Abstract
Rheumatoid arthritis (RA) is a systemic autoimmune disease, caused by both genetic and environmental factors. Recently, investigators have focused on the gut microbiota, which is thought to be an environmental agent affecting the development of RA. Here we review the evidence from animal and human studies that supports the role of the gut microbiota in RA. We and others have demonstrated that the abundance of Prevotella copri is increased in some early RA. We have also used gnotobiotic experiments to show that dysbiosis in RA patients contributed to the development of Th17 cell-dependent arthritis in intestinal microbiota-humanized SKG mice. On the other hand, Prevotella histicola from human gut microbiota suppressed the development of arthritis. In summary, Prevotella species are involved in the pathogenesis of arthritis.
Keywords: rheumatoid arthritis, microbiota, Prevotella copri, gut, Th17 cell
1. Introduction
The human intestine contains over 1000 bacterial species and 1014 bacterial cells [1], but it is difficult to detect most anaerobic bacteria with culture techniques. However, the new sequencing technology, known as “high-throughput microbial DNA sequencing”, has allowed us to better characterize the human microbiota. Recent advances in our understanding of mucosal immunity have clarified the correlation between the gut microbiota and the host immune system [2,3]. Most of the interactions between a host and its commensal microbiota are symbiotic. However, microbial abnormalities, called “dysbiosis” are thought to correlate with various kinds of diseases. Unsurprisingly, intestinal dysbiosis was observed in inflammatory bowel disease [4,5]. Interestingly, dysbiosis is also found in diseases that affect the tissues out of the gut. Recent studies have suggested that the composition of the microbiota is altered in type 1 diabetes, multiple sclerosis, and autism [6,7,8]. Moreover, dysbiosis has been reported in patients with rheumatoid arthritis (RA) in the United States, China, and Finland [9,10,11].
Here, we review previous findings regarding the role of microbiota in human RA and mouse models of arthritis. We have shown that some Japanese RA patients carry increased numbers of Prevotella copri in the intestine and have used gnotobiotic tools to show that a P. copri-dominated microbiota induced Th17 cell-dependent arthritis in mice [12]. In contrast, another study showed that P. histicola suppresses the development of arthritis [13]. These results support the idea that different Prevotella species have different effects on arthritis.
2. Microbiota and RA
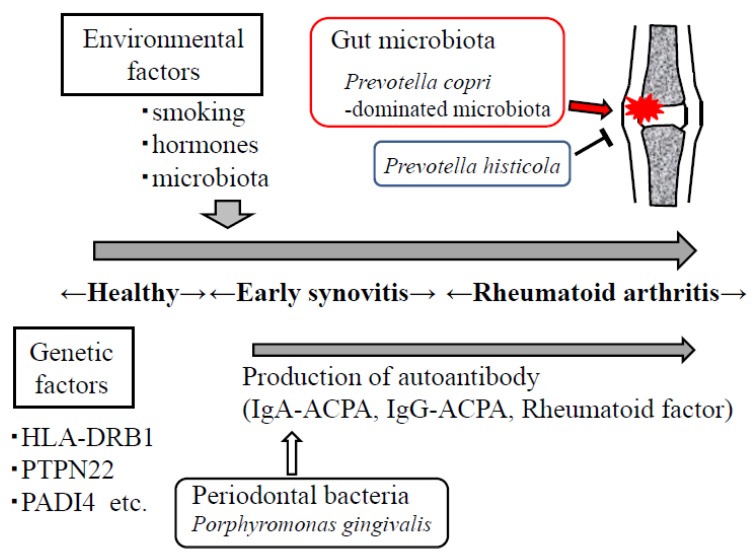
Rheumatoid arthritis is a chronic autoimmune inflammatory disease characterized by auto-antibody production and destruction of bone in multiple joints (Figure 1) [14]. Recent studies have demonstrated that over 100 genetic susceptibility loci are involved in RA [15,16]. However, the environmental factors that affect the development of RA are not fully understood. It was recently shown that an immunoglobulin A (IgA) anti-citrullinated protein antibody (ACPA) is detectable before the onset of arthritis [17,18], suggesting that RA originates at mucosal sites, such as the oral cavity and the gut. Porphyromonas gingivalis, a major pathogenic bacterium of periodontal diseases, may correlate with the development of RA [19,20], because this bacterium is the only known pathogen that expresses a peptidylarginine deiminase and may be related to ACPA [21].
Figure 1.
Environmental factors such as smoking, hormones, gut microbiota, and periodontal bacteria are important in the development of rheumatoid arthritis. Prevotella copri-dominated microbiota may contribute to the development of arthritis, whereas P. histicola suppresses the onset of arthritis.
The gut microbiota contains the largest abundance of microorganisms in our body. The previous experiments in germ-free mice revealed that the gut microbiota shapes the intestinal immune system [22,23]. Recent studies in several countries have found that the composition of the intestinal microbiota is altered in patients with recent-onset RA. Commensal segmented filamentous bacteria (SFB) induce Th17 cells in the intestine and trigger arthritis in mice [24,25]. Therefore, the gut microbiota is thought to be an important environmental factor in the development of arthritis.
3. Animal Models of Arthritis
Several animal studies have clearly demonstrated that gut microbiota plays an important role in arthritis development (Table 1). We and others have shown that SKG mice, which spontaneously develop arthritis under conventional conditions, did not develop arthritis in a germ-free (GF) environment [12,26]. We also showed that the mono-colonization of GF-SKG mice with P. copri was sufficient to induce arthritis with a fungal injection.
Table 1.
Animal models of arthritis known to be correlated with intestinal bacteria.
| Mice Strain | Environmental Condition | Mechanism of Involvement of Arthritis | Intestinal Bacteria Correlated with Induction of Arthritis | Ref. |
|---|---|---|---|---|
| K/BxN | GF:no arthritis SPF:arthritis | Production of GPI-antibody Th17 cell expansion in the intestine | SFB | [24] |
| IL-1ra−/− | GF:no arthritis conventional:arthritis | Activation of TLR2 and TLR4 Th17 cells↑ Treg cells↓ |
Lactobacillus Bifidus | [27] |
| SKG | GF, SPF:no arthritis conventional:arthritis | Production of auto-reactive T cells Activation of innate immunity by fungi | Prevotella-dominated microbiota | [12] |
Note: GF: germ free, SPF: specific pathogen free, GPI: glucose-6-phospate isomerase, SFB: segmented filamentous bacteria, TLR: toll-like receptor, Treg cells: regulatory T cells.
Interleukin-1 (IL-1) receptor antagonist knock-out (IL1rn−/−) mice showed T cell -mediated arthritis in under a specific-pathogen-free (SPF) condition in response to excessive IL-1 signaling [27]. When the mice were reared under GF condition, they did not develop arthritis. However, the mono-colonization of these mice with Lactobacillus induced arthritis via activation of Toll-like receptor 2 (TLR2) and TLR4.
K/BxN T cell receptor transgenic mice developed inflammatory arthritis, with increased numbers of Th17 cells in the small intestine and spleen [24]. The severity of arthritis and the titers of auto-antibodies directed against glucose-6-phospate isomerase were reduced when the mice were reared under GF condition. Mono-colonization with SFB was sufficient to cause the development of Th17 cell-dependent arthritis in this model. Therefore, a particular gut commensal microbiota is sufficient to induce arthritis in mice.
4. Human Microbiota in RA
The role of the gut microbiota in human RA is not fully understood. However, several studies have demonstrated that the composition of the intestinal microbiota is altered in RA patients (Table 2) [9,10,12].
Table 2.
Altered composition of intestinal microbiota observed in human RA patients.
| Country | Increased Bacteria | Reduced Bacteria | Method | Ref. |
|---|---|---|---|---|
| Finland | none | Eubacterium rectale-Clostridium coccoides group Bacteroides fragilis subgroup etc. | 16S rRNA hybridization, DNA staining | [9] |
| United States | Prevotella (Prevotella copri) | Bacteroides | 16S rRNA sequencing | [10] |
| China | Clostridium asparagiforme Lactobacillus salivarius etc. | Veillonella, Haemophilus etc. | Metagenomic shotgun sequence | [11] |
| Japan | Prevotella (Prevotella copri) <1/3 of RA patients> | Bacteroides <1/3 of RA patients> | 16S rRNA sequencing | [12] |
Vaahtovuo et al. investigated the composition of the microbiota in patients with early RA or fibromyalgia using a technique based on flow cytometry, 16S rRNA hybridization, and DNA staining [9]. They found that the Bacteroides fragilis subgroup, the genus Bifidobacterium, and Eubacterium rectale–Clostridium coccoides are reduced in RA patients.
Using 16S rRNA gene sequencing, Scher et al. found that patients with recent-onset RA in North American populations carried an increased abundance of P. copri and a reduced abundance of Bacteroides in the gut [10]. We also confirmed that approximately one-third of Japanese patients with recent-onset RA had an increased abundance of P. copri in the gut [12].
Another study based on metagenomic shotgun sequencing showed that RA patients in China had an increased abundance of L. salivarius in the gut, on the tooth, and in the saliva [11]. However, the abundance of P. copri in the gut was only elevated in the first year after disease onset. The authors showed that the dysbiosis observed in RA patients partly improved after treatment with disease-modifying drugs.
5. Correlation between Prevotella and Arthritis
Prevotella copri was first isolated from human fecal samples in Japan [28]. It is an obligately anaerobic, non-spore-forming Gram-negative bacterium. Interestingly, Scher et al. showed that the abundance of P. copri was elevated in untreated recent-onset RA patients [10]. By contrast, the numbers of P. copri were reduced in patients with chronic RA, patients with psoriatic arthritis, and healthy volunteers. They also found that the relative abundance of P. copri in the intestine correlated with an absence of human leukocyte antigen (HLA)-DRB1. Moreover, P. copri–colonized mice displayed exacerbated colitis when they were treated with dextran sulfate sodium in their drinking water. However, the mechanistic link between the increased number of P. copri in the gut and arthritis is unknown.
Therefore, we produced intestinal microbiota-humanized mice and analyzed the severity of their arthritis [12]. It has been reported that SKG mice develop Th17 cell-dependent autoimmune arthritis, clinically resembling human RA [29]. We used GF-SKG mice, which contain no bacterium in their gut. The GF-SKG mice showed no signs of arthritis when they were treated with a fungal component, suggesting that microbial stimuli are important for disease development. We colonized the GF-SKG mice with fecal samples from RA patients or healthy controls. The mice were kept in separate vinyl isolators. The SKG mice colonized with a P. copri–dominated microbiota from RA patients (RA-SKG mice) showed increased numbers of Th17 cells in the large intestine. We also confirmed that the abundance of P. copri increased in the large intestines of RA-SKG mice, but not in the small intestines. Intriguingly, the RA-SKG mice developed severe arthritis when they were injected with a low dose of zymosan (a fungal component). We also showed that lymphocytes from regional lymph nodes and large intestines of the RA-SKG mice produced high levels of IL-17 in response to the arthritis-related auto-antigen RPL23A. Moreover, bone marrow-derived dendritic cells stimulated with P. copri expressed high levels of IL-6 and IL-23 in an in vitro analysis. In addition, P. copri-monocolonized SKG mice showed Th17 cell-dependent arthritis development upon fungal injection, thus indicating that dysbiosis dominated by P. copri contributes to arthritis development. In the future, more intensive studies are needed to investigate whether P. copri elicits severe arthritis compared to other intestinal bacteria in in vivo experiments.
Very recently, Annalisa et al. used liquid chromatography–tandem mass spectrometry to identify an HLA-DR-presented peptide (T cell epitope) from a 27-kDa P. copri protein (Pc-p27) [30]. The peptide was detected in the synovial tissue, synovial fluid mononuclear cells, and peripheral blood mononuclear cells (PBMCs) of some RA patients. To investigate the Th1/Th17 responses to this peptide, the authors stimulated PBMCs from 40 RA patients with the peptide and evaluated the cytokines produced, with enzyme-linked immunosorbent assays (ELISA). They found that the levels of interferon γ (IFN-γ) were elevated in 42% of RA patients. However, PBMCs from only one RA patient showed increased IL-17 production. They also evaluated the IgG and IgA antibody responses to Pc-p27. IgG antibody responses were observed in 13% of new-onset RA patients and 20% of chronic RA patients. IgA antibody responses were also detected in approximately 10% of both new-onset and chronic RA patients. From these observations, they concluded that P. copri may contribute to the pathogenesis of RA. It will be interesting to analyze whether RA patients showing IgA response to Pc-p27 are also positive for IgA-ACPA.
Interestingly, several studies have shown that the genus Prevotella plays beneficial roles in our body. A recent study suggested that P. histicola isolated from the commensal bacteria in the human gut reduced the severity of collagen-induced arthritis in HLA-DQ8 mice [13]. P. histicola was isolated from the human duodenum and cultured on agar plates for further experiments. HLA-DQ8 mice were immunized with collagen and orally treated with P. histicola. The mice inoculated with P. histicola had a significantly reduced incidence of arthritis as a result of the suppression of the serum levels of several proinflammatory cytokines, such as IL-2, IL-17, and tumor necrosis factor α (TNF-α). The P. histicola-inoculated mice showed increased regulatory T cells in the gut and reduced antigen-specific Th17 responses. The researchers also demonstrated that sequences of P. histicola were very different from those of P. copri. These results suggest that not all Prevotella species are arthritogenic and that some of Prevotella, such as P. histicola, can suppress the development of arthritis.
6. Correlation between Prevotella and Insulin Resistance
A previous report also demonstrated a relationship between P. copri and glucose metabolism. One study showed that a barley-kernel-based bread (BKB) improved glucose metabolism by increasing the abundance of P. copri in the intestine [31]. When the researchers compared the composition of the microbiota in healthy individuals who responded or did not respond to the BKB intervention, they detected an increased abundance of Prevotella in the responders. Moreover, GF mice inoculated with the microbiota of the responders showed improved glucose metabolism, with an increased abundance of P. copri in the gut.
By contrast, another study in Denmark showed that P. copri induced insulin resistance [32]. The researchers investigated the correlation between the gut microbiota and serum metabolites in 277 non-diabetic individuals. They found that P. copri and Bacteroides vulgatus in the gut induced insulin resistance by increasing the serum levels of branched-chain amino acids (BCAA). To investigate whether P. copri directly induces insulin resistance, they inoculated C57BL/6J mice on a high-fat diet with P. copri and analyzed their glucose tolerance. The mice treated with P. copri showed increased levels of serum BCAA and insulin resistance. The researchers speculated that the different outcomes of the two studies were attributable to the different dietary regimens used.
7. Conclusions
We have reviewed the role of the gut microbiota in human RA and in mouse models of arthritis. Several studies have suggested that increased abundance of P. copri was observed in early RA patients. In contrast, another report showed other Prevotella species suppressed the induction of arthritis. Further studies are required to determine the precise mechanistic links between dysbiosis and the development of human RA. The modulation of the gut microbiota may offer a novel therapeutic or preventive approach to RA patients.
Author Contributions
Y.M. and K.T. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Savage D.C. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 2.Lee Y.K., Mazmanian S.K. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch S.V., Pedersen O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 4.Alhagamhmad M.H., Day A.S., Lemberg D.A., Leach S.T. An overview of the bacterial contribution to Crohn disease pathogenesis. J. Med. Microbiol. 2016;65:1049–1059. doi: 10.1099/jmm.0.000331. [DOI] [PubMed] [Google Scholar]
- 5.Michail S., Durbin M., Turner D., Griffiths A.M., Mack D.R., Hyams J., Leleiko N., Kenche H., Stolfi A., Wine E. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm. Bowel Dis. 2012;18:1799–1808. doi: 10.1002/ibd.22860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Needell J.C., Zipris D. The Role of the Intestinal Microbiome in Type 1 Diabetes Pathogenesis. Curr. Diabetes Rep. 2016;16:89. doi: 10.1007/s11892-016-0781-z. [DOI] [PubMed] [Google Scholar]
- 7.Miyake S., Kim S., Suda W., Oshima K., Nakamura M., Matsuoka T., Chihara N., Tomita A., Sato W., Kim S.W., et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS ONE. 2015;10:e0137429. doi: 10.1371/journal.pone.0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharon G., Sampson T.R., Geschwind D.H., Mazmanian S.K. The Central Nervous System and the Gut Microbiome. Cell. 2016;167:915–932. doi: 10.1016/j.cell.2016.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaahtovuo J., Munukka E., Korkeamaki M., Luukkainen R., Toivanen P. Fecal microbiota in early rheumatoid arthritis. J. Rheumatol. 2008;35:1500–1505. [PubMed] [Google Scholar]
- 10.Scher J.U., Sczesnak A., Longman R.S., Segata N., Ubeda C., Bielski C., Rostron T., Cerundolo V., Pamer E.G., Abramson S.B., et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLIFE. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Zhang D., Jia H. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015;21:895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 12.Maeda Y., Kurakawa T., Umemoto E., Motooka D., Ito Y., Gotoh K., Hirota K., Matsushita M., Furuta Y., Narazaki M., et al. Dysbiosis Contributes to Arthritis Development via Activation of Autoreactive T Cells in the Intestine. Arthritis Rheumatol. 2016;68:2646–2661. doi: 10.1002/art.39783. [DOI] [PubMed] [Google Scholar]
- 13.Marietta E.V., Murray J.A., Luckey D.H., Jeraldo P.R., Lamba A., Patel R., Luthra H.S., Mangalam A., Taneja V. Suppression of Inflammatory Arthritis by Human Gut-Derived Prevotella histicola in Humanized Mice. Arthritis Rheumatol. 2016;68:2878–2888. doi: 10.1002/art.39785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris E.D., Jr. Rheumatoid arthritis. Pathophysiology and implications for therapy. N. Engl. J. Med. 1990;322:1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- 15.Okada Y., Wu D., Trynka G., Raj T., Terao C., Ikari K., Kochi Y., Ohmura K., Suzuki A., Yoshida S., et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506:376–381. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stahl E.A., Raychaudhuri S., Remmers E.F., Xie G., Eyre S., Thomson B.P., Habibuw M.R., Vandenbrouke J.P., Dijkmans B.A., Peter K.G., et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat. Genet. 2010;42:508–514. doi: 10.1038/ng.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielen M.M., Van Schaardenburg D., Reesink H.W., Van de Stadt R.J., Van der Horst-Bruinsma I.E., De Koning M.H., Habibuw M.R., Vandenbrouke J.P., Dijkmans B.A. Specific autoantibodies precede the symptoms of rheumatoid arthritis: A study of serial measurements in blood donors. Arthritis Rheum. 2004;50:380–386. doi: 10.1002/art.20018. [DOI] [PubMed] [Google Scholar]
- 18.Rantapaa-Dahlqvist S., De Jong B.A., Berglin E., Hallmans G., Wadell G., Stenlund H., Sundin U., van Venrooji W.J. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–2749. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 19.Lappin D.F., Apatzidou D., Quirke A.M., Oliver-Bell J., Butcher J.P., Kinane D.F., Riggio M.P., Venables P., Mclnnes I.B., Culshaw S. Influence of periodontal disease, Porphyromonas gingivalis and cigarette smoking on systemic anti-citrullinated peptide antibody titres. J. Clin. Periodontol. 2013;40:907–915. doi: 10.1111/jcpe.12138. [DOI] [PubMed] [Google Scholar]
- 20.Hendler A., Mulli T.K., Hughes F.J., Perrett D., Bombardieri M., Houri-Haddad Y., Weiss E.I., Nissim A. Involvement of autoimmunity in the pathogenesis of aggressive periodontitis. J. Dent. Res. 2010;89:1389–1394. doi: 10.1177/0022034510381903. [DOI] [PubMed] [Google Scholar]
- 21.Wegner N., Wait R., Sroka A., Eick S., Nguyen K.A., Lundberg K., Kinloch A., Culshaw S., Potempa J., Venables P.J. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: Implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62:2662–2672. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macpherson A.J., Gatto D., Sainsbury E., Harriman G.R., Hengartner H., Zinkernagel R.M. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 23.Pollard M., Sharon N. Responses of the Peyer's Patches in Germ-Free Mice to Antigenic Stimulation. Infect. Immun. 1970;2:96–100. doi: 10.1128/iai.2.1.96-100.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H.-J., Ivanov I.I., Darce J., Hattori K., Shima T., Umesaki Y., Littman D.R., Benoist C., Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rehaume L.M., Mondot S., Aguirre de Carcer D., Velasco J., Benham H., Hasnain S.Z., Bowman J., Ruutu M., Hansbro P.M., McGuckin M.A., et al. ZAP-70 genotype disrupts the relationship between microbiota and host, leading to spondyloarthritis and ileitis in SKG mice. Arthritis Rheumatol. 2014;66:2780–2792. doi: 10.1002/art.38773. [DOI] [PubMed] [Google Scholar]
- 27.Abdollahi-Roodsaz S., Joosten L.A., Koenders M.I., Devesa I., Roelofs M.F., Radstake T.R., Heuvelmans-Jacobs M., Akira S., Nicklin M.J., Ribeiro-Dias F., et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Investig. 2008;118:205–216. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi H., Shibata K., Sakamoto M., Tomita S., Benno Y. Prevotella copri sp. nov. and Prevotella stercorea sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2007;57:941–946. doi: 10.1099/ijs.0.64778-0. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi N., Takahashi T., Hata H., Nomura T., Tagami T., Yamazaki S., Sakihama T., Matsutani T., Negishi I., Nakatsuru S., et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 30.Pianta A., Arvikar S., Strle K., Drouin E.E., Wang Q., Costello C.E., Steere AC. Evidence of Immune Relevance of Prevotella copri, a Gut Microbe, in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2016;69:964–975. doi: 10.1002/art.40003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovatcheva-Datchary P., Nilsson A., Akrami R., Lee Y.S., De Vadder F., Arora T., Hallen A., Martens E., Björck I., Bäckhed F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell Metab. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen H.K., Gudmundsdottir V., Nielsen H.B., Hyotylainen T., Nielsen T., Jensen B.A., Forslund K., Hildebrand F., Prifti E., Falony G., et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]