267 | 9.1 (6.3 to 12.9) | 3.5 (0.4 to 7.7) | .02 |
|
| ||||||||
| Non-Hispanic white | 54 | 1297 | 5.5 (4.2 to 7.2) | 4 | 72 | 3.7 (1.4 to 9.9) | −1.8 (−4.6 to 5.5) | .46 |
|
| ||||||||
| Minority | 17 | 413 | 5.7 (3.5 to 9.0) | 32 | 195 | 11.2 (7.3 to 16.7) | 5.5 (1.0 to 11.0) | .03 |
|
| ||||||||
| Peripheral neuropathy | 110 | 1720 | 8.5 (7.1 to 10.2) | 58 | 265 | 17.7 (13.6 to 22.7) | 9.2 (4.8 to 14.4) | <.001 |
|
| ||||||||
| Non-Hispanic white | 82 | 1310 | 8.3 (6.8 to 10.3) | 14 | 71 | 15.7 (9.1 to 25.6) | 7.3 (0.4 to 17.6) | .03 |
|
| ||||||||
| Minority | 28 | 410 | 8.8 (6.1 to 12.7) | 44 | 194 | 19.6 (14.5 to 26.1) | 10.8 (4.9 to 17.4) | <.01 |
|
| ||||||||
| Cardiovascular autonomic neuropathy | 197 | 1615 | 14.4 (12.5 to 16.6) | 43 | 252 | 15.7 (11.7 to 20.6) | 1.2 (−3.1 to 6.5) | .62c |
|
| ||||||||
| Non-Hispanic white | 155 | 1226 | 15.2 (12.9 to 17.7) | 18 | 66 | 25.6 (16.6 to 37.4) | 10.4 (1.2 to 22.3) | .03c |
|
| ||||||||
| Minority | 42 | 389 | 12.3 (8.9 to 16.7) | 25 | 186 | 12.7 (8.6 to 18.3) | 0.4 (−5.0 to 6.7) | .90 |
|
| ||||||||
| Arterial stiffness | 137 | 1625 | 11.6 (9.8 to 13.6) | 107 | 205 | 47.4 (40.3 to 54.7) | 35.9 (29 to 42.9) | <.001 |
|
| ||||||||
| Non-Hispanic white | 94 | 1242 | 10.2 (8.4 to 12.4) | 21 | 60 | 30.7 (20.0 to 43.9) | 20.4 (10.1 to 33.0) | <.001 |
|
| ||||||||
| Minority | 43 | 383 | 15.4 (11.5 to 20.3) | 86 | 145 | 55.4 (46.8 to 63.7) | 40.0 (30.9 to 48.5) | <.001 |
|
| ||||||||
| Hypertension | 141 | 1743 | 10.1(8.6 to 11.9) | 66 | 272 | 21.6 (17.1 to 26.9) | 11.5 (6.8 to 16.9) | <.001 |
|
| ||||||||
| Non-Hispanic white | 100 | 1325 | 9.5 (7.8 to 11.5) | 16 | 72 | 20.0 (12.5 to 30.6) | 10.5 (2.7 to 21.3) | .01 |
|
| ||||||||
| Minority | 41 | 418 | 12.2 (8.9 to 16.4) | 50 | 200 | 22.4 (17.1 to 28.9) | 10.3 (4.0 to 17.1) | <.01 |
Logistic regression model estimates set age = 21 y for type 1 and type 2 diabetes.
P values from age-adjusted logistic regression models.
Hosmer-Lemeshow goodness-of-fit test P < .05; indicates poor model fit.
A total of 44 participants (19.9%) with type 2 diabetes had diabetic kidney disease compared with 89 participants (5.8%) with type 1 diabetes (absolute difference, 14.0%; 95% CI, 9.1%–19.9%; P < .001). The prevalence of diabetic retinopathy was also significantly higher for type 2 diabetes participants than for type 1 (type 2 diabetes, n = 36, 9.1%; type 1 diabetes, n = 71, 5.6%; absolute difference, 3.5%; 95% CI, 0.4%–7.7%; P = .02).
The prevalence of peripheral neuropathy was also higher in participants with type 2 diabetes vs type 1 overall (n = 58 vs 110; 17.7%vs 8.5%; absolute difference, 9.2%; 95%CI, 4.8–14.4; P < .001) and in both racial/ethnic groups.
Cardiovascular autonomic neuropathy prevalence was not significantly different overall among type 2 and type 1 diabetes participants (n = 43 vs 197; 15.7% vs 14.4%; absolute difference, 1.2%; 95% CI, −3.1 to 6.5; P = .62). Because of poor model fit for race/ethnicity subsets, strata-specific comparisons of cardiovascular autonomic neuropathy prevalence by diabetes type could not be made.
Arterial stiffness prevalence was significantly higher among participants with type 2 diabetes vs those with type 1 (n = 107 vs 137;47.4%vs 11.6%; absolute difference, 35.9%; 95% CI, 29%–42.9%; P < .001) and among both non-Hispanic whites and minority participants with type 2 diabetes compared with type 1, with an estimated prevalence of 55.4%in minority adolescents and young adults with type 2 diabetes (n = 86).
Hypertension prevalence was significantly higher in participants with type 2 diabetes vs type 1 (n = 66 vs 141; 21.6% vs 10.1%; absolute difference, 11.5%; 95% CI, 6.8%–16.9%; P < .001), including among non-Hispanic white and minority groups.
Overall, 195 teenagers and young adults with type 2 diabetes (72%) and 562 of those with type 1 diabetes (32%) had evidence of at least 1 early diabetes-related complication or comorbidity.
Table 4 includes sequentially adjusted models for the associations of diabetes type and each outcome to determine whether established risk factors contributed to the higher prevalence among those with type 2 diabetes vs type 1. The base model (adjusted for age, sex, duration of diabetes, and clinical site) showed significantly higher rates of complications for type 2 diabetes vs type 1 for all outcomes, except for cardiovascular autonomic neuropathy. Adjustment for race/ethnicity did not attenuate the higher odds of complications associated with type 2 diabetes vs type 1 for any of the outcomes explored. For diabetic kidney disease, retinopathy, and peripheral neuropathy, further adjustment for multiple risk factors did not attenuate the significantly higher odds of complications for type 2 diabetes vs type 1 prevalence, although for retinopathy, inclusion of mean arterial pressure alone attenuated the OR to nonsignificance. For arterial stiffness and hypertension, adjustment for waist-height ratio attenuated the OR to nonsignificance. With inclusion of multiple risk factors in the final model, the associations of diabetes type with arterial stiffness and hypertension were no longer significant.
Table 4.
Multivariable Logistic Regression Models for the Association Between Diabetes Type (2 vs 1) and Outcomes
| No. of Events/No. of Individuals | Model 1: Basea | Model 2: 1 + Race/Ethnicity | Model 3: 1 + Glucose Control (Mean Hemoglobin A1c Level Over Time) | Model 4: 1 + Waist-Height Ratio (Mean Over Time) | Model 5: 1 + BMI (Mean Over Time) | Model 6: 1 + Mean Arterial Pressure (Mean Over Time) | Model 7: 1 + All (Except BMI) | |
|---|---|---|---|---|---|---|---|---|
| Diabetic kidney disease | 133/1762 | |||||||
| OR (95% CI) | 4.03 (2.53–6.43) | 3.16 (1.92–5.22) | 4.21 (2.61–6.80) | 2.93 (1.60–5.39) | 4.27 (2.46–7.42) | 2.88 (1.75–4.75) | 2.58 (1.39–4.81) | |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | .003 | |
| Retinopathy | 107/1977 | |||||||
| OR (95% CI) | 2.05 (1.25–3.39) | 1.94 (1.12–3.37) | 2.00 (1.19–3.36) | 2.39 (1.24–4.61) | 2.36 (1.28–4.32) | 1.56 (0.91–2.67) | 2.24 (1.11–4.50) | |
| P value | .01 | .02 | .01 | .01 | .01 | .10 | .02 | |
| Peripheral neuropathy | 168/1985 | |||||||
| OR (95% CI) | 3.36 (2.21–5.10) | 2.89 (1.83–4.57) | 3.41 (2.24–5.18) | 2.67 (1.55–4.60) | 2.88 (1.76–4.71) | 3.12 (2.00–4.86) | 2.52 (1.43–4.43) | |
| P value | <.001 | <.001 | <.001 | .001 | <.001 | <.001 | .001 | |
| Cardiovascular autonomic neuropathy | 240/1867 | |||||||
| OR (95% CI) | 1.27 (0.85–1.89) | 1.54 (1.00–2.38) | 1.26 (0.85–1.89) | 0.85 (0.50–1.43) | 1.17 (0.74–1.84) | 0.97 (0.64–1.49) | 0.98 (0.57–1.67) | |
| P value | .24b | .05 | .25 | .53 | .50b | .90 | .93 | |
| Arterial stiffness | 244/1830 | |||||||
| OR (95% CI) | 6.72 (4.64–9.72) | 5.52 (3.72–8.20) | 6.77 (4.68–9.81) | 1.36 (0.82–2.24) | 2.78 (1.80–4.29) | 4.16 (2.80–6.16) | 1.07 (0.63–1.84) | |
| P value | <.001 | <.001 | <.001b | .23 | <.001 | <.001 | .80 | |
| Hypertensionc | 207/2015 | |||||||
| OR (95% CI) | 2.53 (1.75–3.68) | 2.19 (1.46–3.28) | 2.55 (1.76–3.71) | 0.93 (0.56–1.54) | 1.37 (0.88–2.12) | 0.85 (0.50–1.45) | ||
| P value | <.001 | <.001b | <.001 | .78 | .17b | .55 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); OR, odds ratio.
Base model adjusted for age, sex, duration (time from initial diagnosis of diabetes to measurement of complication), and clinical site. Risk factor covariates were continuous variables except for race/ethnicity: non-Hispanic white and minority. Numbers of participants vary for each outcome because of missing data for the outcome.
Hosmer-Lemeshow goodness-of-fit test P < .05; indicates poor model fit.
Mean arterial pressure not included in models for hypertension.
Discussion
In this cohort of teenagers and young adults who had received a diagnosis of diabetes during childhood and adolescence, the prevalence of diabetes-related complications was higher among those with type 2 diabetes than with type 1, but the prevalence was substantial in both groups. At an estimated age of 21 years and after 7.9 years’ mean diabetes duration, approximately 1 in 3 teenagers and young adults with type 1 diabetes (32%) and almost 3 of 4 of those with type 2 diabetes (72%) had at least 1 such complication or comorbidity, and these rates are likely to increase. Moreover, these complications disproportionately affected teenagers and young adults with type 2 diabetes. Participants were phenotyped to avoid misclassification by diabetes type and were followed from near onset of diabetes, with multiple risk factor measures over time and a single standardized assessment of diabetes complications and comorbidities. These results were from a US study of adolescents and young adults with youth-onset type 1 and type 2 diabetes whose data were drawn from a population-based registry. The current data, coupled with previouswork,3 suggest that the participants in this analysis are reasonably representative of the general US population with onset of type 1 and type 2 diabetes in childhood or adolescence.
The microvascular complications of diabetic kidney disease, retinopathy, and peripheral neuropathy were significantly elevated among teenagers and young adults with type 2 diabetes vs those with type 1 after adjustment for glycemic control, central obesity, and blood pressure levels over time. In several studies, both diabetic kidney disease and peripheral neuropathy have been reported as elevated in individuals with youth-onset type 2 diabetes.5,6,24–26 There is less consistency in regard to retinopathy, with several studies finding no significant difference in prevalence among youth-onset type 2 diabetes compared with patients with type 1 diabetes.5–7,24,27,28
Two studies found a higher prevalence of retinopathy among patients with type 2 diabetes compared with those with type 1 diabetes,25,29 as did a previous pilot study.30 We found that the higher prevalence of retinopathy (as well as that of diabetic kidney disease) was primarily among minority adolescents and young adults with type 2 diabetes. Although it is possible that obesity played a role in the higher prevalence of retinopathy among patients with type 2 diabetes, the TODAY study found that among young persons with type 2 diabetes, retinopathy prevalence was lower among the highest tertile of obesity than among the lower tertiles.31
In the present study, the OR for retinopathy among patients with type 2 diabetes vs type 1 remained significant when measures of obesity were added in the logistic regression models, but was attenuated and became nonsignificant when arterial pressure was included (Table 4). These analyses suggest that mean arterial pressure may be an important factor that influenced the difference in retinopathy prevalence between type 1 and type 2 diabetes, although after inclusion of mean arterial pressure and other risk factors in the fully adjusted model, the OR indicating higher odds of retinopathy among type 2 diabetes vs type 1 remained significant. Given the young age of this group, only a small proportion received medications to control hypertension or dyslipidemia. For hypertension, this proportion was between 2% and 8%, and for dyslipidemia itwasbetween1%and4%, depending on the visit. In a sensitivity analysis, the association between medication use and complications was minimal.
Because the greater association of microvascular complications in type 2 diabetes compared with type 1 remained significant after adjusting for established risk factors, these data indicate a need to explore other potential pathways, such as inflammatory markers, endothelial dysfunction, advanced glycation end products, endogenous inhibitors of nitric oxide synthase, markers of renal tubular dysfunction, and dietary factors. These results do not imply that glucose control (clearly important for the development of microvascular complications for both type 132 and type 2 diabetes33), obesity, and blood pressure were not important risk factors; however, including these variables in the analytic models did not attenuate the associations of higher prevalence of microvascular complications among teenagers and young adults with type 2 diabetes vs type 1.
The higher prevalence of microvascular complications in patients with type 2 diabetes vs type 1 and the relatively high prevalence among patients with type 1 diabetes present a challenge to clinicians. Patients with type 2 diabetes often have limited access to services and less than optimal participation in satisfactory treatment regimens for multiple economic, behavioral, and social reasons.34 Glycemic control goals are not easy to meet among adolescents, regardless of diabetes type.35,36 It is possible that microvascular complications are more aggressive in adolescents and young adults with type 2 diabetes than in those with type 1 diabetes, resulting in greater prevalence at the same diabetes duration. Incidence data will be required to confirm this observation. Whether the smaller prevalence among adolescents and young adults with type 1 diabetes, especially those of non-Hispanic white background, could be related to more aggressive treatment in general must also be further explored.
Comorbid outcomes included hypertension and arterial stiffness, both of which had higher prevalence among patients with type 2 diabetes and in minorities with either diabetes type. The significantly higher prevalence for both outcomes in unadjusted analyses was reduced to nonsignificance with adjustment for risk factors, primarily waist-height ratio, indicating that differences in central obesity were associated with the type 2 diabetes excess. Given that cardiovascular disease25 and mortality7 have been shown to be higher in young-onset type 2 diabetes than in young-onset type 1 diabetes, as well as in young-onset vs later-onset type 2 diabetes,37 the presence of arterial stiffness and hypertension at a young age, together with an increased prevalence of risk factors9 and diabetic kidney disease, suggests that these patients may be at increased risk for subsequent cardiovascular events.
This study has several strengths. The study population was drawn from what is, to our knowledge, the largest multiethnic population-based registry of pediatric diabetes in the United States and has demographic and clinical characteristics similar to those of the overall population from which it draws.3 Even though the outcomes were based on cross-sectional assessment, risk factors were measured longitudinally at previous visits, allowing the determination of the relationship of glucose control, markers of overall and central obesity, and mean blood pressure levels over time with differences in the prevalence of complications by diabetes type.
This study also has some limitations. First, a single measure of each outcome was used, without repeated testing. Second, there were relatively small numbers of participants with some outcomes, especially in subgroups by race/ethnicity. Third, the analysis of risk factors that might explain differences by type of diabetes did not include all possible factors and pathways. Fourth, it is possible that participants with youth-onset type 2 diabetes had a longer period of undetected glycemia such that the duration of hyperglycemia was longer than for type 1 diabetes. Data are not available in this study to address this question; however, surveys of high-risk minority youths have identified few with undiagnosed type 2 diabetes in cross-sectional surveys of glucose intolerance.38,39 If youth-onset type 2 diabetes had a longer preclinical course, it would be expected that a larger number of such undiagnosed cases would be identified. Fourth, the mean number of measures of risk factors was 3.2 for patients with type 1 diabetes and 3.0 for those with type 2 diabetes, so it is possible that the true excursions of hemoglobin A1c level and blood pressure were underestimated. This could have led to less ability to fully characterize the associations of hemoglobin A1c level and blood pressure with complications according to type of diabetes. Fifth, there were a large number of end points compared, without adjustment for multiple comparisons, so it is possible that some of the statistically significant findings represent type I error.
Conclusions
Among teenagers and young adults who had been diagnosed with diabetes during childhood and adolescence, the prevalence of complications and comorbidities was higher among those with type 2 diabetes compared with type 1, but frequent in both groups. These findings support early monitoring of these patients for development of complications.
Key Points.
Question
What is the prevalence of complications of type 1 diabetes and type 2 diabetes among teenagers and young adults who had been diagnosed during childhood and adolescence?
Findings
In an observational study of 1746 patients with type 1 diabetes and 272 with type 2 diabetes with onset younger than 20 years, the prevalence of diabetic kidney disease, retinopathy, and peripheral neuropathy was significantly greater in patients with type 2 diabetes, even after adjustment for differences in hemoglobin A1c level, body mass index, waist-height ratio, and mean arterial blood pressure.
Meaning
Among teenagers and young adults who had been diagnosed with diabetes during childhood and adolescence, the prevalence of complications was higher among those with type 2 diabetes compared with type 1 diabetes, but frequent in both groups.
Acknowledgments
Funding/Support: The SEARCH for Diabetes in Youth Cohort Study (1UC4DK108173-01) is funded by the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases and supported by the Centers for Disease Control and Prevention (CDC). The Population Based Registry of Diabetes in Youth Study (RFP DP15-002) is funded by the CDC and supported by the NIH, National Institute of Diabetes and Digestive and Kidney Diseases. Sites: Kaiser Permanente Southern California (U18DP006133, U48/CCU919219, U01 DP000246, and U18DP002714), University of Colorado–Denver (U18DP006139, U48/CCU819241-3, U01 DP000247, and U18DP000247-06A1), Cincinnati’s Children’s Hospital Medical Center (U18DP006134, U48/CCU519239, U01 DP000248, and 1U18DP002709), University of North Carolina at Chapel Hill (U18DP006138, U48/CCU419249, U01 DP000254, and U18DP002708), Seattle Children’s Hospital (U18DP006136, U58/CCU019235-4, U01 DP000244, and U18DP002710-01), and Wake Forest University School of Medicine (U18DP006131, U48/CCU919219, U01 DP000250, and 200-2010-35171).
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Marcovina reports consulting for Denka Seiken and MedTest Dx. No other disclosures were reported.
SEARCH for Diabetes in Youth Research Group: California: Jean M. Lawrence, ScD, MPH, MSSA, Corinna Koebnick, PhD, MSc, Kristi Reynolds, PhD, MPH, Kim Holmquist, MPHc, and Xia Li, MS, for the Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena; and David J. Pettitt, MD, Santa Barbara, California. Colorado: Dana Dabelea, MD, PhD, Richard F. Hamman, MD, DrPH, Lisa Testaverde, MS, Anna Bellatorre, PhD, for the Department of Epidemiology, Colorado School of Public Health, University of Colorado–Denver; Georgeanna J. Klingensmith, MD, Marian J. Rewers, MD, PhD, David Maahs, MD, and Paul Wadwa, MD, for the Barbara Davis Center for Childhood Diabetes; Stephen Daniels, MD, PhD, Greta Wilkening, PsyD, Michael G. Kahn, MD, PhD, Department of Pediatrics and Children’s Hospital; Clifford A. Bloch, MD, for the Pediatric Endocrine Associates; Jeffrey Powell, MD, MPH, for the Navajo Area Indian Health Promotion Program; and Kathy Love-Osborne, MD, for the Denver Health and Hospital Authority. Ohio: Lawrence M. Dolan, MD, and Debra A. Standiford, MSN, CNP, for the Cincinnati Children’s Hospital Medical Center. North Carolina: Elizabeth J. Mayer-Davis, PhD, Amy Mottl, MD, MPH, and Joan Thomas, MS, RD, for the University of North Carolina, Chapel Hill; James Amrhein, MD, and Bryce Nelson, MD, for Greenville Health System. South Carolina: Anwar Merchant, ScD, Angela D. Liese, PhD, MPH, Malaka Jackson, MD, and Lisa Knight, MD, for the University of South Carolina; Deborah Bowlby, MD, for the Medical University of South Carolina. Washington: Catherine Pihoker, MD, Maryam Afkarian, MD, Irl Hirsch, MD, Lenna L. Liu, MD, MPH, John Neff, MD, Grace Kim, MD, Craig Taplin, MD, Debika Nandi-Munshi, MD, Joyce Yi-Frazier, PhD, Lina Merjaneh, MD, and Davene Wright, PhD, for the University of Washington; Beth Loots, MPH, MSW, Natalie Beauregard, BA, Sue Kearns, RN, Mary Klingsheim, RN, and Michael Pascual, BA, for Seattle Children’s Hospital; and Carla Greenbaum, MD, for Benaroya Research Institute. Centers for Disease Control and Prevention: Giuseppina Imperatore, MD, PhD, and Sharon H. Saydah, PhD. National Institute of Diabetes and Digestive and Kidney Diseases, NIH: Barbara Linder, MD, PhD. Central Laboratory: Santica M. Marcovina, PhD, ScD, Vinod P. Gaur, PhD, Greg Strylewicz, PhD, and Jessica Harting for the University of Washington Northwest Lipid Research Laboratories. Coordinating Center: Ralph D’Agostino Jr, PhD, Lynne E. Wagenknecht, DrPH, Ronny Bell, PhD, MS, Jasmin Divers, PhD, Timothy Morgan, PhD, Beth Reboussin, PhD, Leora Henkin, MPH, MEd, Maureen T. Goldstein, BA, Carrie Williams, MA, CCRP, Scott Isom, MS, Jeanette Stafford, MS, and June Pierce, AB, for Wake Forest School of Medicine.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC and the National Institute of Diabetes and Digestive and Kidney Diseases.
Additional Contributions: We acknowledge the involvement of the South Carolina Clinical and Translational Research Institute at the Medical University of South Carolina, NIH/National Center for Advancing Translational Sciences (NCATS) grant UL1 TR001450; Seattle Children’s Hospital and the University of Washington, NIH/NCATS grant UL1 TR00423; University of Colorado Pediatric Clinical and Translational Research Center, NIH/NCATS grant UL1 TR000154; the Barbara Davis Center at the University of Colorado at Denver (Diabetes and Endocrinology Research Center NIH grant P30 DK57516); the University of Cincinnati, NIH/NCATS grant UL1 TR001425; and the Children with Medical Handicaps program managed by the Ohio Department of Health. This study includes data provided by the Ohio Department of Health, which should not be considered an endorsement of this study or its conclusions.
Author Contributions: Dr Dabelea had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Dabelea, Mayer-Davis, D’Agostino, Dolan, Imperatore, Linder, Mottl, Pop-Busui, Saydah, Pihoker.
Acquisition, analysis, or interpretation of data: Dabelea, Stafford, Mayer-Davis, D’Agostino, Dolan, Imperatore, Lawrence, Marcovina, Mottl, Black, Pop-Busui, Saydah, Hamman, Pihoker.
Drafting of the manuscript: Dabelea, Stafford, D’Agostino, Pihoker.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Dabelea, Stafford, D’Agostino.
Obtained funding: Dabelea, Mayer-Davis, Dolan, Lawrence, Hamman, Pihoker.
Administrative, technical, or material support: Mayer-Davis, Marcovina, Hamman.
Supervision: Mayer-Davis, Dolan.
Role of the Funder/Sponsor: Drs Linder (NIH), Imperatore, and Saydah (CDC) were participating members of the study steering committee and the writing group for this manuscript because of the cooperative funding agreements. They were involved in the design of the study but not the conduct of the study; they were not involved in the collection, management, and analysis of the data, but were involved in interpretation of the data; they were involved in the preparation, review, and approval of the manuscript and the decision to submit the manuscript for publication.
References
- 1.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996;128(5 pt 1):608–615. doi: 10.1016/s0022-3476(96)80124-7. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Hanson RL, Bennett PH, Roumain J, Knowler WC, Pettitt DJ. Increasing prevalence of type II diabetes in American Indian children. Diabetologia. 1998;41(8):904–910. doi: 10.1007/s001250051006. [DOI] [PubMed] [Google Scholar]
- 3.Dabelea D, Mayer-Davis EJ, Saydah S, et al. SEARCH for Diabetes in Youth Study. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson CC, Gyurus E, Rosenbauer J, et al. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: evidence of non-uniformity over time in rates of increase. Diabetologia. 2012;55(8):2142–2147. doi: 10.1007/s00125-012-2571-8. [DOI] [PubMed] [Google Scholar]
- 5.Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care. 2014;37(2):436–443. doi: 10.2337/dc13-0954. [DOI] [PubMed] [Google Scholar]
- 6.Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29(6):1300–1306. doi: 10.2337/dc05-2470. [DOI] [PubMed] [Google Scholar]
- 7.Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36(12):3863–3869. doi: 10.2337/dc12-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong J, Constantino M, Yue DK. Morbidity and mortality in young-onset type 2 diabetes in comparison to type 1 diabetes: where are we now? Curr Diab Rep. 2015;15(1):566–577. doi: 10.1007/s11892-014-0566-1. [DOI] [PubMed] [Google Scholar]
- 9.Hamman RF, Bell RA, Dabelea D, et al. SEARCH for Diabetes in Youth Study Group. The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care. 2014;37(12):3336–3344. doi: 10.2337/dc14-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingram DD, Parker JD, Schenker N, et al. United States Census 2000 population with bridged race categories. Vital Health Stat 2. 2003;(135):1–55. [PubMed] [Google Scholar]
- 11.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 12.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases consortia. J Clin Endocrinol Metab. 2010;95(7):3360–3367. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lampasona V, Schlosser M, Mueller PW, et al. Diabetes antibody standardization program: first proficiency evaluation of assays for autoantibodies to zinc transporter 8. Clin Chem. 2011;57(12):1693–1702. doi: 10.1373/clinchem.2011.170662. [DOI] [PubMed] [Google Scholar]
- 14.Dabelea D, D’Agostino RB, Jr, Mason CC, et al. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia. 2011;54(1):78–86. doi: 10.1007/s00125-010-1911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dabelea D, Pihoker C, Talton JW, et al. SEARCH for Diabetes in Youth Study. Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care. 2011;34(7):1628–1633. doi: 10.2337/dc10-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornstad P, Cherney DZ, Maahs DM. Update on estimation of kidney function in diabetic kidney disease. Curr Diab Rep. 2015;15(9):57–69. doi: 10.1007/s11892-015-0633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein R, Klein BEK, Magli YL, et al. An alternative method of grading diabetic retinopathy. Ophthalmology. 1986;93(9):1183–1187. doi: 10.1016/s0161-6420(86)33606-6. [DOI] [PubMed] [Google Scholar]
- 18.Jaiswal M, Lauer A, Martin CL, et al. SEARCH for Diabetes in Youth Study Group. Peripheral neuropathy in adolescents and young adults with type 1 and type 2 diabetes from the SEARCH for Diabetes in Youth follow-up cohort: a pilot study. Diabetes Care. 2013;36(12):3903–3908. doi: 10.2337/dc13-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herman WH, Pop-Busui R, Braffett BH, et al. DCCT/EDIC Research Group. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29(7):937–944. doi: 10.1111/j.1464-5491.2012.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaiswal M, Urbina EM, Wadwa RP, et al. Reduced heart rate variability among youth with type 1 diabetes: the SEARCH CVD study. Diabetes Care. 2013;36(1):157–162. doi: 10.2337/dc12-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urbina EM, Wadwa RP, Davis C, et al. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr. 2010;156(5):731–737. 737.e1. doi: 10.1016/j.jpeds.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 22.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2) suppl 4th report:555–576. [PubMed] [Google Scholar]
- 23.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 24.Scott A, Toomath R, Bouchier D, et al. First national audit of the outcomes of care in young people with diabetes in New Zealand: high prevalence of nephropathy in Maori and Pacific Islanders. N Z Med J. 2006;119(1235):U2015. [PubMed] [Google Scholar]
- 25.Luk AOY, Lau ESH, So WY, et al. Prospective study on the incidences of cardiovascular-renal complications in Chinese patients with young-onset type 1 and type 2 diabetes. Diabetes Care. 2014;37(1):149–157. doi: 10.2337/dc13-1336. [DOI] [PubMed] [Google Scholar]
- 26.Yokoyama H, Okudaira M, Otani T, et al. Higher incidence of diabetic nephropathy in type 2 than in type 1 diabetes in early-onset diabetes in Japan. Kidney Int. 2000;58(1):302–311. doi: 10.1046/j.1523-1755.2000.00166.x. [DOI] [PubMed] [Google Scholar]
- 27.Rajalakshmi R, Amutha A, Ranjani H, et al. Prevalence and risk factors for diabetic retinopathy in Asian Indians with young onset type 1 and type 2 diabetes. J Diabetes Complications. 2014;28(3):291–297. doi: 10.1016/j.jdiacomp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Song SH. Complication characteristics between young-onset type 2 versus type 1 diabetes in a UK population. BMJ Open Diabetes Res Care. 2015;3(1):e000044. doi: 10.1136/bmjdrc-2014-000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGrath NM, Parker GN, Dawson P. Early presentation of type 2 diabetes mellitus in young New Zealand Maori. Diabetes Res Clin Pract. 1999;43(3):205–209. doi: 10.1016/s0168-8227(99)00003-0. [DOI] [PubMed] [Google Scholar]
- 30.Mayer-Davis EJ, Davis C, Saadine J, et al. SEARCH for Diabetes in Youth Study Group. Diabetic retinopathy in the SEARCH for Diabetes in Youth Cohort: a pilot study. Diabet Med. 2012;29(9):1148–1152. doi: 10.1111/j.1464-5491.2012.03591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.TODAY Study Group. Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care. 2013;36(6):1772–1774. doi: 10.2337/dc12-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gubitosi-Klug RA DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: summary and future directions. Diabetes Care. 2014;37(1):44–49. doi: 10.2337/dc13-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 34.Anderson BJ, McKay SV. Barriers to glycemic control in youth with type 1 diabetes and type 2 diabetes. Pediatr Diabetes. 2011;12(3 pt 1):197–205. doi: 10.1111/j.1399-5448.2010.00667.x. [DOI] [PubMed] [Google Scholar]
- 35.Badaru A, Klingensmith GJ, Dabelea D, et al. Correlates of treatment patterns among youth with type 2 diabetes. Diabetes Care. 2014;37(1):64–72. doi: 10.2337/dc13-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pihoker C, Badaru A, Anderson A, et al. SEARCH for Diabetes in Youth Study Group. Insulin regimens and clinical outcomes in a type 1 diabetes cohort: the SEARCH for Diabetes in Youth study. Diabetes Care. 2013;36(1):27–33. doi: 10.2337/dc12-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Saeed AH, Constantino MI, Molyneaux L, et al. An inverse relationship between age of type 2 diabetes onset and complication risk and mortality: the impact of youth-onset type 2 diabetes. Diabetes Care. 2016;39(5):823–829. doi: 10.2337/dc15-0991. [DOI] [PubMed] [Google Scholar]
- 38.Dolan LM, Bean J, D’Alessio D, et al. Frequency of abnormal carbohydrate metabolism and diabetes in a population-based screening of adolescents. J Pediatr. 2005;146(6):751–758. doi: 10.1016/j.jpeds.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 39.Baranowski T, Cooper DM, Harrell J, et al. STOPP-T2D Prevention Study Group. Presence of diabetes risk factors in a large US eighth-grade cohort. Diabetes Care. 2006;29(2):212–217. doi: 10.2337/diacare.29.02.06.dc05-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]