Abstract
Aptamers are nucleic acids referred to as chemical antibodies as they bind to their specific targets with high affinity and selectivity. They are selected via an iterative process known as ‘selective evolution of ligands by exponential enrichment’ (SELEX). Aptamers have been developed against numerous cancer targets and among them, many tumor cell-membrane protein biomarkers. The identification of aptamers targeting cell-surface proteins has mainly been performed by two different strategies: protein- and cell-based SELEX, when the targets used for selection were proteins and cells, respectively. This review aims to update the literature on aptamers targeting tumor cell surface protein biomarkers, highlighting potentials, pitfalls of protein- and cell-based selection processes and applications of such selected molecules. Aptamers as promising agents for diagnosis and therapeutic approaches in oncology are documented, as well as aptamers in clinical development.
Keywords: aptamer, SELEX, cell-surface biomarker
1. Introduction
Nucleic acid aptamers are single stranded DNA (ssDNA) or RNA molecules. They are selected through a fully in vitro well-established iterative process known as ‘selective evolution of ligands by exponential enrichment’ (SELEX) [1,2,3]. A large library of 1014–1015 ssDNA or RNA sequences of 20–50 variable bases is combined with a target, under temperature and buffer conditions dependent on requirements. During the partitioning step, nucleic acid molecules which bind to the target are selected while the vast majority of sequences which do not bind are washed away. Binding molecules are amplified thanks to the fixed primer-binding sequences flanking the variable region, by PCR or RT-PCR according to the nature of the oligonucleotide, ssDNA or RNA. Double-stranded DNA molecules are then separated from ssDNA in DNA-SELEX, or in vitro transcribed in RNA-SELEX by means of a T7-RNA polymerase binding site in the fixed region. The three steps (selection, partitioning and amplification) constitute a round of SELEX. Negative selection to environmental elements (usually supports of the SELEX process, like filters, beads…) and/or counter selection to target’s counterparts (like related proteins or cells) is usually performed, either before or after the positive selection, to eliminate non-specific binding nucleic acids. The rounds of selection are repeated several times, increasing stringency progressively, until molecules with the desired binding properties are cloned and sequenced. Those selected oligonucleotides are named aptamers, from the greek aptus (“to fit”) and the suffix “-mer”. Databases dedicated to aptamers provide information on targets of specific aptamers, length, molecular weight, extinction coefficient, GC (guanine-cytosine) content, chemistry, primary sequences, predictive secondary structures, as well as affinities, assay buffers… (for example, [4,5,6]).
More than 900 aptamers have been generated from SELEX [7] towards a wide range of targets such as inorganic ions, small organic ligands, amino-acids, nucleotides and derivatives, oligonucleotides, antibiotics, peptides, proteins, sugars, parasites, virus, cells and tissues; the most common targets remaining proteins. In oncology, the aptamer field is rapidly expanding. On 3 May 2017, Medline listed 1398 entries for ‘aptamer and cancer’, including 167 reviews, with more than half entries published in the last 4 years. Deregulation of cell-surface membrane protein expressions and activities are hallmarks of cancer cells. Receptors that are uniquely expressed, overexpressed or mutated in tumors are major pharmaceutical targets for therapies in the era of personalized medicine. Advances in genomic, proteomic, metabolomic techniques led to the discovery of tumor biomarkers including cell surface receptors (tyrosine kinase receptors, cell adhesion receptors, cell death receptors) [8,9,10]. A vast majority of drugs are currently designed to target such proteins/receptors and to inhibit their oncogenic activities [11]. Recent data emphasized the high inter-and intra-tumoral heterogeneity of histologically similar tumors, at genomic but also protein levels of relevant targets [12,13]. Failure of clinical trials for targeted therapies in non-stratified patient populations may be linked to this underappreciated tumor heterogeneity. Highly specific tools, such as aptamers, may represent molecular probes for labeling the targets on tumor samples and thus helping to patient stratification. In addition, accessibility of cell surface receptors for targeting with aptamers will allow not only molecular imaging but also the direct delivery of drugs to relevant cancer cells [14].
The success of a SELEX process relates to the identification of aptamers of high affinities and specificities for their targets. Identification of aptamers is more complex for cell-surface proteins which activities depend on their conformation and on their cellular environment than for non-amphipatic molecules. To target cell surface biomarkers, it can therefore be important when possible to choose selection conditions and processes mimicking as far as possible the targets natural environment. This review will thus focus on the main selection strategies which have been used to identify aptamers to tumor cell-surface proteins, highlighting differences, success, and limitations of the SELEX processes. Tumor cell-surface biomarkers used as targets for SELEX are listed in Table 1. This review includes three more tables describing characteristics and applications of aptamers identified by protein- and cell-based SELEX, for which targets are proteins and cells, respectively. For cell-based SELEX, a distinction has been made between pre- and post-identified targets. Powerful potential applications of aptamers as diagnostic and therapeutic agents in the field of oncology will be briefly discussed and recent developments of the few aptamers already in clinical trials in oncology described.
Table 1.
Major functions of biomarkers cited in this review.
| Biomarker | Major Functions of Biomarker in Cancer |
|---|---|
| Cell-adhesion molecules | |
| Epithelial cell adhesion molecule-EpCAM (CD326) | EpCam is a pleiotropic molecule able to promote and prevent epithelial cell-cell adhesion. Induces proliferation, up-regulates proto-oncogene (c-myc, cyclins A/E) and is involved in migration, proliferation, differentiation and metastasis. Circulating tumor cell marker Implicated in self-renewal of stem cells |
| Carcinoembryonic antigen-CEA (CD66e) | Implicated in cell adhesion, anoikis resistance, promotion of metastasis |
| Integrin αvβ3 | Essential for endothelial cell survival. Involved in cell growth and migration, tumor invasion, metastasis and angiogenesis |
| Integrin α6β4 | Implicated in tumor cell growth, invasion and metastasis |
| Integrin αv | Endothelial adhesion receptor. Implicated in angiogenesis |
| Integrin α4 | Prognostic marker in lymphocytic leukemia |
| E-and P-Selectin | Implicated in tumor cell adhesion to vascular endothelium, migration, proliferation of cancer cells and step of metastasis formation |
| L-Selectin | Roles in cell adhesion and in initiation of cell-cell interaction in vasculature system. Initiates events leading to leukocyte extravasations for the blood. Biomarker of naïve T cells |
| Tyrosine kinase receptors | |
| EGFR | EGFR is a receptor tyrosine kinase expressed at the surface of cell. Involved in proliferation, invasion, angiogenesis and metastasis. Associated with poor prognosis |
| EGFRVIII | The most common form of EGFR mutation. Associated with poor survival of patients. Promotes tumor cell motility |
| PDGFR β | Cell surface tyrosine kinase receptor. Implicated in proliferation, migration and angiogenesis |
| HER-2 | Belongs to ErbB family (EGFR, ErbB2). Implicated in formation, progression of human tumor, aggressiveness and resistance to therapy. Implicated in poor prognosis |
| HER-3 | Member of the receptor tyrosine kinase. Contributes to increased drug resistance in HER2 cells |
| c-MET | Receptor tyrosine kinase involved in development and progression of cancer. Induces cell proliferation, cell survival, cell motility and invasion |
| Receptor tyrosine kinase-RETC634Y | Mutated form of RET (proto-oncogene) |
| Neurotrophin receptor TrkB | Cancer cell resistance to chemotherapy. Promotes growth of cancer cells. Implicated in cell proliferation, differentiation, survival, and invasion |
| TGFβ III receptor | Role in signal transduction. Role in disease is poorly understood but correlates with higher tumor grade |
| Protein Tyrosine Kinase 7-PTK7 | Biomarker for leukemia |
| Insulin Receptor | Receptor tyrosine kinase. Implicated in activation of MAPK/ERK and PI3K/AKT pathways Implicated in cancer development and progression |
| Axl | Belongs to TAM family of tyrosine kinase receptors. Implicated in invasiveness and metastasis |
| Cell membrane-associated enzyme | |
| Prostate specific membrane antigen-PSMA | Prostate tumor cell marker |
| Mucines | |
| MUC-1 | Cell surface mucin glycoprotein |
| Tumor necrosis factor receptor (TNF-R) and co-stimulatory receptors | |
| T-cell receptor OX40 | Member of TNF family receptors. Co-stimulatory receptor on CD4+ T cells. Increases immune response to cancer and foreign entities. Implicated in exacerbating effects of inflammation through the increased cytokine production (IL2) and in cell survival of CD4+ OX40+ T cells |
| T-cell receptor 4-1BB | Co-stimulatory receptor implicated in survival and expansion of activated T cells |
| Receptor activator of NF-κB-RANK | NFkB is a member of the tumor necrosis factor (TNF) receptor family. Implicated in osteoclast differentiation and in focal bone erosion and bone malignancies |
| CD28 | Co-stimulatory receptor implicated in activation of T lymphocytes |
| Surface transmembrane glycoproteins | |
| CD133 | Cancer stem cells marker |
| CD30 TNFRSF8 | Hematological malignancies biomarker |
| Others | |
| Cytotoxic T cell antigen-4-CTLA-4 | Receptor expressed on activated T cells. Reduction in T-cell responses and activation |
| B-cell–activating factor (BAFF)-receptor (BAFF-R) | BAFF is a member of the tumor necrosis factor (TNF) family cytokines. BAFF receptor is expressed on B-cells. Implicated in proliferation, maturation and cell survival of cancer B cells |
| CD124 (IL-4Rα) | Implicated in survival and in suppressor activity of myeloid derived suppressor cells |
| VCAM-1 | Member of the immunoglobulin-like super family. Implicated in recruitment of immune cells to inflammation sites |
| Toll-like receptor 3 ectodomain | Implicated in the production of INF-β and inflammatory cytokine |
| hyaluronic acid (HA) binding domain of CD44 | Belongs to the proteoglycan family of transmembrane glycoproteins. Receptor for hyaluronic acid. Role in tumor growth and metastasis. Marker for subpopulations of tumor initiating cells and implicated in chemo resistance |
| Angiopoietin-1 | Promotes endothelial cell survival and vascular impermeability. Prevents increase in endothelial cell adhesion molecule expression |
| Angiopoietin-2 | Potentialisation of proangiogenic growth factors |
| CD71 | Responsible for cellular iron transport. Rapidly proliferative cells require more iron. |
| Cofactor for enzyme involved in DNA synthesis of proliferative cells | |
| Multidrug resistant-associated protein 1-MRP1 | MRP1 correlates with chemotherapy drug resistance. Expressed in cancer stem cells |
| CD16α | Implicated in antibody dependent cellular cytotoxicity and recruitment of NK cells. NK cells are important for suppressing tumor metastasis and outgrowth |
| Alkaline phosphatase placental-like 2-ALPPL-2 | Implicated in cell growth and invasion. Ectopically expressed on cell surface and in cell secretion |
| Nucleolin | Multifunctional protein interacting with DNA and RNA. Involved in rRNA maturation, ribosome assembly and exportation of ribosome components to the cytoplasm |
| Role in mRNA stabilization (example: role in stabilization of bcl-2 mRNA in human leukemia). Overexpressed on the surface of certain cancer cells | |
| Immunoglobulin heavy mu chain | Major component of the B-cell antigen receptor in Burkitt-s lymphoma cells |
| Implicated in transforming healthy cells and in cancer progression | |
2. Generalities on Aptamers
Owing to their three-dimensional conformation and the strong shape complementarity between aptamers and their targets, aptamers are known to recognize their specific target with high affinity and selectivity. Analogous to antibodies in their range of target recognition and variety of applications, they are referred as ‘chemical antibodies’ [15]. Aptamers though possess several advantages over antibodies: Temperature stability, self-refolding, easy production, and presumably lack of immunogenicity/toxicity… Due to their molecular weight, which is comprised between 10 and 30 Da, aptamers penetrate tissues faster and more efficiently than the 150 kDa antibodies [16]. Aptamers are chemically synthetized, rapidly, at reasonable cost, with no or low variation from batch to batch, and independently of any biological systems. Risks of viral and bacterial contamination are thus eliminated. Aptamers are thermally stable. Denatured at 95 °C, they refold into their correct three-dimensional conformation when cooled down to room temperature. To date, aptamers have never elicited toxicity in therapeutic applications.
RNA aptamers fold into more diverse three-dimensional structures than ssDNA aptamers, because of their 2′-hydroxyl (2′-OH) group on ribose and the non-Watson-Crick base pairing. The chemical production of ssDNA aptamers is easier and cheaper than RNA aptamers. But the preparation of ssDNA molecules during the SELEX process is more challenging than RNAs [17,18]. Nucleic aptamers suffer from two major disadvantages: their nuclease sensitivity, especially for RNA aptamers that can be cleared from the circulation within minutes or seconds [19], which limits their development as pharmaceuticals. Further, the chemical diversity of a 4 nucleotides based DNA and RNA library may be limited compared to that of a 20 amino acids based library. Developments allowed overcoming these weaknesses. The 2′-OH group is the naturally occurring cleavage site prone to nucleophilic attack. Typical modifications that can be inserted during the SELEX process include masking the nuclease-sensitive 2′OH group by replacing it with 2′fluoro (2′-F) or 2′-amino (2′-NH2). Such modified pyrimidines are incorporated via an efficient engineered mutant T7 RNA polymerase [20]. Other modified nucleotides can also be incorporated in SELEX libraries like 2′-O-methyl nucleotides, locked nucleic acid (LNA), hexitol nucleic acid (HNA) [21,22,23,24,25]. Modifications, if they do not alter much specificity and target binding properties of aptamers, can be introduced post-selection. Those modifications include 2′-F, 2′-NH2, 2′-thio (2′-SH), 2′-azido (2′-N3), 2′-hydroxymethyl (2′-CH2OH) or 2′-methoxy (2′-O-Me) groups, LNA [26]. Synthetic nucleic acids, named xeno nucleic acids (XNA), increase the resistance and also the diversity of such modified aptamers [27,28]. Gold et al. [29] demonstrated that the success rate of a conventional SELEX on difficult proteins is lower than 30% and improved this rate up to 84%, by the incorporation of four modified nucleotide triphosphate analogs: 5-benzylaminocarbonyl-dU(BndU), 5-naphthylmethylaminocarbonyl-dU (NapdU), 5- tryptaminocarbonyl-dU (TrpdU), and 5-isobutylaminocarbonyl-dU (iBudU). The idea was to expand the chemical diversity of aptamers by adding functional groups that mimic amino acid side chains [30]. These modifications enhance protein binding through direct hydrophobic contacts with the target protein, resulting in increased binding affinities and slower complex dissociation rates compared to unmodified aptamers. So called Slow Off-rate Modified Aptamers (SOMAmers), are developed by the SomaLogic Company (Boulder, CO, USA) to currently more than 1300 proteins. SOMAmers are used in SOMAscanTM assay, a highly sensitive proteomic tool for discovering and validation of biomarkers. Capping aptamers with inverted nucleotides is another alternative to reduce exonuclease degradation [31]. The native phosphodiester backbone of nucleotides can also be modified with boranophosphate or phosphorothioates [32,33,34]. The company NOXXON Pharma AG (Berlin, Germany) takes advantage of spiegelmers (‘spiegel’ means ‘mirror’ in German) to prevent enzymatic degradation in biological fluids [19,35]. Spiegelmers are artificial RNA-like molecules built from L-ribose units, images of natural D-units, highly resistant to nuclease degradation and much more stable in vivo, with lifetimes greater than 60 hours in plasma. The SELEX is performed with D-RNA libraries combined with mirror images of peptides or small proteins artificially synthesized with D-aminoacids. Once aptamers are identified, they are converted to stable L-RNAs and they bind to the natural L-form of peptide or protein targets with equally high affinities. Three spiegelmers are actually in clinical trials, of which one in oncology (described in Section 5). This process is however limited to artificially synthesized targets. To increase their size, consequently reducing their systemic clearance and prolonging their in vivo half-lives, ssDNA and RNA aptamers are often coupled to bulky groups, such as poly (D,L-lactic-co-glycolic acid) (PLGA), poly ethylene glycol (PEG), liposomes, steptavidin or cholesterol [36,37,38,39,40,41].
3. Protein- and Cell-Based SELEX Processes
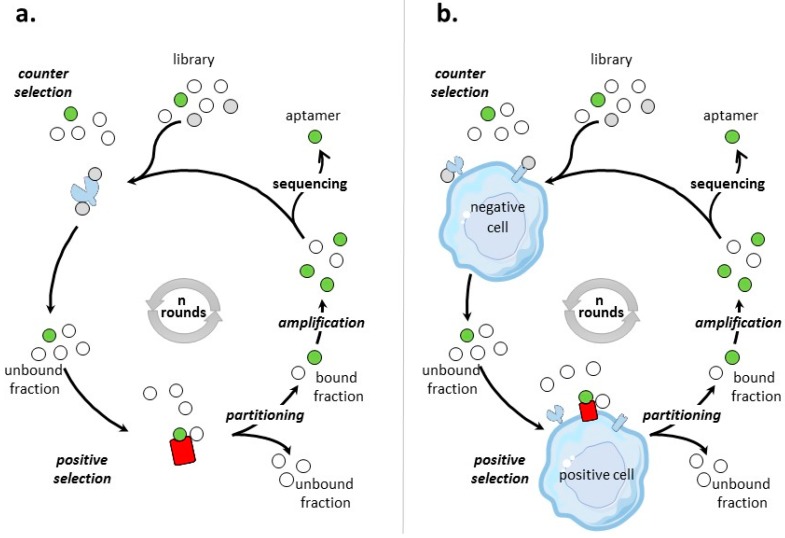
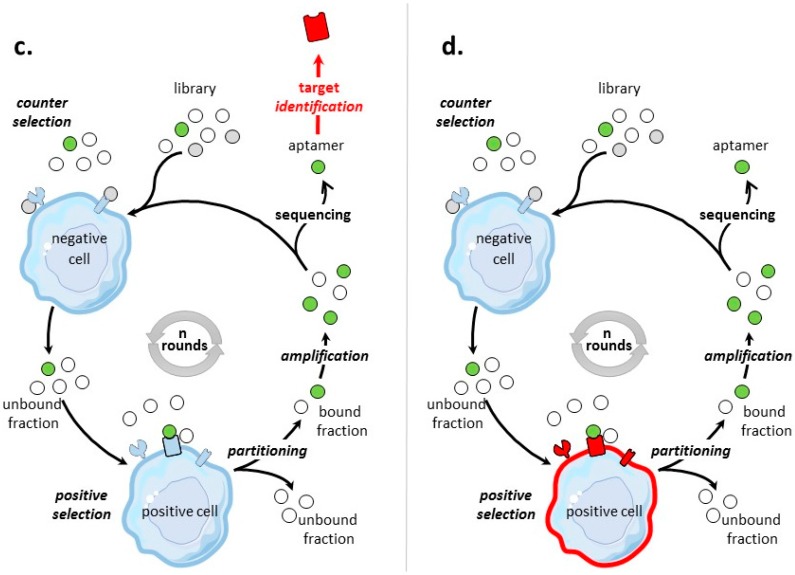
Different SELEX processes have been developed for the selection of aptamers targeting tumor cell-surface protein biomarkers. Figure 1 illustrates the classical protein- and cell-based SELEX procedures, the two main methods that are used to identify aptamers to tumor cell-surface biomarkers for which targets are proteins and cells, respectively.
Figure 1.
Scheme of protein- and cell-based SELEX processes. Briefly, the process of SELEX involves first a selection step: the nucleic acid library is incubated with a target (positive selection), which can be preceded or followed by a counter selection phase to remove non-specific nucleic acid molecules. During the partitioning step, bound and unbound fractions are separated. The bound fraction is amplified to obtain an enriched pool for next round of selection. This process is repeated for n rounds until the pool is enriched for sequences that specifically bind the target. These nucleic acid molecules are cloned and sequenced. Individual sequences are aptamers. (a) Protein-based SELEX. The pre-identified purified protein used as target for the protein-based SELEX is colored in red. (b) Cell-based SELEX on a pre-identified tumor cell-surface biomarker colored in red. (c) Cell-based SELEX on a post-identified tumor cell-surface biomarker. The target, identified at the end of the SELEX process, is colored in red. (d) Cell-based SELEX to a tumor cell type. In this case, no particular cell-surface biomarkers are identified and aptamers are specific to the cell’s molecular signature. The whole cell used for positive selection is colored in red.
3.1. Aptamers Selected by Protein-Based SELEX
Protein SELEX is the simplest form of SELEX process (Figure 1a). Table 2 provides an inventory of most of tumor cell-surface protein biomarkers selected by protein-SELEX. Aptamers selected by protein-based SELEX have been reported for cell-adhesion molecules, tyrosine kinase receptors, cell membrane-associated enzymes, mucins, T-cell receptors, co-stimulator receptors, surface transmembrane glycoproteins… Some of the biomarkers have been subjects to different SELEX procedures, like integrin αvβ3, MUC-1, L-selectin and CD44 and the tyrosine kinase receptors EGFR, HER-2, and c-Met. Many factors varied, like the nature and the number of random nucleotides of the library, the separation procedures, the numbers of selection rounds. All of these selection procedures lead to aptamers of high affinities for their targets, usually in the low nanomolar range.
Table 2.
Aptamers to tumor cell-surface biomarkers selected by protein-based SELEX (updated since 2011 [42,43,44]).
| Biomarker | Target Used for Protein-SELEX | Aptamer Library | Number of Variable Nucleotides | Number of Rounds of Selection | Separation Method | Name of Selected Aptamers | KD | Applications | Year | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Cell-adhesion molecules | ||||||||||
| Epithelial cell adhesion molecule-EpCAM (CD326) | His-tagged C-terminal domain of EpCAM | 2′F-RNA | 40 | 12 | affinity chromatography | Truncated EpCAM RNA aptamer | 55 nM to human cells | Target stem cell marker. Potential applications: development of targeted cancer nanomedecine and molecular imaging agents | 2011 | [45] |
| EpCAM | His-tagged C-terminal domain of EpCAM | DNA | 40 | 12 | affinity chromatography | Truncated aptamer SYL3C | 38 and 67 nM to cells | Imaging (confocal) Cancer cell capture |
2013 | [46] |
| Carcinoembryonic antigen- CEA (CD66e) | His-tagged recombinant protein of full-length CEA | 2′F-RNA | 40 | 17 | affinity chromatography | Group I, II and III | low nM range | Inhibition of cell migration/invasion in vivo. Promotion of cell anoikis resistance in vitro Inhibition of liver metastasis in vivo |
2012 | [47] |
| Integrin αvβ3 | purified αvβ3 integrin | 2′F-RNA | 50 | 15–17 | affinity chromatography | Apt-avb3 | nM range | Inhibition of endothelial cell adhesion and proliferation Reduction of endothelial cell tube formation Inhibition of cancer cell proliferation and adhesion (HUVEC) Increases endothelial cell apoptosis |
2005 | [48,49] |
| Integrin αvβ3 | purified αvβ3 integrin | 2′F-RNA | 50 | 6 | MAI-SELEX 1 | αV-1 and β3-1 | 8.9–10.5 nM | Recognizes distinct binding sites on a single target ( αV or β3) with minimal cross-reactivity Potential applications: molecular diagnosis and targeted therapies |
2012 | [50] |
| E-and P-Selectin | recombinant human E-selectin/IgG-Fc-chimeras | DNA | 50 | 17 | affinity chromatography | SDA | 100 nM | Inhibition of cancer cell adhesion Potential applications in therapies during metastasis formation |
2014 | [51] |
| L-Selectin | L-selectin-Ig chimera | 2′-NH2 RNA | 40 | 14 | affinity chromatography | 14.12 | 0.2–3 nM range | Preferential blokade of a specific selectin | 1996 | [52] |
| L-Selectin | L-selectin–IgG fusion protein | DNA | 40 | 17 | affinity chromatography | LD201, LD174 and LD196 | 1.8, 5.5 and 3.1 nM | Inhibition of lymphocyte rolling on endothelial cells | 1996 | [53] |
| Tyrosine kinase receptors | ||||||||||
| EGFR | purified extracellular domain of human EGFR | RNA | 62 | 12 | not documented | J18 | 7 nM | Drug delivery (internalization of gold nanoparticules) Potential application: delivery of siRNA and cancer detection |
2010 | [54] |
| EGFR | human EGFR-Fc protein | 2′F-RNA | 62 | 9 + 7–9 rounds with a 30% doped sequence (from aptamer E01) | affinity chromatography | E07 Internalized | 2.4 nM | Prevention of proliferation of tumor cells (blocks receptor autophosphorylation) Drug delivery (Gemcitabine) and induces cell death |
2011 | [55,56] |
| EGFR | purified extracellular domain of EGFR | DNA | 40 | 11 | affinity chromatography | Tutu-22 | 56 nM | Regognizes EGFR-positive cancer cells with strong affinity and selectivity Potential applications: development of novel targeted cancer detection, imaging and therapy |
2014 | [57] |
| EGFRVIII | histidine-tagged EGFRvIII ectodomain (E. coli. system) | 2′F-RNA | 40 | 12 | affinity chromatography | E21 | 33 nM | Disruption of post-translational modifications of immature EGFRvIII Induction of apoptosis |
2009 | [58] |
| HER-2 | recombinant glutathione S-transferase (GST)-tagged ErbB2 protein (22–122 amino acids) | 2′F-RNA | 50 | 15 | affinity chromatography | SE15-8 | low nM range | High specificity to ErbB2 and not to other members of the ErbB family Potential applications: drug delivery and imaging for in vivo diagnosis |
2011 | [59] |
| HER-2 | peptide from the juxtamembrane region of HER2 extracellular domain | DNA | 40 | multiple | affinity chromatography | HB5 | 18.9 nM | Drug delivery (Doxorubicin) | 2012 | [60] |
| HER-2 | 20-amino acid HER2 peptide | Thio-DNA | 21 | 12 | affinity chromatography | HY6 | 172 nM | Potential application: targeted therapy | 2015 | [61] |
| HER-2 | His-tagged Her2–extra cellular domain (E. coli system) | DNA | 40 | 15 | membrane filtration | ECD_Apt1 | 6.33 nM | Potential applications: theranostic (non invasive cancer diagnosis), therapeutics and monitoring patient compliance | 2017 | [62] |
| HER-3 | extracellular domains of HER3 produced in S2 insect cells | RNA | 49 | 15 | membrane filtration and gel shift assay | A30 | 0.1 nM range | Inhibition of HER3 activation and growth of tumor cells Potential application: anticancer drug |
2003 | [63] |
| c-MET | c-Met-Fc | DNA | 40 | 12 | membrane filtration | CLN3 and CLN4 | 91 pm and 11 nM | Mediates tumor cell lysis Recruits NK cells to tumor and induces ADCC |
2011 | [64] |
| c-MET | c-Met-Fc | 2′F-RNA | 40 | 16 | membrane filtration | CLN64 | 1 nM | Inhibition of tumor cell migration Potential application: therapeutics and diagnosis |
2015 | [65] |
| Cell membrane-associated enzyme | ||||||||||
| Prostate specific membrane antigen-PSMA | 706 extracellular amino acids of PMSA | 2′F-RNA | 40 | 6 | affinity chromatography | A9, A10, A10-3 after minimization & optimization | low nM range | Promotion of tumor regression Delivery of siRNA Potential application: diagnosis and therapies |
2002 | [66,67] |
| Mucins | ||||||||||
| MUC-1 | MUC-1 peptides of 2 lenghts: 9 and 60 amino acids | DNA | 25 | 10 | affinity chromatography | S1.3/S2.2 | low nM range to 0.1 nM | Potential application: detection by fluorescent microscopy | 2006 | [68] |
| MUC-1 | His-tagged unglycosylated form of the MUC1 protein containing five tandem repeats of the VTR (E. coli system) | DNA | 25 | 10 | affinity chromatography | MUC1-5TR-1, 2, 3, 4 | 47–85 nM | Potential application: diagnosis assays for early or metastatic diseases | 2008 | [69] |
| Tumor necrosis factor receptor (TNF-R) and co-stimulatory receptors | ||||||||||
| T-cell receptor OX40 | extracellular domain of OX40-Fc fusion protein | 2′F-RNA | 40 | 9–11 | affinity chromatography | 9C7, 11F11 | 2-10 nM for purified OX40 protein and # 50 nM for OX40 on activated T cells | Increasing proliferation of T lymphocytes and production of IFN-γ. Potential application: therapeutics in association with dendritic cell-based vaccines (adoptive cellular therapy) | 2013 | [70] |
| T-cell receptor OX40 | murine extracellular domain of OX40-Fc fusion protein | 2′F-RNA | 40 | 11 | affinity chromatography | 9.8 | 8 nM | Induces nuclear localization of NFκB, cytokine production and cell proliferation. Increases dendritic cell based tumor vaccine effects | 2008 | [71] |
| T-cell receptor 4-1BB | murine extracellular domain of 4-1BB-Fc fusion protein | 2′F-RNA | 40 | 12 | affinity chromatography | M12-23 (multimeric aptamer) | 40 nM | Inhibition of tumor growth in vivo. Potential application: therapeutic manipulation of the immune system | 2008 | [72] |
| Receptor activator of NF-κB-RANK | recombinant human soluble RANK/IgG1Fc chimera | RNA | 40 | 7 | affinity chromatography | apt1, apt2 and apt3 | 0.33, 1.8 and 5.8 μM. 100 nM for the 2′-F version of aptamers | Potential application: therapeutics against osteoclastogenesis | 2004 | [73] |
| CD28 2 | murine recombinant CD28-Fc fusion protein | 2′F-RNA | 25 | 9 | affinity chromatography | CD28Apt2 and CD28Apt7 | 60 nM for CD28Apt7-dimer | Potentialisation of antitumor vaccine efficacy Reduction of tumor progression and increased overall survival (in vivo) Potential application: enhancing vaccine-induced immune responses |
2013 | [74] |
| Others | ||||||||||
| Cytotoxic T cell Antigen-4-CTLA-4 | murine CTLA-4/Fc fusion protein | 2′F-RNA | 40 | 9 | membrane filtration | M9-9 | 30–60 nM | Increases tumor immunity (in vivo) Potential application: immunotherapy |
2003 | [75] |
| B-cell–activating factor (BAFF)-receptor (BAFF-R) | Human recombinant BAFF-R protein | 2′F-RNA | 50 | 12 | membrane filtration | R-1, R-2 and R-14 | 47, 95 and 96 nM | Delivery of siRNA. Potential application: combinatorial therapeutics | 2013 | [76] |
| CD124 (IL-4Rα) | recombinant ILR4α protein enzymatically cleaved | 2′F-RNA | 40 | 5 | affinity chromatography | cL42 | 14 nM for recombinant protein and 788 nM for MCS2 cells | Induction of MDSCS apoptosis Promotes CD8+ T cell infiltration and reduces the number of MDSCs infiltration. Reduction of tumor progression in vivo |
2012 | [77] |
| VCAM-1 | N-terminal fragment of VCAM-1 | 2′F-RNA | 40 | 12 | affinity chromatography | 12.11 | 10 nM | Potential application: imaging | 2007 | [78] |
| Toll-like receptor 3 ectodomain | Toll-like receptor 3 ectodomain with N-terminal FLAG and C-terminal His | RNA | 40 | 7 | membrane filtration | Family-I and Family II | # 3 nM | Aptamer without agonist and antagonist effects | 2006 | [79] |
| hyaluronic acid (HA) binding domain of CD44 | HA-binding domain of human CD44 (cell-free expression system) | Thio-DNA | 30 | 10 | affinity chromatography | TA1-TA6 | 180–295 nM | Potential applications targeted therapy and imaging | 2010 | [80] |
| CD44 | GST-tagged human recombinant full length CD44 protein | 2′F-RNA | 45 | 11 | affinity chromatography | Apt1 | 81.3 nM | Potential applications therapeutic (targeted delivery againt stem cells) and diagnosis | 2013 | [81] |
| Angiopoietin-1 | recombinant human Ang1 | 2′F-RNA | 40 | 9 | membrane filtration | ANG9-4 | 2.8 nM | Inhibition of cell endothelial cell survival | 2008 | [82] |
| Angiopoietin-2 | recombinant human Ang2 | 2′F-RNA | 40 | 11 | membrane filtration | 11-1 and truncated 11-1.41 | 3.1 and 2.2 nM | Inhibition of angiogeneis (in vivo) | 2003 | [83] |
1 Integrin αvβ3 is a heterodimeric transmembrane protein composed of α and β chains, for which the selection procedure of a 2′-fluoro aptamer has been patented [48]. In order to select for aptamers specific to homodimer αv and β3, Gong et al [50], developed a strategy called MAI-SELEX (MAI for multivalent aptamer isolation). Two distinct selection stages were employed, the first being a classical affinity selection on the purified full-length αvβ3 integrin. The second module, for specificity, leads selection to β3 as integrin αIIbβ3 served as a protein decoy. Two aptamers, specific for αv and β3 were identified with affinities in the low nanomolar range. This selection strategy applied to heterodimeric proteins is limited to the availability of decoy proteins. 2 Aptamer, GR1, targets CD28. This G-rich oligonucleotide, which, alike AS1411 [84], has not selected by SELEX, inhibits CD28 T cell responses in vitro and in vivo [85].
Cell surface proteins used as targets for protein-based SELEX are either full-length or truncated versions of full length proteins, generally recombinant ectodomains coupled to tags (His-tags, Fc fragments of antibody or GST), facilitating purification and selection by affinity. Peptides can also be used as targets. In that case, the advantages linked to the exact knowledge of the aptatope, and to the facility of production of large amounts of targets can be offset by the limitations of these peptides as protein mimics. Cell-surface proteins are amphipatic membrane proteins, and therefore not easily extracted from the lipidic membrane, purified and solubilized. Even though membrane proteins can be purified and solubilized, large amount of proteins are needed for a whole protein-SELEX procedure. Further, full-length membrane proteins or ectodomains, expressed in prokaryotic or lower eukaryotic systems, may lack or have different post-translational modifications (phosphorylation, glycosylation, ubiquitination, methylation, myristoylation, acetylation…). For example, the 2′F-RNA aptamer E21, elicited against the EGFRvIII ectodomain produced in bacteria, did not bind to the native protein expressed from eucaryotic cells because glycosylation, a post-translational modification present only in eukaryotic systems, significantly alters the structure of the target protein [58].
Some of the biomarkers have been subjects to different SELEX, like MUC-1, that allowed comparison between aptamers targeting proteins and peptides mimics of proteins. For example, Ferreira et al. [68] selected ssDNA aptamers targeting two peptides of the native MUC1. They used the 9-amino-acid immune-dominant antibody-binding epitope APDTRPAPG which contains only part of the variable tandem repeat region of MUC-1, as well as a larger peptide (60 amino acids long) which comprised three complete tandem repeats of the MUC1 region. From a preceding experience with an antibody binding to MUC1, the short peptide was weakly binding to the antibody compared to longer peptides, possibly due to increased flexibility resulting in higher entropic cost [86]. The two SELEX on the two peptides of different lengths resulted in identical aptamer sequences with affinities as low as 0.1 nM. Moreover, aptamers selected on the short peptide have been shown to successfully recognize and bind to the native MUC1 at the surface of the MCF7 breast cancer cell line. In comparison, the same group has selected aptamers to the MUC-1 protein with higher KD (47–85 nM) than those obtained for aptamers to peptides, which might be due to an unstructured MUC-1 protein [69].
Overall, the main disadvantage of protein SELEX is that purified and solubilized membrane proteins, recombinant proteins and peptides may not adopt the same state (in terms of lengths, conformation, post-translational modifications…), than the cell-surface biomarker in its endogenous environment. Thus, some aptamers selected against purified membrane proteins failed to recognize their targets in whole cells [58,78], limiting their use for medical applications [87]. Moreover, some biomarkers like receptors require co-receptors for proper folding, that prevents the use of protein-SELEX. Other processes, like cell-based SELEX using whole living cells as targets may overcome these limitations.
3.2. Aptamers Selected by Cell-Based SELEX
The general process of cell-SELEX is shown in Figure 1b–d. In cell-based SELEX, the target of the selection is embedded in its native environment in cells. PubMed indicates 829 entries with ‘cell SELEX’ of which more than 1/3 are on ‘cancer and cell SELEX’. There has been growing developments in cell-SELEX these last years. Recent reviews detail advances in aptamer selection technology, particularly the high-throughput next-generation sequencing and bioinformatics [88], the cell-SELEX process [89], the evolution of complex target SELEX [90]. For example, FACS (Fluorescence activated cell sorting) SELEX aims at removing, from the cell suspension, dead cells which could have an impact on the selection [91,92,93]. In Internalized cell-SELEX, aptamers are selected on their ability to bind to cell-surface target and to internalize in the cell [94,95,96,97,98,99,100]. Nucleic acid sequences which do not internalize are eliminated. To best mimic the natural environment of targets, Souza et al. [101] developed 3D cell-SELEX, in which aptamers are selected on spheroid cells in 3D cell culture by magnetic levitation method to mimic the tissue microenvironment in vitro.
3.2.1. Cell-Based SELEX to Identify Aptamers to Tumor Cell-Surface Protein Biomarkers
Depending on the objective, knowledge of the target identity prior to selection can be useful, or not. Therefore, different strategies can be distinguished:
Pre-Identified Tumor Cell-Surface Biomarkers
When the objective of a cell-based SELEX is the identification of aptamers to a clearly identified cell-surface biomarker (Figure 1b), the SELEX procedure is sometimes termed TECS-SELEX for ‘target expressed on cell surface-SELEX’ [102]. In Table 3, selections by cell-based SELEX realized on pre-identified tumor cell-surface biomarkers are described. Many identical tumor biomarkers, in particular tyrosine kinase receptors, EpCAM and integrins, were used as pre-identified targets for protein-and cell-based SELEX (Table 2 and Table 3). As an example of solid tumor targeted by cell-based SELEX, Delac et al. [103] reviewed aptamers designed to target glioblastoma cells. Some of the key cell surface molecules that have been identified as glioblastoma biomarkers (transferrin receptor, αvβ3 integrin, EGFR, EGFRvIII) [104], have already been targets of protein-and/or cell-based SELEX (Table 2 and Table 3).
Table 3.
Aptamers selected by cell-based SELEX on pre-identified tumor cell-surface biomarkers.
| Biomarker | Aptamer Library | Number of Variable Positions | KD Range | Number of Positive Selection Rounds (selection method) 1 |
Cells Used for Positive Selection | Cells Used for Counter-SELEX | Applications | References |
|---|---|---|---|---|---|---|---|---|
| Tyrosine kinase receptors | ||||||||
| EGFRvIII | DNA | 30 | 0.62–37.57 nM | 14 | U87-EGFRvIII, U87-MG, GBM cells overexpressing EGFRvIII |
U87-MG | Potential applications: delivery of chemical drug and diagnosis | [117] |
| EGFRvIII | DNA | 40 | 3–16 nM | 11 | U87-MG, GBM cells overexpressing EGFRvIII | U87-MG | Imaging (radiolabeled 188Re, in vivo) Potential applications: diagnosis in stratifying patient and monitoring treatment |
[118] |
| HER-2 | RNA | 40 | 94.6 nM | 20 | HER-2-overexpressing SK-BR-3 cell line | MDA-MB-231, a HER-2-underexpressing breast cancer cell line | Potential application: therapy | [113] |
| HER-2 | 2′F-RNA | 20 | 46–82 nM | 9 (cell-internalization SELEX) |
N202.1A mammary carcinoma clonal cell lines expressing high levels of surface HER-2/neu. |
N202.1 E clonal cell line has no detectable surface expression of the HER2/neu oncoprotein. | Drug delivery (Bcl-2 siRNA) Induces chemosensibilisation and reduces drug resistance |
[100] |
| HER-2 | DNA | 50 | Not documented | 4 rounds | Cleared extract of ErbB-2-overexpressing N87 cells | Not documented | Acceleration of ErbB-2 degradation in lysosomes Inhibition of growth of tumor cell in vitro and tumor mass in vivo |
[116] |
| HER-2 | DNA | 30 | 5–23 nM | 8 rounds of protein SELEX followed by 7 rounds of cell -SELEX (hybrid-SELEX) |
HER-2 overexpressed in SKOV3 ovarian cancer cells | No cells | PET imaging (radio labeled, in vivo) | [109] |
| HER-2 | DNA | 40 | Not documented | 16 | HER2- overexpressing breast cancer cell line, SK-BR3 |
HER2 negative breast cancer cell line, MDA-MB468 |
Development of a new method to monitor the enrichment of aptamers in a given round of cell-SELEX | [114] |
| Receptor tyrosine kinase-RETC634Y | 2′F RNA | 50 | Tens of nM | 15 rounds of selection on cells, followed by 7 rounds on purified protein (hybrid-SELEX) |
RETC634Y mutant receptor expressed in PC12 cells (PC12/MEN2A) | PC12 | Potential applications: diagnosis, imaging and therapy | [108] |
| Neurotrophin receptor TrkB | 2′F RNA | 20 | 2 nM | 4 (cell-internalization SELEX) |
TrkB-expressing HEK cells | HEK | Neuroprotective effects Potential in therapy for neuro degenerative disease |
[119] |
| Other kinase receptors | ||||||||
| TGFβ III receptor | 2′F RNA | 60 | 1 nM | 11 | TGFβ III receptor ectopically expressed on CHO-K1 cells | CHO-K1 | Potential application: therapy through inhibition of TGFβIII receptor | [102] |
| Transferrin receptors (TfR) | ||||||||
| CD71 | 2′F-RNA | 50 | nM range | 4 rounds of protein-SELEX on the His-tagged recombinant protein and 1 round on cells (hybrid SELEX combined with cell-internalization SELEX) |
HeLa cells, a human cervical cancer cell line known to express TfR |
NO cells | Delivery of siRNA Targets cell in liposomes |
[94] |
| ATP-binding cassette (ABC) transporters | ||||||||
| Multidrug resistant-associated protein 1-MRP1 | 2′F-RNA | 25 | 50 nM | 10 rounds of peptide-SELEX followed by 1 round of cell-SELEX (hybrid-SELEX) |
chemotherapy-resistant tumor cell line that has high MRP1 expression (H69AR) |
Parental cell line H69 | Reduction of cell growth in vitro and improved survival in vivo | [111] |
| Cell adhesion molecules | ||||||||
| Epithelial cell adhesion molecule-EpCAM (CD326) | DNA | 40 | μM range | 7 (FACS-SELEX) |
EpCAM over-expressed in HepG2 cells | HepG2 cells | Potential application: stem cell marker | [120] |
| Integrin α6β4 | DNA | 39 | 139 nM | 5 rounds of cell-SELEX followed by 7 rounds of protein-SELEX (hybrid-SELEX) |
PC-3 cells | PC-3 β4 integrin (ITGB4) knockdown cells | Imaging (confocal) Potential application: drug delivery |
[110] |
| Integrin αv | RNA | 35 & 40 | 359–408 nM | 13 (Isogenic cell-SELEX) |
Human HEK293 cells manipulated to generate positive αv selection cells by overexpressing ITGAV |
Human HEK293 cells depleted in ITGAV with microRNA-mediated silencing. |
Potential application: targeting channels, transporters… | [112] |
| Fc receptors | ||||||||
| CD16α | DNA | 40 | 6–429 nM | 9 rounds of protein-SELEX and 6 rounds of cell-SELEX (hybrid-SELEX) |
CD16_-6His and CD16_ Val-158 or Phe-158 alloforms expressed on recombinant Jurkat cells |
Jurkat E6.1 cell line | ADCC (tumor cell lysis) | [64] |
| Surface transmembrane glycoproteins | ||||||||
| CD133 | 2′F RNA | 40 | Not determined | 6 | HEK293T expressing CD133 | His-tagged irrelevant protein expressed in HEK293T | Potential application: target cancer stem cells and molecular imaging | [121] |
| CD30 TNFRSF8 |
DNA | 30 | nM range | 20 rounds of cell-SELEX followed by 5 rounds on purified His-tagged CD30 protein (hybrid-SELEX) |
CD30-positive K299 T-cells lymphoma | Jurkat cells | Potential application: therapy | [115] |
1 Selection method is specified if it is different from classical cell-SELEX.
Different procedures have been developed to direct the recognition of the target by the aptamer. For example, ligand-guided selection (LIGS) SELEX guides the selection to a specific epitope of a known cell-surface target. Zumrut et al. [105,106] used antibodies as secondary ligands to elute specific aptamers. The antibody interacts with its cognate antigen to outcompete aptamers specific for a pre-determined cell-surface receptor and replace specific aptamers from an enriched SELEX pool. Immunoprecipitation (IP) SELEX is an approach combining cell-SELEX and protein-nucleic acid immunoprecipitation to ensure that aptamers bind to a pre-identified target [7,107]. Hybrid-SELEX, also called cross-over SELEX, is a combinatorial approach to guide selection towards a pre-identified target. Usually, the first rounds of selection are realized on cells which express the target by cell-SELEX, and then rounds of selection are realized on the same version of the target in its purified form by protein-based SELEX. A reverse hybrid-SELEX combines first protein-SELEX followed by cell-SELEX. Hybrid-or reverse hybrid-SELEX have been used to isolate aptamers targeting the RETC634Y mutant receptor tyrosine kinase overexpressed in PC12 cells [108], the HER-2 tyrosine kinase receptor expressed in SKOV3 ovarian cancer cells [109], CD16α on Jurkat cells overexpressing CD16 alloforms [64], integrin α6β4 on PC-3 cells [110], the transferrin receptor CD71 expressed in HeLa cells [94]. In this last example, a combination of protein-SELEX and cell-internalized SELEX was realised. Soldevilla et al. [111] used another variant of reverse hybrid-SELEX, combining cell-SELEX to peptide-SELEX. Ten rounds of selection were first realized by peptide-SELEX, followed by one round of cell-SELEX on a chemotherapy-resistant tumor cell line that highly express MRP1 (H69AR).
Cell-based SELEX involves positive selection to collect aptamers that interact specifically with the target cells and usually counter selection to eliminate nonspecific nucleic acid sequences binding to negative cells. Different human tumor cell lines have been used for positive selection: glioblastoma (U251, U87MG), breast carcinoma (SKBR-3, N202.1), ovarian cancer (SKOV3), gastric cancer (N87), lung carcinoma (A549, H69), lymphoma (Jurkat, K299T), liver cancer (HepG2), prostate adenocarcinoma (PC-3), cervical cancer (HeLa). The PC12 cell line derived from pheochromocytoma of the rat adrenal medulla has also been used, as well as other non-tumoral cells lines, like Chinese hamster ovary (CHO), Human embryonic kidney (HEK) cells. The choice of the couple of cells used for positive- and counter-selections is of great importance for cell-based SELEX on pre-identified cell-surface protein. The two cell type should display a high difference in target expression levels, so that aptamers interact specifically with the target on positive cells and does not interact with cells used for counter-selection which does not display or displays lower levels of the target protein. For positive selection, the pre-identified target can be naturally expressed or over-expressed at the surface of the cell line. Cells used for counter selection are chosen in light of positive cells. In the literature, cell lines used for counter selection were:
(i) isogenic to cells used for positive selection. Most of the cell-based SELEX process used cells overexpressing the target of interest for the positive selection, and the parental cell line for counter selection (Table 3). As an example over many others, a cell-based SELEX was realized on Chinese hamster ovary (CHO)-K1 cells ectopically expressing human transforming growth factor-β type III receptor while CHO-K1 cells were used for counter selection [102]. In two examples described in Table 3, both on integrins, cells used for counter-selection were under-expressing the target. In the first example, the parental isogenic cell lines are used for the positive selection. Berg et al. [110] used this strategy to select an ssDNA aptamer specific for α6β4 integrin in its native state. Five rounds of positive cell selection were realized on PC-3 cells. PC-3 β4 integrin (ITGB4) knockdown cells were used in a preselection step to deplete aptamers specific for cell surface marker other than β4 sub-unit. However, the counter-selection strategy was found to be insufficient. And seven more rounds of protein-SELEX were performed on a recombinant α6β4 protein to select for α6β4-specific aptamers. In the second example, HEK293 cells lines are used. For positive selection, HEK293 cells were manipulated to generate positive αv selection cells by overexpressing ITGAV. For counter selection, the same cell line was depleted in ITGAV with microRNA-mediated silencing. Takahashi et al. [112] named this strategy Icell-SELEX for isogenic cell SELEX.
(ii) unrelated to cells used for positive selection. Two studies [113,114] related the use for positive selection of the breast cancer cell line SK-BR3 modified to overexpress HER-2. For counter-selection, the human breast cell types MDA-MB-231 and MDA-MB-468, respectively underexpressing and negative for HER-2 were used. Twelve and sixteen rounds of selection were performed to select RNA- [113] and ssDNA- [114] aptamers. According to Dastjerdi et al. [114], the use of the HER2 underexpressing cell line (MDA-MB-231) in Kang et al’s study [113], instead of a HER2-negative cell line may have compromised counter selection. A hybrid-SELEX strategy was used to get aptamers to the surface transmembrane glycoprotein CD30 [115]. For the cell-SELEX, lymphoma cells K299T and Jurkat cells were used for positive and counter-selection, respectively.
(iv) inexistent or not documented [116]. ssDNA aptamers to HER-2 have recently been selected through a hybrid-SELEX [109], combining 8 rounds a protein-SELEX using the His-tagged extracellular domain of HER-2 and 7 rounds of cell-SELEX on HER-2 over-expressed in SKOV3 cancer cells. Human serum albumin was used for negative screening in protein-SELEX and added to ssDNA pools during cell-SELEX, but no cells were used for counter-selection. Post-selection, HER2-negative MCF7, MDA-MB-435 and MDA-MB-231 cells were used to test the specificity of aptamers. Seven aptamer candidates from hybrid-SELEX were radiolabeled with 18F and further screened in vivo by PET imaging in a SKOV3 tumor model. Two aptamers, Heraptamer-1 and-2 which had relatively high tumor uptake ratios, are promising ligands for HER-2 imaging in cancer. A similar approach was used by Wilner et al. [94]. Four rounds of protein-SELEX were performed using the His-tagged recombinant transferring receptor CD71 (TfR) as target, followed by 1 round of cell-SELEX on HeLa cells, a human cervical cancer cell line known to express TfR. Following the first round, a negative selection step was performed in which the library was pre-incubated with Ni-NTA agarose prior to the positive selection. But the cell-SELEX procedure did not include counter-selection steps.
Post-Identified Tumor Cell-Surface Biomarkers—Discovery of Tumor Biomarkers
Aptamers are valuable tools to isolate tumor-specific biomarkers [122,123,124], as cell-based SELEX can also be used to identify targets after the selection (Figure 1c). The idea is first to select and identify aptamers specific for a tumor cell type, and then to use these high-affinity aptamers as probes to identify their cell-surface targets. These cell-surface targets, specific for tumor cells under study, are identified as (new) biomarkers. The main challenge is the identification of the protein target, usually by affinity purification followed by mass-spectrometry. The first post cell-SELEX identification of a tumor biomarker was realized in 2003 by Daniels et al. [125]. The U251 glioblastoma cell line was used as target for positive selection. Counter selection rounds were not included in SELEX. Twenty-one rounds of cell-SELEX using a 34 nucleotide long random library of ssDNA were performed to select aptamer GBI-10, shown to be the ligand for Tenascin-C, which is not a cell-surface receptor, but an extracellular matrix protein. Table 4 presents some (certainly not all) tumor cell-surface protein biomarkers which have been identified post-SELEX.
Table 4.
Aptamers Selected by Cell-Based SELEX on Post-Identified Tumor Cell-Surface Biomarkers.
| Biomarker | Aptamer Library | Applications | References |
|---|---|---|---|
| EGFR | 2′F-RNA | Induces EGFR-mediated signal pathways causing selective cell death Inhibits tumor growth in vivo Combined cetuximab-aptamer treatment shows clear synergy in inducing apoptosis in vitro and in vivo Potential application: translational therapy |
[130] |
| PDGFR β | 2′F-RNA | Inhibition of receptor signaling and of glioblastoma-derived tumor growth Inhibition of cell migration and proliferation Induction of differentiation |
[99] |
| Alkaline phosphatase placental-like 2-ALPPL-2 | 2′F-RNA | Targets ALPPL-2 in both membrane bound and secretary forms Potential applications: diagnosis, imaging and therapy |
[131] |
| selectin L and integrin α4 | DNA | Potential application: therapeutic intervention | [132] |
| CD44/CD24 | DNA | Potential applications: disruption of therapeutic resistance, invasion and angiogenesis | [133] |
| CD44 | DNA | Potential applications: cancer detection, imaging and drug delivery | [134] |
| Protein Tyrosine Kinase 7-PTK7 | DNA | Potential application: drug delivery | [135] |
| Insulin Receptor | 2′-fluoro RNA | Inhibition of IR signaling Reduction of cell viability Potential applications: targeted therapies |
[136] |
| Axl | 2′F-RNA | Interferes with cell migration and invasion Inhibition of spheroid formation and cell transformation Inhibition of tumor growth |
[137] |
| Nucleolin | DNA | Interferes with multiple biological activities in tumor cells Induction of apoptosis via down-regulation of bcl-2 proteins |
[84,127,128,129] |
| Immunoglobulin heavy mu chain | DNA | Role in identification of cell membrane receptor with increased expression levels Potential applications: early diagnosis, targeted therapy and mechanistic studies |
[138] |
The ssDNA aptamer AS1411 in clinical trials as a novel treatment for cancer is original compared to all other aptamers cited in this review, as its discovery does not result from a SELEX approach. It is referred as an aptamer as its activity arises from binding to a specific target via shape-specific recognition. Briefly, Bates et al. [84], in the late 1990’s, tested G-rich oligonucleotides (GRO) in a variety of cell lines and showed that they had unexpected anti-proliferative effect. AS1411 is a 26-nucleotide GRO, which forms quadruplex. It blocks the activation of secondary targets (NF-κB, and B-cell lymphoma 2, BCL2), sensitizes tumor cells to chemotherapy, has direct anti-proliferative effects in vitro, is efficiently internalized even at nanomolar doses… [126,127]. Later, aptamer AS1411 was found to target nucleolin [127,128], a multifunctional protein that was first considered to be nucleolar. Nucleolin was found to be highly expressed by cancer cells, intracellularly but also at the cell surface. A recent review described uses and mechanisms of the G-quadruplex oligonucleotide AS1411 as a targeting agent [129].
Berezovski et al. [139] described a technology for biomarker discovery, named aptamer-facilitated biomarker discovery (AptaBiD). It involves working with pool of aptamers rather than individual sequences. It is based on three steps: (i) cell-SELEX is performed to select ssDNA aptamers to biomarkers differentially expressed at the cell surfaces; (ii) a pool of biotinylated aptamers is used to isolate biomarkers from the cells, and then (iii) biomarkers are identified. AptaBiD was used to discover previously unknown surface biomarkers that distinguish live mature and immature dendritic cells [139] and to select DNA aptamers on lung adenocarcinoma cells derived from post-operative tissues [140].
Undetermined Targets
By cell-based SELEX process, aptamers have also been selected for tumor cell types (Figure 1d), without pre- or post-identification of targets. In this respect, aptamers are specific to the molecular signature of a tumor cell type. Such cell-based SELEX have been performed on several tumor cells, including breast cancer cells [141], colorectal cancer cells [142], small-cell lung cancer [143,144], non-small cell lung cancer [145], liver cancer cells [146], leukemia cells [147,148], gastric cancer cells [149], pancreatic ductal adenocarcinoma cells [150], prostate cancer cells [101], metastatic cells [151,152], and tumor initiating cells [153]. For example, J.N. Rich’s team [153], identified tumor initiating cell (TIC) specific ssDNA aptamers. They used a cell-based SELEX approach with positive selection for CD133(+) TICs and counter selection for CD133(-) non-TICs both from human GBM xenografts in mice. In addition, counter selection was also made on human non-tumoral neural progenitor cells. Selected aptamers specifically bound to TICs with dissociation constants (KD) in the low nanomolar range. These aptamers internalized into glioblastoma TICs that self-renew, proliferate, and initiate tumors.
3.2.2. Advantages and Limitations of Cell-Based SELEX
Aptamer binding is dependent on the target conformation which is conditioned by its environment. Full-length purified recombinant proteins, ectodomains or peptidic fragments of proteins used in protein-based SELEX may not be relevant targets if they have to be used in clinical developments. In cell-based SELEX, cell-surface targets post-translationally processed are in their endogenous environment, as close as possible to their natural distributions and conformations. Cell-based SELEX does not necessitate the production and purification steps usually required for protein-based SELEX and therefore the method may be useful to target proteins difficult to be produced and/or purified. Moreover, aptamers can be selected without prior knowledge of cell-surface targets. In that case, the aptamer acts as a bait to identify its target, which can be a new tumor protein biomarker.
But cell-based SELEX is a complex process, much more difficult than protein-based SELEX [122]. Cells lines need to be available, cultivable and stable. Aptamers might be difficult to generate for less expressed target proteins on cells. For most of the tumor cell-based selections described in this review, SELEX required modifications of cell lines, like over- and/or under-expression of the cell-surface protein target for selection and/or counter-selection. Over- or under-expression of a protein might deregulate expression of other proteins. This can trigger modifications to the cell line that might not represent anymore the disease cellular context. It is commonly admitted that more rounds of positive selection are performed in cell-SELEX compared to protein-SELEX and more rounds of counter selection are required to improve the selectivity of aptamers.
Post-SELEX characterization of the target is often required to check if the predetermined target is the ‘real’ target of selected aptamers, or to identify the target if it has not been identified prior selection. Selected aptamers are used as bait, a posteriori, to purify and then to identify their targets. Briefly, biotinylated-aptamers are used to retain whole live cells on streptavidin beads. The cell lysate is then loaded on a SDS-PAGE gel. After electrophoresis and silver staining, proteins that have a higher intensity than controls (extracted proteins with empty beads and extracted proteins with a scrambled nucleic acid sequence) are extracted from the gel and analyzed by nano-LC-MS/MS mass spectroscopy [132,154]. Aptamer-mediated target identification is not an easy process, due to the inherent properties of lipid embedded proteins (hydrophobicity, poor solubility, resistance to digestion…), even though significant advances have occurred to address these challenges [155].
Other methods, indirect compared to the method described above, have been used to identify a protein target post-cell-based SELEX. For example, Esposito et al. [130] used different techniques to characterize an aptamer’s target. They realized a whole-cell-based SELEX strategy on human NSCLC to select 2′-fluoro RNA aptamers, which distinguish A549 cells (used for positive selection) from H460 cells (used for counter selection). The best candidate, CL4, was used to identify functional targets using a phospho-RTK array analysis, which provided convincing evidence that the target could likely be EGFR and/or ErbB3. To definitively identify the target, authors performed first a filter binding analysis with the soluble extracellular domains of EGFR and ErbB3, which showed a strong affinity for the EGFR protein (in its monomeric and dimeric forms), while no binding was observed for the ErbB3 protein. Binding of the radiolabeled CL4 aptamer to EGFR was confirmed on NIH3T3 cells overexpressing EGFR and compared to NIH3T3 cells. Binding to A549 cells was decreased by interfering with EGFR expression and by high concentration of EGF. Binding of radiolabeled CL4 on A549 cells was competed by soluble EGFR but not by soluble ErbB3. The specific interaction of CL4 with EGFR on cell surface was further analyzed by affinity purification on streptavidin coated beads of extracts from A549 cells treated with biotin-labeled CL4 followed by immunoblotting with anti-EGFR antibodies. Then, CL4 was detected on stable tumor derived cell lines that express high levels of EGFR. Later [156] this aptamer has been shown to bind to the EGFRvIII mutant, even though the mutant lacks most of domains I and III in the extracellular part of the protein. Other methods have been used to demonstrate the binding of CL4 to glioblastoma U87MG cells stably transfected with the EGFRvIII mutant: reverse transcription polymerase chain reaction, confocal microscopy with fluorescent FAM-labelled aptamer and flow cytometry. The binding was shown to inhibit EGFRvIII autophosphorylation and to affect migration, invasion and proliferation of glioblastoma cells.
Cell-based SELEX might lead to the selection of aptamers against other proteins than the pre-identified target. A cell-based SELEX was performed by Cibiel et al. [157] to identify aptamers against CHO-K1 cells expressing the Endothelin type B receptor (ETBR), using the isogenic CHO-K1 cell line for counter selection rounds. None of the selected aptamers could discriminate between both cell lines. Nevertheless, the authors decided to study the 2’-fluoro RNA aptamer ACE4. The band excised from the PAGE-gel revealed that ACE4 did not interact with ETBR, but with Annexin-2, a calcium dependent phospholipid-binding protein which is up-regulated in various tumor types and plays multiple roles in regulating cellular functions (angiogenesis, proliferation, cell migration and adhesion). The high affinity aptamer, although not initially planned to bind to the post-SELEX identified tumor biomarker Annexin A2, could however be a promising tool for biomedical applications. In another example [158], a panel of G-rich ssDNA aptamers was selected by cell-SELEX against EGFR-transfected A549 cell line, using A549 cells for counter selection, and their targets was suggested to be nucleolin.
4. Applications of Aptamers in Oncology
Besides their role for biomarker discovery above mentioned, aptamers have many applications in oncology. Some relevant applications of aptamers in the cancer field will be briefly described.
4.1. Aptamers as Detection and Imaging Reagent
Current approaches of protein biomarker detection include gel electrophoresis and conventional immunoassays (for capture and/or detection) such as enzyme-linked immunosorbent assay (ELISA), surface plasmon resonance, mass-sensing BioCD protein array, surface enhanced Raman spectroscopy (SERS), microfluidics and colorimetric, electrochemical and fluorescence assays (flow cytometry, europium or gold nanoparticle based detection, protein microarray, quantum dots…). Most of these approaches still lack accuracy, sensitivity and specificity required for diagnostic application. Advantages and limitations of these methods have been compared for the prostate specific antigen (PSA) biomarker detection [159]. Different cancer biosensors and their characteristics were recently reported [160]. Aptamers are a class of bio-recognition element which can be used as molecular tools for clinical diagnosis [161,162]. For localization of proteins on tissue sections to aid in histopathological diagnosis, aptahistochemistry methods have been developed [163]. Soluble tumor biomarkers (notably CEA, EGFR, HER2, MUC1, PSA) were detected on aptamer-based analytical platforms with low limits of detection [164]. Aptamers can be used in sufficiently sensitive diagnosis assays for the detection and capture of circulating tumor cells [165,166]. Upon the binding to their targets, conformational changes undergone by aptamers can be converted into measurable signals. So called aptasensors match the requirements of fast and portable molecular devices. The most current applications of aptasensors in oncology concerns biomarker detection [167]. A recent review reports the development of aptasensors for analysis of tumor biomarkers using electrochemical, electrochemoluminescence and photoelectrochemical transduction. In particular, nano-structure-based aptasensors significantly improved analytical performances [168]. Aptasensors can also be used to detect exoxomes [169]. Aptamers can be used as imaging agents [170,171,172,173], for example to track cell status and functions [174]. In particular, glioblastoma-specific aptamers were developed by either targeting the whole cell surface or known glioma biomarkers. Coupled with radionuclide or fluorescent labels, bioconjugates or nanoparticles, they represent a noninvasive manner for defining the tumor tissue border [103].
4.2. Aptamers in Therapy
Applications of aptamers as targeted therapeutics have been reviewed recently [15,175,176]. Nucleic acid aptamers can serve as antagonist or agonist and have therefore a direct therapeutic effect by themselves by activating or blocking key cellular functions upon interaction with their cellular targets. Particularly, aptamers have been successfully used as agonists of co-stimulatory receptors, like CD28, OX40 and 4-1BB, in aptamer-mediated cancer immunotherapy [177,178,179]. Multivalent aptamers which induce receptor multimerization, are often more efficient than monovalent aptamers to trigger downstream signaling [87]. Aptamers can also be used as delivery tools of therapeutic agents [15,180,181,182,183]. Internalized receptors carry along loaded aptamers [184]. Aptamers are thus cargoes which deliver therapeutics inside the cytosol of specific targeted cells where therapeutics exert their intracellular actions. Aptamer chimera, composed of an aptamer and a therapeutic nucleic acid, may target cancer cells and deliver anti-cancer nucleic acids, e.g. small interfering RNA, micro RNA, antimicroRNA and small hairpin RNA, for aptamer-mediated gene therapy [185]. Aptamers can also act as carriers for anti-tumor drugs or toxins [186,187]. Therapeutic agents are either non-covalently or covalently conjugated to aptamers. Doxorubicin, a chemotherapeutic agent extensively used in the treatment of various cancers, has often been used as a drug non-covalently conjugated to aptamers containing CG/GC sequences or covalently conjugated to aptamer through a functional linker [87]. Other aptamer-drug or-toxin conjugates include gemcitabine, docetaxel, daunorubicin, cisplatin, gelonin. For aptamer-mediated therapeutics delivery, aptamers have also been associated to nanoparticles [87,188,189,190,191,192,193]. Such aptamer nanocomplexes can be composed of copolymers, liposomes, metal nanomaterials (like gold nanoparticles, [194]), or virus-like particles.
5. Aptamers in Clinical Trials
Eleven aptamers have entered clinical stages [175,195] for eye disorders (three aptamers), coagulation (four aptamers), inflammation (two aptamers) and cancer (two aptamers). The majority of aptamer targets in clinical trials are extracellular proteins. The advantage linked to an extracellular protein is that the aptamer activity can be reversed by the use of an oligonucleotide antidote. Pegnivacogin is an antithrombotic aptamer that binds and inhibits coagulation factor IXa. The pegnivacogin dose-dependent antithrombotic activity can be reversed with an antidote, anivamersen, a 15-nucleotide long 2′-O-methyl RNA [196,197]. Pegnivacogin is a 2′-fluoro-2′-O-methyl 31 nucleotide long RNA aptamer, protected from exonuclease degradation by a 3′ inverted deoxythymidine cap. It is branched with a 40 kDa PEG carrier. PEGylation is generally used to increase aptamers molecular weight, prolong their circulating half-life by limiting renal clearance, enhance their stability and decrease their toxic accumulation in tissues and the volume of distribution of therapeutic molecules [198,199,200]. Tested in a phase IIb study, in patients with acute coronary syndrome, pegnivacogin administration resulted in allergic reactions after the first dose in three patients of 640. Two of them presented an anaphylactic reaction and one presented an isolated dermal reaction. These observations induced an early termination of the trial [201]. A phase III clinical trial (NCT01848106), enrolling 3232 patients undergoing percutaneous coronary intervention, terminated at an early stage, due to severe allergic reactions reported in 1% of patients receiving pegnivacogin versus <1% of patients treated with bivalirudin [202,203,204]. However, it was observed that pegnivacogin did not induce inflammation response or histamine release. This aptamer, or its degradation, was therefore not responsible for the observed severe allergic reactions. Patients with allergic reactions had high levels of antibody to PEG, induced by complement activation and trypase release [205]. In 1983 was first reported the induction of antibodies against PEG in animals [206], but induction of PEG immunogenicity became clearer these last years as more PEGylated therapeutics enter clinical trials [207,208,209]. Currently formulated with a 40 kDa PEG carrier, pegnivacogin is associated with severe allergic reactions [203].
Pegaptanib sodium or Macugen [210] was the first aptamer-based drug approved by the U.S. Food and Drug Administration, in December 2004. Pegaptanib sodium is a 2′-fluoropyrimidine RNA-based aptamer, 28 nucleotides in length that terminates in a pentylamino linker, to which two 20-kiloDalton monomethoxy PEG units are covalently attached. It recognizes the abundant isoform of vascular endothelial growth factor, VEGF165. As an inhibitor of VEGF165-associated vessel formation, pegaptanib sodium is an anti-angiogenic medicine for the treatment of wet age-related macular degeneration. Pegaptanib sodium has also potential therapeutic effects as an agent for solid cancers characterized by extensive angiogenesis [15].
In oncology, two aptamers have undergone clinical trials. The first one was AS1411 (AGRO100), a DNA quadruplex targeting nucleolin. This multifunctional protein, located in the nucleolus but overexpressed at the membrane of several cancer cells, is implicated in cancer development by interacting with key oncogenes (bcl-2, Rb, p53, Akt-1) and by transferring specific extracellular ligands into the cells. The interaction of AS1411 and nucleolin induces internalization, inhibition of DNA synthesis and induction of apoptosis [127,211,212,213,214]. In a phase I study (September 2003 and July 2004), AS1411was tested in advanced cancer. Results showed that 8/16 patients had a stable disease during 2–9 months and one patient presented a complete response after more than 6 months [215]. In another phase I study (NCT00881244, September 2003–April 2009) [216], AS1411 was tested in patients with advanced solid tumors. AS1411 was well-tolerated. It induced a partial and a complete response in twelve patients with metastatic renal-cell carcinoma (RCC) and a stabilization of disease (>2 months) for seven patients. In 2008, Aptamer AS1411 was tested in a phase II study, in monotherapy, in patients with advanced metastatic RCC who had failed treatment with >1prior tyrosine kinase inhibitor (NCT00740441, August 2008–September 2009). AS1411 has been administrated at 40 mg/kg/j for the 4 first days of a 28 days cycle for two cycles. Out of 35 patients, 33 finished the two cycles. One patient presented a partial response. The OOR was only 2.9% (24 months). Twelve (34.3%) patients presented a stabilization of disease (5.5 month) and 21 patients (60%) presented a progression of disease. Adverse effects were observed on 12 grade 1 or 2 patients. This study showed a low level of activity of AS1411 in patients with metastatic RRC. An important and durable response has however been observed without toxicity [217]. AS1411 has also been evaluated in a phase II study combined with cytarabine (chemotherapy) for the treatment of patients with primary refractory or relapsed acute myeloid leukemia (NCT00512083, December 2009–February 2011). Few patients presented a durable remission. But the drug combination was proved to be safe (40 mg/kg/day for 7 days), well tolerated and shows promising signs of activity [218].
NOX-A12 (olaptesed pegol) is in clinical development for the treatment of chronic lymphatic leukemia and multiple myeloma [219,220,221]. NOX-A12 is a 45 nucleotide long L-stereoisomer RNA aptamer (Spiegelmer®, NOXXON Pharma AG, Berlin, Germany), which is a specific antagonist of CXCL12/SDF-1 (C-X-C motif ligand 12/stromal cell-derived factor 1). This chemokine, via interaction with the receptors CXCR4 and CXCR7, induces attraction and activation of immune and non-immune cells, migration and adhesion of tumor cells to the protective tissue microenvironment. In tumor cells expressing a high level of CXCR4, the disruption of the CXCR4-CXCL2 interaction induces a diminution of the interactions of cancer cells with the bone marrow environment. This cell mobilization sensitizes cancer cells to therapy. NOX-A12 has been evaluated in two phase I and two phase II clinical studies. In a phase IIa study, in combination with bendamustine and rituximab (chemotherapy) in patients with relapsed/refractory chronic lymphocytic leukemia (CCL) (NCT01486797 march 2012 and January 2016) [222], results showed an effective long-term mobilization of CLL cell and an increased CLL cells circulating in the periphery blood. In term of clinical efficacy, all patients responded to the combination of chemotherapy and NOX-A12. The ORR was 100%. A partial response was achieved for 19/28 patients (68%) and a complete remission for 4/28 patient (14%). Among them, 8 high-risk patients, who had relapsed within 24 months after the first fludarabine/bendamustine treatment or presenting TP53-deletion/mutation, presented a partial or complete response. NOX-A12 was safe and well tolerated. In another independent phase IIa clinical study, NOX-A12 has been evaluated in combination with a proteasome inhibitor, bortezomib, and a corticosteroid, dexamethasone (VD) in previously treated patients with multiple myeloma. This study started in March 2012 and finished in October 2015 (NCT01521533) [223]. A mobilization of myeloma cells was shown in plasma. The OOR was estimated at 68%. Five patients (18%) presented a very good partial response, 2/28 (7%) a complete response and 12/28 (43%) a partial response. These studies showed that aptamer NOX-A12 merits further study in randomized controlled trials.
6. Conclusions
Due to their pleiotropic characteristics and improvements in the SELEX technology, aptamers, which can be selected to virtually any type of targets, are thought to gain rapidly an important place in clinical applications and may replace in many cases animal-derived antibodies. Aptamers as synthetic molecules are believed to save cost and time in R&D and manufacture. The targets of aptamers actually in clinical trials [175] are extracellular proteins (VEGF165, C5, PDGFβ, coagulation factor IXa, A1 domain of von Willebrand factor, thrombin, TFPI, CXCL12, CCL2, hepcidin peptide hormone), except nucleolin which is highly expressed at the surface of several tumor cells. Most of the aptamers in clinical trials were identified by protein-SELEX. Combination of protein-and cell-based selection processes may allow the discovery of highly selective but also conformation-dependent aptamers against tumor cell membrane biomarkers. Other selection processes more difficult to settle than protein-and cell-based SELEX and for which targets cannot be determined before selection, have been described to target a tumor under near-in vivo conditions. Aptamers have been selected on tissue sections [224,225], and very recently by Morph-X-Select (morphology based tissue aptamer selection, a combination of image-directed laser micro-dissection technology with tissue SELEX) on targeted tissue sections for ovarian cancer biomarker discovery [226]. Such selected DNA thio-aptamers have shown specificity to tumor vasculature of human ovarian tissue and human microvascular endothelial cells but not to the tumor stromal cells. Aptamers selected in living animals, through in vivo tissue-specific SELEX, have been shown to localize to hepatic colon cancer metastase [227] or to enter the brain after peripheral delivery [228]. These processes should prove helpful to characterize new tumor cell surface protein biomarkers, post-selection. Exciting new fields for aptamer applications in oncology emerge nowadays including detection of circulating tumor cells or tumor exosomes in biological fluid, and specific delivery of therapeutics inside cells with possibilities of co-targeting multiple oncogenic pathways. New opportunities for aptamers as multipotent tools will be characterized and will provide the pavement for approved clinical applications in the near future.
Acknowledgments
We are grateful to Pierre Fechter for valuable comments of the manuscript. This study was supported by the CNRS-French National Center for Scientific Research, the University of Strasbourg (PEPS-IDEX W15RPE24) and SATT-Conectus. Marie-Cécile Mercier is a doctoral fellow from Hospital of Strasbourg.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ellington A.D., Szostak J.W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 2.Robertson D.L., Joyce G.F. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature. 1990;344:467–468. doi: 10.1038/344467a0. [DOI] [PubMed] [Google Scholar]
- 3.Tuerk C., Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 4.Ponomarenko J.V., Orlova G.V., Frolov A.S., Gelfand M.S., Ponomarenko M.P. SELEX_DB: A database on in vitro selected oligomers adapted for recognizing natural sites and for analyzing both SNPs and site-directed mutagenesis data. Nucleic Acids Res. 2002;30:195–199. doi: 10.1093/nar/30.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aptamer Database Apta-Index™. [(accessed on 1 June 2017)]; Available online: http://www.aptagen.com/aptamer-index/aptamer-list.aspx.
- 6.Cruz-Toledo J., McKeague M., Zhang X., Giamberardino A., McConnell E., Francis T., DeRosa M.C., Dumontier M. Aptamer base: A collaborative knowledge base to describe aptamers and SELEX experiments. Database. 2012;2012:bas006. doi: 10.1093/database/bas006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lao Y.-H., Phua K.K.L., Leong K.W. Aptamer nanomedicine for cancer therapeutics: Barriers and potential for translation. ACS Nano. 2015;9:2235–2254. doi: 10.1021/nn507494p. [DOI] [PubMed] [Google Scholar]
- 8.Jacobi N., Seeboeck R., Hofmann E., Eger A. ErbB family signalling: A paradigm for oncogene addiction and personalized oncology. Cancers. 2017;9:33. doi: 10.3390/cancers9040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemech C., Arkenau H.-T. Biomarkers in advanced colorectal cancer: Challenges in translating clinical research into practice. Cancers. 2011;3:1844–1860. doi: 10.3390/cancers3021844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciriello G., Miller M.L., Aksoy B.A., Senbabaoglu Y., Schultz N., Sander C. Emerging landscape of oncogenic signatures across human cancers. Nat. Genet. 2013;45:1127–1133. doi: 10.1038/ng.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camorani S., Crescenzi E., Gramanzini M., Fedele M., Zannetti A., Cerchia L. Aptamer-mediated impairment of EGFR-integrin αvβ3 complex inhibits vasculogenic mimicry and growth of triple-negative breast cancers. Sci. Rep. 2017;7:46659. doi: 10.1038/srep46659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szász A.M., Győrffy B., Marko-Varga G. Cancer heterogeneity determined by functional proteomics. Semin. Cell Dev. Biol. 2017;64:132–142. doi: 10.1016/j.semcdb.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Ceccarelli M., Barthel F.P., Malta T.M., Sabedot T.S., Salama S.R., Murray B.A., Morozova O., Newton Y., Radenbaugh A., Pagnotta S.M., et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164:550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monaco I., Camorani S., Colecchia D., LOCATELLI E., Calandro P., Oudin A., Niclou S., Arra C., Chiariello M., Cerchia L., et al. Aptamer functionalization of nanosystems for glioblastoma targeting through the blood-brain-barrier. J. Med. Chem. 2017;60:4510–4516. doi: 10.1021/acs.jmedchem.7b00527. [DOI] [PubMed] [Google Scholar]
- 15.Zhou G., Wilson G., Hebbard L., Duan W., Liddle C., George J., Qiao L. Aptamers: A promising chemical antibody for cancer therapy. Oncotarget. 2016;7:13446–13463. doi: 10.18632/oncotarget.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang D., Zheng C., Zhou S.-F., Qiao S., Tran P.H.-L., Pu C., Li Y., Kong L., Kouzani A.Z., Lin J., et al. Superior performance of aptamer in tumor penetration over antibody: Implication of aptamer-based theranostics in solid tumors. Theranostics. 2015;5:1083–1097. doi: 10.7150/thno.11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avci-Adali M., Paul A., Wilhelm N., Ziemer G., Wendel H.P. Upgrading SELEX technology by using lambda exonuclease digestion for Single-Stranded DNA generation. Molecules. 2009;15:1–11. doi: 10.3390/molecules15010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paul A., Avci-Adali M., Ziemer G., Wendel H.P. Streptavidin-coated magnetic beads for DNA strand separation implicate a multitude of problems during cell-SELEX. Oligonucleotides. 2009;19:243–254. doi: 10.1089/oli.2009.0194. [DOI] [PubMed] [Google Scholar]
- 19.Eulberg D., Klussmann S. Spiegelmers: Biostable aptamers. ChemBioChem. 2003;4:979–983. doi: 10.1002/cbic.200300663. [DOI] [PubMed] [Google Scholar]
- 20.Padilla R., Sousa R. Efficient synthesis of nucleic acids heavily modified with non-canonical ribose 2′-groups using a mutant T7 RNA polymerase (RNAP) Nucleic Acids Res. 1999;27:1561–1563. doi: 10.1093/nar/27.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burmeister P.E., Lewis S.D., Silva R.F., Preiss J.R., Horwitz L.R., Pendergrast P.S., McCauley T.G., Kurz J.C., Epstein D.M., Wilson C., et al. Direct in vitro selection of a 2′-O-methyl aptamer to VEGF. Chem. Biol. 2005;12:25–33. doi: 10.1016/j.chembiol.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Pinheiro V.B., Taylor A.I., Cozens C., Abramov M., Renders M., Zhang S., Chaput J.C., Wengel J., Peak-Chew S.-Y., McLaughlin S.H., et al. Synthetic genetic polymers capable of heredity and evolution. Science. 2012;336:341–344. doi: 10.1126/science.1217622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuwahara M., Obika S. In vitro selection of BNA (LNA) aptamers. Artif. DNA PNA XNA. 2013;4:39–48. doi: 10.4161/adna.25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kasahara Y., Irisawa Y., Ozaki H., Obika S., Kuwahara M. 2′,4′-BNA/LNA aptamers: CE-SELEX using a DNA-based library of full-length 2′-O,4′-C-methylene-bridged/linked bicyclic ribonucleotides. Bioorg. Med. Chem. Lett. 2013;23:1288–1292. doi: 10.1016/j.bmcl.2012.12.093. [DOI] [PubMed] [Google Scholar]
- 25.Veedu R.N., Wengel J. Locked nucleic acid nucleoside triphosphates and polymerases: On the way towards evolution of LNAaptamers. Mol. Biosyst. 2009;5:787–792. doi: 10.1039/b905513b. [DOI] [PubMed] [Google Scholar]
- 26.Shigdar S., Macdonald J., O’Connor M., Wang T., Xiang D., Al.Shamaileh H., Qiao L., Wei M., Zhou S.-F., Zhu Y., et al. Aptamers as theranostic agents: Modifications, serum stability and functionalisation. Sensors. 2013;13:13624–13637. doi: 10.3390/s131013624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anosova I., Kowal E.A., Dunn M.R., Chaput J.C., Van Horn W.D., Egli M. The structural diversity of artificial genetic polymers. Nucleic Acids Res. 2016;44:1007–1021. doi: 10.1093/nar/gkv1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinheiro V.B., Holliger P. Towards XNA nanotechnology: New materials from synthetic genetic polymers. Trends Biotechnol. 2014;32:321–328. doi: 10.1016/j.tibtech.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold L., Ayers D., Bertino J., Bock C., Bock A., Brody E.N., Carter J., Dalby A.B., Eaton B.E., Fitzwater T., et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE. 2010;5:e15004. doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eaton B.E. The joys of in vitro selection: Chemically dressing oligonucleotides to satiate protein targets. Curr. Opin. Chem. Biol. 1997;1:10–16. doi: 10.1016/S1367-5931(97)80103-2. [DOI] [PubMed] [Google Scholar]
- 31.Sun H., Zu Y. A Highlight of recent advances in aptamer technology and its application. Molecules. 2015;20:11959–11980. doi: 10.3390/molecules200711959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andreola M.-L., Calmels C., Michel J., Toulmé J.-J., Litvak S. Towards the selection of phosphorothioate aptamers. Eur. J. Biochem. 2000;267:5032–5040. doi: 10.1046/j.1432-1327.2000.01557.x. [DOI] [PubMed] [Google Scholar]
- 33.Lato S.M., Ozerova N.D.S., He K., Sergueeva Z., Shaw B.R., Burke D.H. Boron-containing aptamers to ATP. Nucleic Acids Res. 2002;30:1401–1407. doi: 10.1093/nar/30.6.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keefe A.D., Cload S.T. SELEX with modified nucleotides. Curr. Opin. Chem. Biol. 2008;12:448–456. doi: 10.1016/j.cbpa.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 35.Vater A., Klussmann S. Turning mirror-image oligonucleotides into drugs: The evolution of Spiegelmer® therapeutics. Drug Discov. Today. 2015;20:147–155. doi: 10.1016/j.drudis.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Farokhzad O.C., Cheng J., Teply B.A., Sherifi I., Jon S., Kantoff P.W., Richie J.P., Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl. Acad. Sci. USA. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee I.-H., An S., Yu M.K., Kwon H.-K., Im S.-H., Jon S. Targeted chemoimmunotherapy using drug-loaded aptamer–dendrimer bioconjugates. J. Control. Release. 2011;155:435–441. doi: 10.1016/j.jconrel.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 38.Baek S.E., Lee K.H., Park Y.S., Oh D.-K., Oh S., Kim K.-S., Kim D.-E. RNA aptamer-conjugated liposome as an efficient anticancer drug delivery vehicle targeting cancer cells in vivo. J. Control. Release. 2014;196:234–242. doi: 10.1016/j.jconrel.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Willis M.C., Collins B., Zhang T., Green L.S., Sebesta D.P., Bell C., Kellogg E., Gill S.C., Magallanez A., Knauer S., et al. Liposome-anchored vascular endothelial growth factor aptamers. Bioconjug. Chem. 1998;9:573–582. doi: 10.1021/bc980002x. [DOI] [PubMed] [Google Scholar]
- 40.Farokhzad O.C., Jon S., Khademhosseini A., Tran T.-N.T., LaVan D.A., Langer R. Nanoparticle-aptamer bioconjugates. Cancer Res. 2004;64:7668–7672. doi: 10.1158/0008-5472.CAN-04-2550. [DOI] [PubMed] [Google Scholar]
- 41.Healy J.M., Lewis S.D., Kurz M., Boomer R.M., Thompson K.M., Wilson C., McCauley T.G. Pharmacokinetics and biodistribution of novel aptamer compositions. Pharm. Res. 2004;21:2234–2246. doi: 10.1007/s11095-004-7676-4. [DOI] [PubMed] [Google Scholar]
- 42.Wang J., Li G. Aptamers against cell surface receptors: Selection, modification and application. Curr. Med. Chem. 2011;18:4107–4116. doi: 10.2174/092986711797189628. [DOI] [PubMed] [Google Scholar]
- 43.Dua P., Kim S., Lee D. Nucleic acid aptamers targeting cell-surface proteins. Methods. 2011;54:215–225. doi: 10.1016/j.ymeth.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Cibiel A., Dupont D.M., Ducongé F. Methods to identify aptamers against cell surface biomarkers. Pharmaceuticals. 2011;4:1216–1235. doi: 10.3390/ph4091216. [DOI] [Google Scholar]
- 45.Shigdar S., Lin J., Yu Y., Pastuovic M., Wei M., Duan W. RNA aptamer against a cancer stem cell marker epithelial cell adhesion molecule. Cancer Sci. 2011;102:991–998. doi: 10.1111/j.1349-7006.2011.01897.x. [DOI] [PubMed] [Google Scholar]
- 46.Song Y., Zhu Z., An Y., Zhang W., Zhang H., Liu D., Yu C., Duan W., Yang C.J. Selection of DNA aptamers against epithelial cell adhesion molecule for cancer cell imaging and circulating tumor cell capture. Anal. Chem. 2013;85:4141–4149. doi: 10.1021/ac400366b. [DOI] [PubMed] [Google Scholar]
- 47.Lee Y.J., Han S.R., Kim N.Y., Lee S., Jeong J., Lee S. An RNA aptamer that binds carcinoembryonic antigen inhibits hepatic metastasis of colon cancer cells in mice. Gastroenterology. 2012;143:155–165.e8. doi: 10.1053/j.gastro.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 48.Ruckman J., Gold L., Stephens A., Janjic N. Nucleic acid ligands to integrins. 6,331,394. U.S. Patent. 2001 Dec 18;
- 49.Mi J., Zhang X., Giangrande P.H., McNamara II J.O., Nimjee S.M., Sarraf-Yazdi S., Sullenger B.A., Clary B.M. Targeted inhibition of αvβ3 integrin with an RNA aptamer impairs endothelial cell growth and survival. Biochem. Biophys. Res. Commun. 2005;338:956–963. doi: 10.1016/j.bbrc.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 50.Gong Q., Wang J., Ahmad K.M., Csordas A., Zhou J., Nie J., Stewart R., Thomson J.A., Rossi J.J., Soh H.T. Selection strategy to generate aptamer pairs that bind to distinct sites on protein targets. Anal. Chem. 2012;84:5365–5371. doi: 10.1021/ac300873p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faryammanesh R., Lange T., Magbanua E., Haas S., Meyer C., Wicklein D., Schumacher U., Hahn U. SDA, a DNA aptamer Inhibiting E- and P-Selectin mediated adhesion of cancer and leukemia cells, the first and pivotal step in transendothelial migration during metastasis formation. PLoS ONE. 2014;9:e93173. doi: 10.1371/journal.pone.0093173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Connell D., Koenig A., Jennings S., Hicke B., Han H.L., Fitzwater T., Chang Y.F., Varki N., Parma D., Varki A. Calcium-dependent oligonucleotide antagonists specific for L-selectin. Proc. Natl. Acad. Sci. USA. 1996;93:5883–5887. doi: 10.1073/pnas.93.12.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hicke B.J., Watson S.R., Koenig A., Lynott C.K., Bargatze R.F., Chang Y.F., Ringquist S., Moon-McDermott L., Jennings S., Fitzwater T., et al. DNA aptamers block L-selectin function in vivo. Inhibition of human lymphocyte trafficking in SCID mice. J. Clin. Invest. 1996;98:2688–2692. doi: 10.1172/JCI119092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li N., Larson T., Nguyen H.H., Sokolov K.V., Ellington A.D. Directed evolution of gold nanoparticle delivery to cells. Chem. Commun. Camb. Engl. 2010;46:392–394. doi: 10.1039/B920865H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li N., Nguyen H.H., Byrom M., Ellington A.D. Inhibition of cell proliferation by an anti-EGFR aptamer. PLoS ONE. 2011;6:e20299. doi: 10.1371/journal.pone.0020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ray P., Cheek M.A., Sharaf M.L., Li N., Ellington A.D., Sullenger B.A., Shaw B.R., White R.R. Aptamer-mediated delivery of chemotherapy to pancreatic cancer cells. Nucleic Acid Ther. 2012;22:295–305. doi: 10.1089/nat.2012.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang D.-L., Song Y.-L., Zhu Z., Li X.-L., Zou Y., Yang H.-T., Wang J.-J., Yao P.-S., Pan R.-J., Yang C.J., et al. Selection of DNA aptamers against epidermal growth factor receptor with high affinity and specificity. Biochem. Biophys. Res. Commun. 2014;453:681–685. doi: 10.1016/j.bbrc.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y., Kuan C.-T., Mi J., Zhang X., Clary B.M., Bigner D.D., Sullenger B.A. Aptamers selected against the unglycosylated EGFRvIII ectodomain and delivered intracellularly reduce membrane-bound EGFRvIII and induce apoptosis. Biol. Chem. 2009;390:137–144. doi: 10.1515/BC.2009.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim M.Y., Jeong S. In Vitro Selection of RNA Aptamer and Specific Targeting of ErbB2 in Breast Cancer Cells. Nucleic Acid Ther. 2011;21:173–178. doi: 10.1089/nat.2011.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Z., Duan J.-H., Song Y.-M., Ma J., Wang F.-D., Lu X., Yang X.-D. Novel HER2 aptamer selectively delivers cytotoxic drug to HER2-positive breast cancer cells in vitro. J. Transl. Med. 2012;10:148. doi: 10.1186/1479-5876-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu Y., Duan J., Cao B., Zhang L., Lu X., Wang F., Yao F., Zhu Z., Yuan W., Wang C., et al. Selection of a novel DNA thioaptamer against HER2 structure. Clin. Transl. Oncol. 2015;17:647–656. doi: 10.1007/s12094-015-1292-0. [DOI] [PubMed] [Google Scholar]
- 62.Sett A., Borthakur B.B., Bora U. Selection of DNA aptamers for extra cellular domain of human epidermal growth factor receptor 2 to detect HER2 positive carcinomas. Clin. Transl. Oncol. 2017:1–13. doi: 10.1007/s12094-017-1629-y. [DOI] [PubMed] [Google Scholar]
- 63.Chen C.B., Chernis G.A., Hoang V.Q., Landgraf R. Inhibition of heregulin signaling by an aptamer that preferentially binds to the oligomeric form of human epidermal growth factor receptor-3. Proc. Natl. Acad. Sci. USA. 2003;100:9226–9231. doi: 10.1073/pnas.1332660100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boltz A., Piater B., Toleikis L., Guenther R., Kolmar H., Hock B. Bi-specific aptamers mediating tumor cell lysis. J. Biol. Chem. 2011;286:21896–21905. doi: 10.1074/jbc.M111.238261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Piater B., Doerner A., Guenther R., Kolmar H., Hock B. Aptamers binding to c-Met inhibiting tumor cell migration. PLoS ONE. 2015;10:e0142412. doi: 10.1371/journal.pone.0142412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lupold S.E., Hicke B.J., Lin Y., Coffey D.S. Identification and characterization of nuclease-stabilized rna molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 2002;62:4029–4033. [PubMed] [Google Scholar]
- 67.McNamara J.O., Andrechek E.R., Wang Y., Viles K.D., Rempel R.E., Gilboa E., Sullenger B.A., Giangrande P.H. Cell type–specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 2006;24:1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 68.Ferreira C.S.M., Matthews C.S., Missailidis S. DNA aptamers that bind to MUC1 tumour marker: design and characterization of MUC1-binding single-stranded DNA aptamers. Tumour Biol. 2006;27:289–301. doi: 10.1159/000096085. [DOI] [PubMed] [Google Scholar]
- 69.Ferreira C.S.M., Papamichael K., Guilbault G., Schwarzacher T., Gariepy J., Missailidis S. DNA aptamers against the MUC1 tumour marker: Design of aptamer–antibody sandwich ELISA for the early diagnosis of epithelial tumours. Anal. Bioanal. Chem. 2008;390:1039–1050. doi: 10.1007/s00216-007-1470-1. [DOI] [PubMed] [Google Scholar]
- 70.Pratico E.D., Sullenger B.A., Nair S.K. Identification and characterization of an agonistic aptamer against the T Cell costimulatory receptor, OX40. Nucleic Acid Ther. 2013;23:35–43. doi: 10.1089/nat.2012.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dollins C.M., Nair S., Boczkowski D., Lee J., Layzer J.M., Gilboa E., Sullenger B.A. Assembling OX40 Aptamers on a molecular scaffold to create a receptor-activating aptamer. Chem. Biol. 2008;15:675–682. doi: 10.1016/j.chembiol.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McNamara J.O., Kolonias D., Pastor F., Mittler R.S., Chen L., Giangrande P.H., Sullenger B., Gilboa E. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J. Clin. Investig. 2008;118:376–386. doi: 10.1172/JCI33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mori T., Oguro A., Ohtsu T., Nakamura Y. RNA aptamers selected against the receptor activator of NF-κB acquire general affinity to proteins of the tumor necrosis factor receptor family. Nucleic Acids Res. 2004;32:6120–6128. doi: 10.1093/nar/gkh949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pastor F., Soldevilla M.M., Villanueva H., Kolonias D., Inoges S., de Cerio A.L., Kandzia R., Klimyuk V., Gleba Y., Gilboa E., et al. CD28 aptamers as powerful immune response modulators. Mol. Ther. Nucleic Acids. 2013;2:e98. doi: 10.1038/mtna.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santulli-Marotto S., Nair S.K., Rusconi C., Sullenger B., Gilboa E. Multivalent RNA aptamers that inhibit Ctla-4 and enhance tumor immunity. Cancer Res. 2003;63:7483–7489. [PubMed] [Google Scholar]
- 76.Zhou J., Tiemann K., Chomchan P., Alluin J., Swiderski P., Burnett J., Zhang X., Forman S., Chen R., Rossi J. Dual functional BAFF receptor aptamers inhibit ligand-induced proliferation and deliver siRNAs to NHL cells. Nucleic Acids Res. 2013;41:4266–4283. doi: 10.1093/nar/gkt125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roth F., Fuente A.C.D.L., Vella J.L., Zoso A., Inverardi L., Serafini P. Aptamer-mediated blockade of IL4Rα triggers apoptosis of MDSCs and limits tumor progression. Cancer Res. 2012;72:1373–1383. doi: 10.1158/0008-5472.CAN-11-2772. [DOI] [PubMed] [Google Scholar]
- 78.Chauveau F., Aissouni Y., Hamm J., Boutin H., Libri D., Ducongé F., Tavitian B. Binding of an aptamer to the N-terminal fragment of VCAM-1. Bioorg. Med. Chem. Lett. 2007;17:6119–6122. doi: 10.1016/j.bmcl.2007.09.046. [DOI] [PubMed] [Google Scholar]
- 79.Watanabe T., Ito K., Matsumoto M., Seya T., Nishikawa S., Hasegawa T., Fukuda K. Isolation of RNA aptamers against human Toll-like receptor 3 ectodomain. Nucleic Acids Symp. Ser. 2006:251–252. doi: 10.1093/nass/nrl125. [DOI] [PubMed] [Google Scholar]
- 80.Somasunderam A., Thiviyanathan V., Tanaka T., Li X., Neerathilingam M., Lokesh G.L.R., Mann A., Peng Y., Ferrari M., Klostergaard J., et al. Combinatorial selection of DNA thioaptamers targeted towards the HA binding domain of human CD44. Biochemistry. 2010;49:9106–9112. doi: 10.1021/bi1009503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ababneh N., Alshaer W., Allozi O., Mahafzah A., El-Khateeb M., Hillaireau H., Noiray M., Fattal E., Ismail S. In vitro selection of modified RNA aptamers against CD44 cancer stem cell marker. Nucleic Acid Ther. 2013;23:401–407. doi: 10.1089/nat.2013.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.White R.R., Roy J.A., Viles K.D., Sullenger B.A., Kontos C.D. A nuclease-resistant RNA aptamer specifically inhibits angiopoietin-1-mediated Tie2 activation and function. Angiogenesis. 2008;11:395–401. doi: 10.1007/s10456-008-9122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.White R.R., Shan S., Rusconi C.P., Shetty G., Dewhirst M.W., Kontos C.D., Sullenger B.A. Inhibition of rat corneal angiogenesis by a nuclease-resistant RNA aptamer specific for angiopoietin-2. Proc. Natl. Acad. Sci. USA. 2003;100:5028–5033. doi: 10.1073/pnas.0831159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bates P.J., Laber D.A., Miller D.M., Thomas S.D., Trent J.O. Discovery and development of the g-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009;86:151–164. doi: 10.1016/j.yexmp.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tam R.C., Phan U.T., Milovanovic T., Pai B., Lim C., Bard J., He L. Oligonucleotide-mediated inhibition of CD28 expression induces human T-cell hyporesponsiveness and manifests impaired contact hypersensitivity in mice. J. Immunol. 1997;158:200–208. [PubMed] [Google Scholar]
- 86.Spencer D.I., Missailidis S., Denton G., Murray A., Brady K., Matteis C.I., Searle M.S., Tendler S.J., Price M.R. Structure/activity studies of the anti-MUC1 monoclonal antibody C595 and synthetic MUC1 mucin-core-related peptides and glycopeptides. Biospectroscopy. 1999;5:79–91. doi: 10.1002/(SICI)1520-6343(1999)5:2<79::AID-BSPY2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 87.Sun H., Zhu X., Lu P.Y., Rosato R.R., Tan W., Zu Y. Oligonucleotide aptamers: New tools for targeted cancer therapy. Mol. Ther. Nucleic Acids. 2014;3:e182. doi: 10.1038/mtna.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Blind M., Blank M. Aptamer selection technology and recent advances. Mol. Ther. Nucleic Acids. 2015;4:e223. doi: 10.1038/mtna.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shangguan D., Bing T., Zhang N. Cell-SELEX: Aptamer selection against whole cells. In: Tan W., Fang X., editors. Aptamers Selected by Cell-SELEX for Theranostics. Springer; Berlin/Heidelberg, Germany: 2015. pp. 13–33. [Google Scholar]
- 90.Mallikaratchy P. Evolution of complex target SELEX to identify aptamers against mammalian cell-surface antigens. Molecules. 2017;22:215. doi: 10.3390/molecules22020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mayer G., Ahmed M.-S.L., Dolf A., Endl E., Knolle P.A., Famulok M. Fluorescence-activated cell sorting for aptamer SELEX with cell mixtures. Nat. Protoc. 2010;5:1993–2004. doi: 10.1038/nprot.2010.163. [DOI] [PubMed] [Google Scholar]
- 92.Raddatz M.-S.L., Dolf A., Endl E., Knolle P., Famulok M., Mayer G. Enrichment of cell-targeting and population-specific aptamers by fluorescence-activated cell sorting. Angew. Chem. Int. Ed. 2008;47:5190–5193. doi: 10.1002/anie.200800216. [DOI] [PubMed] [Google Scholar]
- 93.Avci-Adali M., Metzger M., Perle N., Ziemer G., Wendel H.P. Pitfalls of cell-systematic evolution of ligands by exponential enrichment (SELEX): Existing dead cells during in vitro selection anticipate the enrichment of specific aptamers. Oligonucleotides. 2010;20:317–323. doi: 10.1089/oli.2010.0253. [DOI] [PubMed] [Google Scholar]
- 94.Wilner S.E., Wengerter B., Maier K., de Lourdes Borba Magalhães M., Del Amo D.S., Pai S., Opazo F., Rizzoli S.O., Yan A., Levy M. An RNA alternative to human transferrin: a new tool for targeting human cells. Mol. Ther. Nucleic Acids. 2012;1:e21. doi: 10.1038/mtna.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yan A., Levy M. Cell Internalization SELEX: in vitro selection for molecules that internalize into cells. In: Lafontaine D., Dubé A., editors. Therapeutic Applications of Ribozymes and Riboswitches. Humana Press; New York, NY, USA: 2014. pp. 241–265. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 96.Gourronc F.A., Rockey W.M., Thiel W.H., Giangrande P.H., Klingelhutz A.J. Identification of RNA aptamers that Internalize into HPV-16 E6/E7 transformed tonsillar epithelial cells. Virology. 2013;446:325–333. doi: 10.1016/j.virol.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou J., Bobbin M.L., Burnett J.C., Rossi J.J. Current progress of RNA aptamer-based therapeutics. Front. Genet. 2012;3:234. doi: 10.3389/fgene.2012.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thiel W.H., Bair T., Peek A.S., Liu X., Dassie J., Stockdale K.R., Behlke M.A., Miller F.J., Giangrande P.H. Rapid identification of cell-specific, internalizing RNA aptamers with bioinformatics analyses of a cell-based aptamer selection. PLoS ONE. 2012;7:e43836. doi: 10.1371/journal.pone.0043836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Camorani S., Esposito C.L., Rienzo A., Catuogno S., Iaboni M., Condorelli G., de Franciscis V., Cerchia L. Inhibition of receptor signaling and of glioblastoma-derived tumor growth by a novel PDGFRβ aptamer. Mol. Ther. 2014;22:828–841. doi: 10.1038/mt.2013.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thiel K.W., Hernandez L.I., Dassie J.P., Thiel W.H., Liu X., Stockdale K.R., Rothman A.M., Hernandez F.J., McNamara J.O., Giangrande P.H. Delivery of chemo-sensitizing siRNAs to HER2+-breast cancer cells using RNA aptamers. Nucleic Acids Res. 2012;40:6319–6337. doi: 10.1093/nar/gks294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Souza A.G., Marangoni K., Fujimura P.T., Alves P.T., Silva M.J., Bastos V.A.F., Goulart L.R., Goulart V.A. 3D cell-SELEX: Development of RNA aptamers as molecular probes for PC-3 tumor cell line. Exp. Cell Res. 2016;341:147–156. doi: 10.1016/j.yexcr.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 102.Ohuchi S.P., Ohtsu T., Nakamura Y. Selection of RNA aptamers against recombinant transforming growth factor-β type III receptor displayed on cell surface. Biochimie. 2006;88:897–904. doi: 10.1016/j.biochi.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 103.Delač M., Motaln H., Ulrich H., Lah T.T. Aptamer for imaging and therapeutic targeting of brain tumor glioblastoma. Cytometry A. 2015;87:806–816. doi: 10.1002/cyto.a.22715. [DOI] [PubMed] [Google Scholar]
- 104.Wadajkar A.S., Dancy J.G., Hersh D.S., Anastasiadis P., Tran N.L., Woodworth G.F., Winkles J.A., Kim A.J. Tumor-targeted nanotherapeutics: overcoming treatment barriers for glioblastoma. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016 doi: 10.1002/wnan.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zumrut H.E., Ara M.N., Maio G.E., Van N.A., Batool S., Mallikaratchy P.R. Ligand-guided selection of aptamers against T-cell receptor-cluster of differentiation 3 (TCR-CD3) expressed on Jurkat.E6 cells. Anal. Biochem. 2016;512:1–7. doi: 10.1016/j.ab.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zumrut H.E., Ara M.N., Fraile M., Maio G., Mallikaratchy P. Ligand-guided selection of target-specific aptamers: A screening technology for identifying specific aptamers against cell-surface proteins. Nucleic Acid Ther. 2016;26:190–198. doi: 10.1089/nat.2016.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chang Y.-C., Kao W.-C., Wang W.-Y., Wang W.-Y., Yang R.-B., Peck K. Identification and characterization of oligonucleotides that inhibit Toll-like receptor 2-associated immune responses. FASEB J. 2009;23:3078–3088. doi: 10.1096/fj.09-129312. [DOI] [PubMed] [Google Scholar]
- 108.Pestourie C., Cerchia L., Gombert K., Aissouni Y., Boulay J., Franciscis V.D., Libri D., Tavitian B., Ducongé F. Comparison of different strategies to select aptamers against a transmembrane protein target. Oligonucleotides. 2006;16:323–335. doi: 10.1089/oli.2006.16.323. [DOI] [PubMed] [Google Scholar]
- 109.Zhu G., Zhang H., Jacobson O., Wang Z., Chen H., Yang X., Niu G., Chen X. Combinatorial screening of DNA aptamers for molecular imaging of HER2 in cancer. Bioconjug. Chem. 2017;28:1068–1075. doi: 10.1021/acs.bioconjchem.6b00746. [DOI] [PubMed] [Google Scholar]
- 110.Berg K., Lange T., Mittelberger F., Schumacher U., Hahn U. Selection and characterization of an α6β4 Integrin blocking DNA aptamer. Mol. Ther. Nucleic Acids. 2016;5:e294. doi: 10.1038/mtna.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soldevilla M.M., Villanueva H., Casares N., Lasarte J.J., Bendandi M., Inoges S., de Cerio A.L.-D., Pastor F. MRP1-CD28 bi-specific oligonucleotide aptamers: Target costimulation to drug-resistant melanoma cancer stem cells. Oncotarget. 2016;7:23182–23196. doi: 10.18632/oncotarget.8095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Takahashi M., Sakota E., Nakamura Y. The efficient cell-SELEX strategy, Icell-SELEX, using isogenic cell lines for selection and counter-selection to generate RNA aptamers to cell surface proteins. Biochimie. 2016;131:77–84. doi: 10.1016/j.biochi.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 113.Kang S.K., Huh Y.M., Lee D. Isolation of RNA aptamers targeting HER-2-overexpressing breast cancer cells using cell-SELEX. Bull. Korean Chem. Soc. 2009;30:1827–1831. [Google Scholar]
- 114.Dastjerdi K., Tabar G.H., Dehghani H., Haghparast A. Generation of an enriched pool of DNA aptamers for an HER2-overexpressing cell line selected by Cell SELEX. Biotechnol. Appl. Biochem. 2011;58:226–230. doi: 10.1002/bab.36. [DOI] [PubMed] [Google Scholar]
- 115.Parekh P., Kamble S., Zhao N., Zeng Z., Portier B.P., Zu Y. Immunotherapy of CD30-expressing lymphoma using a highly stable ssDNA aptamer. Biomaterials. 2013;34:8909–8917. doi: 10.1016/j.biomaterials.2013.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mahlknecht G., Maron R., Mancini M., Schechter B., Sela M., Yarden Y. Aptamer to ErbB-2/HER2 enhances degradation of the target and inhibits tumorigenic growth. Proc. Natl. Acad. Sci. USA. 2013;110:8170–8175. doi: 10.1073/pnas.1302594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tan Y., Shi Y., Wu X., Liang H., Gao Y., Li S., Zhang X., Wang F., Gao T. DNA aptamers that target human glioblastoma multiforme cells overexpressing epidermal growth factor receptor variant III in vitro. Acta Pharmacol. Sin. 2013;34:1491–1498. doi: 10.1038/aps.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu X., Liang H., Tan Y., Yuan C., Li S., Li X., Li G., Shi Y., Zhang X. Cell-SELEX aptamer for highly specific radionuclide molecular imaging of glioblastoma in vivo. PLoS ONE. 2014;9:e90752. doi: 10.1371/journal.pone.0090752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang Y.Z., Hernandez F.J., Gu B., Stockdale K.R., Nanapaneni K., Scheetz T.E., Behlke M.A., Peek A.S., Bair T., Giangrande P.H., et al. RNA Aptamer-Based Functional Ligands of the Neurotrophin Receptor, TrkB. Mol. Pharmacol. 2012;82:623–635. doi: 10.1124/mol.112.078220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim J.W., Kim E.Y., Kim S.Y., Byun S.K., Lee D., Oh K.-J., Kim W.K., Han B.S., Chi S.-W., Lee S.C., et al. Identification of DNA aptamers toward epithelial cell adhesion molecule via cell-SELEX. Mol. Cells. 2014;37:742–746. doi: 10.14348/molcells.2014.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shigdar S., Qiao L., Zhou S.-F., Xiang D., Wang T., Li Y., Lim L.Y., Kong L., Li L., Duan W. RNA aptamers targeting cancer stem cell marker CD133. Cancer Lett. 2013;330:84–95. doi: 10.1016/j.canlet.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 122.Chen M., Yu Y., Jiang F., Zhou J., Li Y., Liang C., Dang L., Lu A., Zhang G. Development of cell-SELEX technology and its application in cancer diagnosis and therapy. Int. J. Mol. Sci. 2016;17:2079. doi: 10.3390/ijms17122079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang Y.M., Donovan M.J., Tan W. Using aptamers for cancer biomarker discovery. J. Nucleic Acids. 2013;2013 doi: 10.1155/2013/817350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jin C., Qiu L., Li J., Fu T., Zhang X., Tan W. Cancer Biomarker Discovery Using DNA Aptamers. Analyst. 2016;141:461–466. doi: 10.1039/C5AN01918D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Daniels D.A., Chen H., Hicke B.J., Swiderek K.M., Gold L. A tenascin-C aptamer identified by tumor cell-SELEX: Systematic evolution of ligands by exponential enrichment. Proc. Natl. Acad. Sci. USA. 2003;100:15416–15421. doi: 10.1073/pnas.2136683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ireson C.R., Kelland L.R. Discovery and development of anticancer aptamers. Mol. Cancer Ther. 2006;5:2957–2962. doi: 10.1158/1535-7163.MCT-06-0172. [DOI] [PubMed] [Google Scholar]
- 127.Soundararajan S., Chen W., Spicer E.K., Courtenay-Luck N., Fernandes D.J. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008;68:2358–2365. doi: 10.1158/0008-5472.CAN-07-5723. [DOI] [PubMed] [Google Scholar]
- 128.Soundararajan S., Wang L., Sridharan V., Chen W., Courtenay-Luck N., Jones D., Spicer E.K., Fernandes D.J. Plasma membrane nucleolin is a receptor for the anticancer aptamer AS1411 in MV4-11 leukemia cells. Mol. Pharmacol. 2009;76:984–991. doi: 10.1124/mol.109.055947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bates P.J., Reyes-Reyes E.M., Malik M.T., Murphy E.M., O’Toole M.G., Trent J.O. G-quadruplex oligonucleotide AS1411 as a cancer-targeting agent: Uses and mechanisms. Biochim. Biophys. Acta. 2017;1861:1414–1428. doi: 10.1016/j.bbagen.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 130.Esposito C.L., Passaro D., Longobardo I., Condorelli G., Marotta P., Affuso A., de Franciscis V., Cerchia L. A neutralizing rna aptamer against egfr causes selective apoptotic cell death. PLoS ONE. 2011;6:e24071. doi: 10.1371/journal.pone.0024071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dua P., Kang H.S., Hong S.-M., Tsao M.-S., Kim S., Lee D. Alkaline phosphatase ALPPL-2 Is a novel pancreatic carcinoma-associated protein. Cancer Res. 2013;73:1934–1945. doi: 10.1158/0008-5472.CAN-12-3682. [DOI] [PubMed] [Google Scholar]
- 132.Bing T., Shangguan D., Wang Y. Facile discovery of cell-surface protein targets of cancer cell aptamers. Mol. Cell. Proteom. 2015;14:2692–2700. doi: 10.1074/mcp.M115.051243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu M., Zhou L., Zheng X., Quan Y., Wang X., Zhou X., Ren J. A novel molecular marker of breast cancer stem cells identified by cell-SELEX method. Cancer Biomark. 2015;15:163–170. doi: 10.3233/CBM-140450. [DOI] [PubMed] [Google Scholar]
- 134.Sefah K., Bae K.-M., Phillips J.A., Siemann D.W., Su Z., McClellan S., Vieweg J., Tan W. Cell-based selection provides novel molecular probes for cancer stem cells. Int. J. Cancer J. Int. Cancer. 2013;132:2578–2588. doi: 10.1002/ijc.27936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xiao Z., Shangguan D., Cao Z., Fang X., Tan W. Cell-specific internalization study of an aptamer from whole cell selection. Chem. Weinh. Bergstr. Ger. 2008;14:1769–1775. doi: 10.1002/chem.200701330. [DOI] [PubMed] [Google Scholar]
- 136.Iaboni M., Fontanella R., Rienzo A., Capuozzo M., Nuzzo S., Santamaria G., Catuogno S., Condorelli G., de Franciscis V., Esposito C.L. Targeting insulin receptor with a novel internalizing aptamer. Mol. Ther. Nucleic Acids. 2016;5:e365. doi: 10.1038/mtna.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cerchia L., Esposito C.L., Camorani S., Rienzo A., Stasio L., Insabato L., Affuso A., de Franciscis V. Targeting axl with an high-affinity inhibitory aptamer. Mol. Ther. 2012;20:2291–2303. doi: 10.1038/mt.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mallikaratchy P., Tang Z., Kwame S., Meng L., Shangguan D., Tan W. Aptamer directly evolved from live cells recognizes membrane bound immunoglobin heavy mu chain in burkitt’s lymphoma cells. Mol. Cell. Proteom. 2007;6:2230–2238. doi: 10.1074/mcp.M700026-MCP200. [DOI] [PubMed] [Google Scholar]
- 139.Berezovski M.V., Lechmann M., Musheev M.U., Mak T.W., Krylov S.N. Aptamer-facilitated biomarker discovery (AptaBiD) J. Am. Chem. Soc. 2008;130:9137–9143. doi: 10.1021/ja801951p. [DOI] [PubMed] [Google Scholar]
- 140.Zamay G.S., Kolovskaya O.S., Zamay T.N., Glazyrin Y.E., Krat A.V., Zubkova O., Spivak E., Wehbe M., Gargaun A., Muharemagic D., et al. Aptamers selected to postoperative lung adenocarcinoma detect circulating tumor cells in human blood. Mol. Ther. 2015;23:1486–1496. doi: 10.1038/mt.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang K., Tang L., Sefah K., Zhao Z., Zhu G., Sun W., Goodison S., Tan W. Novel aptamer developed for breast cancer cell internalization. Chem. Med. Chem. 2012;7:79–84. doi: 10.1002/cmdc.201100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sefah K., Meng L., Lopez-Colon D., Jimenez E., Liu C., Tan W. DNA aptamers as molecular probes for colorectal cancer study. PLoS ONE. 2010;5:e14269. doi: 10.1371/journal.pone.0014269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen H.W., Medley C.D., Smith J.E., Sefah K., Shangguan D., Tang Z., Meng L., Tan W. Molecular recognition of small cell lung cancer cells using aptamers. Chem. Med. Chem. 2008;3:991–1001. doi: 10.1002/cmdc.200800030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kunii T., Ogura S., Mie M., Kobatake E. Selection of DNA aptamers recognizing small cell lung cancer using living cell-SELEX. Analyst. 2011;136:1310–1312. doi: 10.1039/c0an00962h. [DOI] [PubMed] [Google Scholar]
- 145.Zhao Z., Xu L., Shi X., Tan W., Fang X., Shangguan D. Recognition of subtype non-small cell lung cancer by DNA aptamers selected from living cells. Analyst. 2009;134:1808–1814. doi: 10.1039/b904476k. [DOI] [PubMed] [Google Scholar]
- 146.Shangguan D., Meng L., Cao Z.C., Xiao Z., Fang X., Li Y., Cardona D., Witek R.P., Liu C., Tan W. Identification of liver cancer-specific aptamers using whole live cells. Anal. Chem. 2008;80:721–728. doi: 10.1021/ac701962v. [DOI] [PubMed] [Google Scholar]
- 147.Shangguan D., Li Y., Tang Z., Cao Z.C., Chen H.W., Mallikaratchy P., Sefah K., Yang C.J., Tan W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc. Natl. Acad. Sci. USA. 2006;103:11838–11843. doi: 10.1073/pnas.0602615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sefah K., Tang Z., Shangguan D., Chen H., Lopez-Colon D., Li Y., Parekh P., Martin J., Meng L., Tan W. Molecular recognition of acute myeloid leukemia using aptamers. Leukemia. 2009;23:235–244. doi: 10.1038/leu.2008.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang X., Zhang J., Ma Y., Pei X., Liu Q., Lu B., Jin L., Wang J., Liu J. A cell-based single-stranded DNA aptamer specifically targets gastric cancer. Int. J. Biochem. Cell Biol. 2014;46:1–8. doi: 10.1016/j.biocel.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 150.Wu X., Zhao Z., Bai H., Fu T., Yang C., Hu X., Liu Q., Champanhac C., Teng I.-T., Ye M., Tan W. DNA aptamer selected against pancreatic ductal adenocarcinoma for in vivo imaging and clinical tissue recognition. Theranostics. 2015;5:985–994. doi: 10.7150/thno.11938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rong Y., Chen H., Zhou X.-F., Yin C.-Q., Wang B.-C., Peng C.-W., Liu S.-P., Wang F.-B. Identification of an aptamer through whole cell-SELEX for targeting high metastatic liver cancers. Oncotarget. 2016;7:8282–8294. doi: 10.18632/oncotarget.6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zueva E., Rubio L.I., Ducongé F., Tavitian B. Metastasis-focused cell-based SELEX generates aptamers inhibiting cell migration and invasion. Int. J. Cancer. 2011;128:797–804. doi: 10.1002/ijc.25401. [DOI] [PubMed] [Google Scholar]
- 153.Kim Y., Wu Q., Hamerlik P., Hitomi M., Sloan A.E., Barnett G.H., Weil R.J., Leahy P., Hjelmeland A.B., Rich J.N. Aptamer Identification of Brain Tumor Initiating Cells. Cancer Res. 2013;73:4923–4936. doi: 10.1158/0008-5472.CAN-12-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Quang N., Miodek A., Cibiel A., Ducongé F. Selection of aptamers against whole living cells: From cell-SELEX to identification of biomarkers. In: Tiller T., editor. Synthetic Antibodies. Springer; New York, NY, USA: 2017. pp. 253–272. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 155.Griffin N.M., Schnitzer J.E. Overcoming key technological challenges in using mass spectrometry for mapping cell surfaces in tissues. Mol. Cell. Proteom. 2011;10:R110.000935. doi: 10.1074/mcp.R110.000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Camorani S., Crescenzi E., Colecchia D., Carpentieri A., Amoresano A., Fedele M., Chiariello M., Cerchia L. Aptamer targeting EGFRvIII mutant hampers its constitutive autophosphorylation and affects migration, invasion and proliferation of glioblastoma cells. Oncotarget. 2015;6:37570–37587. doi: 10.18632/oncotarget.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cibiel A., Nguyen Quang N., Gombert K., Thézé B., Garofalakis A., Ducongé F. From Ugly Duckling to Swan: Unexpected identification from Cell-SELEX of an anti-annexin A2 aptamer targeting tumors. PLoS ONE. 2014;9:e87002. doi: 10.1371/journal.pone.0087002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu L., Zhang Z., Zhao Z., Liu Q., Tan W., Fang X. Cellular internalization and cytotoxicity of aptamers selected from lung cancer cell. Am. J. Biomed. Sci. 2013;5:47–58. [Google Scholar]
- 159.Nimse S.B., Sonawane M.D., Song K.-S., Kim T. Biomarker detection technologies and future directions. Analyst. 2016;141:740–755. doi: 10.1039/C5AN01790D. [DOI] [PubMed] [Google Scholar]
- 160.Jayanthi V.S.P.K.S.A., Das A.B., Saxena U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosens. Bioelectron. 2017;91:15–23. doi: 10.1016/j.bios.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 161.Ozalp V.C., Kavruk M., Dilek O., Tahir A. Aptamers: Molecular Tools for Medical Diagnosis. Curr. Top. Med. Chem. 2015;15:1125–1137. doi: 10.2174/1568026615666150413154233. [DOI] [PubMed] [Google Scholar]
- 162.Prakash J.S., Rajamanickam K. Aptamers and Their Significant Role in Cancer Therapy and Diagnosis. Biomedicines. 2015;3:248–269. doi: 10.3390/biomedicines3030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bukari B.A., Citartan M., Ch’ng E.S., Bilibana M.P., Rozhdestvensky T., Tang T.-H. Aptahistochemistry in diagnostic pathology: technical scrutiny and feasibility. Histochem. Cell Biol. 2017;147:545–553. doi: 10.1007/s00418-017-1561-9. [DOI] [PubMed] [Google Scholar]
- 164.Sun H., Tan W., Zu Y. Aptamers: versatile molecular recognition probes for cancer detection. Analyst. 2016;141:403–415. doi: 10.1039/C5AN01995H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dickey D.D., Giangrande P.H. Oligonucleotide Aptamers: a Next-Generation Technology for the Capture and Detection of Circulating Tumor Cells. Methods. 2016;97:94–103. doi: 10.1016/j.ymeth.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zamay A.S., Zamay G.S., Kolovskaya O.S., Zamay T.N., Berezovski M.V. Aptamer-Based Methods for Detection of Circulating Tumor Cells and Their Potential for Personalized Diagnostics. Adv. Exp. Med. Biol. 2017;994:67–81. doi: 10.1007/978-3-319-55947-6_3. [DOI] [PubMed] [Google Scholar]
- 167.Hong P., Li W., Li J. Applications of Aptasensors in Clinical Diagnostics. Sensors. 2012;12:1181–1193. doi: 10.3390/s120201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ravalli A., Voccia D., Palchetti I., Marrazza G. Electrochemical, Electrochemiluminescence, and Photoelectrochemical Aptamer-Based Nanostructured Sensors for Biomarker Analysis. Biosensors. 2016;6 doi: 10.3390/bios6030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhou Q., Rahimian A., Son K., Shin D.-S., Patel T., Revzin A. Development of an aptasensor for electrochemical detection of exosomes. Methods. 2016;97:88–93. doi: 10.1016/j.ymeth.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 170.Jin C., Zheng J., Li C., Qiu L., Zhang X., Tan W. Aptamers Selected by Cell-SELEX for Molecular Imaging. J. Mol. Evol. 2015;81:162–171. doi: 10.1007/s00239-015-9716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dougherty C.A., Cai W., Hong H. Applications of Aptamers in Targeted Imaging: State of the Art. Curr. Top. Med. Chem. 2015;15:1138–1152. doi: 10.2174/1568026615666150413153400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ku T.-H., Zhang T., Luo H., Yen T.M., Chen P.-W., Han Y., Lo Y.-H. Nucleic acid aptamers: An emerging tool for biotechnology and biomedical sensing. Sensors. 2015;15:16281–16313. doi: 10.3390/s150716281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mankoff D.A., Link J.M., Linden H.M., Sundararajan L., Krohn K.A. Tumor receptor imaging. J. Nucl. Med. 2008;49:149S–163S. doi: 10.2967/jnumed.107.045963. [DOI] [PubMed] [Google Scholar]
- 174.Wiraja C., Yeo D., Lio D., Labanieh L., Lu M., Zhao W., Xu C. Aptamer technology for tracking cells’ status & function. Mol. Cell. Ther. 2014;2 doi: 10.1186/2052-8426-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhou J., Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat. Rev. Drug Discov. 2017;16:181–202. doi: 10.1038/nrd.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xiang D., Shigdar S., Qiao G., Wang T., Kouzani A.Z., Zhou S.-F., Kong L., Li Y., Pu C., Duan W. Nucleic acid aptamer-guided cancer therapeutics and diagnostics: the next generation of cancer medicine. Theranostics. 2015;5:23–42. doi: 10.7150/thno.10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pastor F. Aptamers: A New Technological Platform in Cancer Immunotherapy. Pharmaceuticals. 2016;9:64. doi: 10.3390/ph9040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Soldevilla M.M., Villanueva H., Pastor F. Aptamers: A Feasible Technology in Cancer Immunotherapy. J. Immunol. Res. 2016;2016 doi: 10.1155/2016/1083738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Khedri M., Rafatpanah H., Abnous K., Ramezani P., Ramezani M. Cancer immunotherapy via nucleic acid aptamers. Int. Immunopharmacol. 2015;29:926–936. doi: 10.1016/j.intimp.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 180.Wu X., Chen J., Wu M., Zhao J.X. Aptamers: Active targeting ligands for cancer diagnosis and therapy. Theranostics. 2015;5:322–344. doi: 10.7150/thno.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee J.W., Kim H.J., Heo K. Therapeutic aptamers: Developmental potential as anticancer drugs. BMB Rep. 2015;48:234–237. doi: 10.5483/BMBRep.2015.48.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhu H., Li J., Zhang X.-B., Ye M., Tan W. Nucleic acid aptamer-mediated drug delivery for targeted cancer therapy. ChemMedChem. 2015;10:39–45. doi: 10.1002/cmdc.201402312. [DOI] [PubMed] [Google Scholar]
- 183.Catuogno S., Esposito C.L., de Franciscis V. Aptamer-mediated targeted delivery of therapeutics: An update. Pharmaceuticals. 2016;9:69. doi: 10.3390/ph9040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gopinath S.C.B., Lakshmipriya T., Chen Y., Arshad M.K.M., Kerishnan J.P., Ruslinda A.R., Al-Douri Y., Voon C.H., Hashim U. Cell-targeting aptamers act as intracellular delivery vehicles. Appl. Microbiol. Biotechnol. 2016;100:6955–6969. doi: 10.1007/s00253-016-7686-2. [DOI] [PubMed] [Google Scholar]
- 185.Xiang D., Shigdar S., Qiao G., Zhou S.-F., Li Y., Wei M.Q., Qiao L., Al. Shamaileh H., Zhu Y., Zheng C., et al. Aptamer-mediated cancer gene therapy. Curr. Gene Ther. 2015;15:109–119. doi: 10.2174/1566523214666141224095105. [DOI] [PubMed] [Google Scholar]
- 186.Parashar A. Aptamers in Therapeutics. J. Clin. Diagn. Res. JCDR. 2016;10:BE01–BE06. doi: 10.7860/JCDR/2016/18712.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhu G., Niu G., Chen X. Aptamer–Drug Conjugates. Bioconjug. Chem. 2015;26:2186–2197. doi: 10.1021/acs.bioconjchem.5b00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu B., Zhang J., Liao J., Liu J., Chen K., Tong G., Yuan P., Liu Z., Pu Y., Liu H. Aptamer-Functionalized Nanoparticles for Drug Delivery. J. Biomed. Nanotechnol. 2014;10:3189–3203. doi: 10.1166/jbn.2014.1839. [DOI] [PubMed] [Google Scholar]
- 189.Meng H.-M., Liu H., Kuai H., Peng R., Mo L., Zhang X.-B. Aptamer-integrated DNA nanostructures for biosensing, bioimaging and cancer therapy. Chem. Soc. Rev. 2016;45:2583–2602. doi: 10.1039/C5CS00645G. [DOI] [PubMed] [Google Scholar]
- 190.Benedetto G., Vestal C.G., Richardson C. Aptamer-Functionalized Nanoparticles as “Smart Bombs”: The Unrealized Potential for Personalized Medicine and Targeted Cancer Treatment. Target. Oncol. 2015;10:467–485. doi: 10.1007/s11523-015-0371-z. [DOI] [PubMed] [Google Scholar]
- 191.Zhu J., Huang H., Dong S., Ge L., Zhang Y. Progress in Aptamer-Mediated Drug Delivery Vehicles for Cancer Targeting and Its Implications in Addressing Chemotherapeutic Challenges. Theranostics. 2014;4:931–944. doi: 10.7150/thno.9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cansiz S., Zhang L., Wu C., Wu Y., Teng I.-T., Hou W., Wang Y., Wan S., Cai R., Jin C., et al. DNA Aptamer Based Nanodrugs: Molecular engineering for efficiency. Chem. Asian J. 2015;10:2084–2094. doi: 10.1002/asia.201500434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mathew A., Maekawa T., Sakthikumar D. Aptamers in Targeted Nanotherapy. Curr. Top. Med. Chem. 2015;15:1102–1114. doi: 10.2174/1568026615666150413153525. [DOI] [PubMed] [Google Scholar]
- 194.Wang C.-C., Wu S.-M., Li H.-W., Chang H.-T. Biomedical Applications of DNA-Conjugated Gold Nanoparticles. ChemBioChem. 2016;17:1052–1062. doi: 10.1002/cbic.201600014. [DOI] [PubMed] [Google Scholar]
- 195.Nimjee S.M., White R.R., Becker R.C., Sullenger B.A. Aptamers as therapeutics. Annu. Rev. Pharmacol. Toxicol. 2017;57:61–79. doi: 10.1146/annurev-pharmtox-010716-104558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rusconi C.P., Scardino E., Layzer J., Pitoc G.A., Ortel T.L., Monroe D., Sullenger B.A. RNA aptamers as reversible antagonists of coagulation factor IXa. Nature. 2002;419:90–94. doi: 10.1038/nature00963. [DOI] [PubMed] [Google Scholar]
- 197.Vavalle J.P., Rusconi C.P., Zelenkofske S., Wargin W.A., Ortel T.L., Alexander J.H., Povsic T.J., Becker R.C. The effect of the REG2 Anticoagulation System on thrombin generation kinetics: a pharmacodynamic and pharmacokinetic first-in-human study. J. Thromb. Thrombolysis. 2014;38:275–284. doi: 10.1007/s11239-014-1081-6. [DOI] [PubMed] [Google Scholar]
- 198.Woodruff R.S., Sullenger B.A. Modulation of the coagulation cascade using aptamers. Arterioscler. Thromb. Vasc. Biol. 2015;35:2083–2091. doi: 10.1161/ATVBAHA.115.300131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tan L., Neoh K.G., Kang E.-T., Choe W.S., Su X. PEGylated Anti-MUC1 Aptamer-Doxorubicin Complex for Targeted Drug Delivery to MCF7 Breast Cancer Cells. Macromol. Biosci. 2011;11:1331–1335. doi: 10.1002/mabi.201100173. [DOI] [PubMed] [Google Scholar]
- 200.Taghdisi S.M., Danesh N.M., Emrani A.S., Tabrizian K., ZandKarimi M., Ramezani M., Abnous K. Targeted delivery of Epirubicin to cancer cells by PEGylated A10 aptamer. J. Drug Target. 2013;21:739–744. doi: 10.3109/1061186X.2013.812095. [DOI] [PubMed] [Google Scholar]
- 201.Povsic T.J., Vavalle J.P., Aberle L.H., Kasprzak J.D., Cohen M.G., Mehran R., Bode C., Buller C.E., Montalescot G., Cornel J.H., et al. A Phase 2, randomized, partially blinded, active-controlled study assessing the efficacy and safety of variable anticoagulation reversal using the REG1 system in patients with acute coronary syndromes: results of the RADAR trial. Eur. Heart J. 2013;34:2481–2489. doi: 10.1093/eurheartj/ehs232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Povsic T.J., Lawrence M.G., Lincoff A.M., Mehran R., Rusconi C.P., Zelenkofske S.L., Huang Z., Sailstad J., Armstrong P.W., et al. Pre-existing anti-PEG antibodies are associated with severe immediate allergic reactions to pegnivacogin, a PEGylated aptamer. J. Allergy Clin. Immunol. 2016;138:1712–1715. doi: 10.1016/j.jaci.2016.04.058. [DOI] [PubMed] [Google Scholar]
- 203.Lincoff A.M., Mehran R., Povsic T.J., Zelenkofske S.L., Huang Z., Armstrong P.W., Steg P.G., Bode C., Cohen M.G., Buller C., et al. Effect of the REG1 anticoagulation system versus bivalirudin on outcomes after percutaneous coronary intervention (REGULATE-PCI): A randomised clinical trial. Lancet. 2016;387:349–356. doi: 10.1016/S0140-6736(15)00515-2. [DOI] [PubMed] [Google Scholar]
- 204.Verheugt F.W.A. An anticoagulant too good to be true for revascularisation. Lancet. 2016;387:314–315. doi: 10.1016/S0140-6736(15)00727-8. [DOI] [PubMed] [Google Scholar]
- 205.Ganson N.J., Povsic T.J., Sullenger B.A., Alexander J.H., Zelenkofske S.L., Sailstad J.M., Rusconi C.P., Hershfield M.S. Pre-existing anti–polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J. Allergy Clin. Immunol. 2016;137:1610–1613. doi: 10.1016/j.jaci.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Richter A.W., Akerblom E. Antibodies against polyethylene glycol produced in animals by immunization with monomethoxy polyethylene glycol modified proteins. Int. Arch. Allergy Appl. Immunol. 1983;70:124–131. doi: 10.1159/000233309. [DOI] [PubMed] [Google Scholar]
- 207.Ganson N.J., Kelly S.J., Scarlett E., Sundy J.S., Hershfield M.S. Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res. Ther. 2006;8:R12. doi: 10.1186/ar1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hershfield M.S., Ganson N.J., Kelly S.J., Scarlett E.L., Jaggers D.A., Sundy J.S. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res. Ther. 2014;16:R63. doi: 10.1186/ar4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Armstrong J.K., Hempel G., Koling S., Chan L.S., Fisher T., Meiselman H.J., Garratty G. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–111. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 210.Ruckman J., Green L.S., Beeson J., Waugh S., Gillette W.L., Henninger D.D., Claesson-Welsh L., Janjic N. 2′-Fluoropyrimidine RNA-based Aptamers to the 165-Amino Acid Form of Vascular Endothelial Growth Factor (VEGF165) Inhibition of Receptor Binding and VEGF-induced Vascular Permeability through Interactions Requiring the Exon 7-encoded Domain. J. Biol. Chem. 1998;273:20556–20567. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
- 211.Chen W., Sridharan V., Soundararajan S., Otake Y., Stuart R., Jones D., Fernandes D. Activity and Mechanism of Action of AS1411 in Acute Myeloid Leukemia Cells. Blood. 2007;110:1604. [Google Scholar]
- 212.Xu X., Hamhouyia F., Thomas S.D., Burke T.J., Girvan A.C., McGregor W.G., Trent J.O., Miller D.M., Bates P.J. Inhibition of DNA Replication and Induction of S Phase Cell Cycle Arrest by G-rich Oligonucleotides. J. Biol. Chem. 2001;276:43221–43230. doi: 10.1074/jbc.M104446200. [DOI] [PubMed] [Google Scholar]
- 213.Abdelmohsen K., Gorospe M. RNA-binding protein nucleolin in disease. RNA Biol. 2012;9:799–808. doi: 10.4161/rna.19718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Xu Z., Joshi N., Agarwal A., Dahiya S., Bittner P., Smith E., Taylor S., Piwnica-Worms D., Weber J., Leonard J.R. Knocking down nucleolin expression in gliomas inhibits tumor growth and induces cell cycle arrest. J. Neurooncol. 2012;108:59–67. doi: 10.1007/s11060-012-0827-2. [DOI] [PubMed] [Google Scholar]
- 215.Laber D.A., Sharma V.R., Bhupalam L., Taft B., Hendler F.J., Barnhart K.M. Update on the first phase I study of AGRO100 in advanced cancer. J. Clin. Oncol. 2005;23:3064. doi: 10.1200/jco.2005.23.16_suppl.3064. [DOI] [Google Scholar]
- 216.Laber D.A., Taft B.S., Kloecker G.H., Bates P.J., Trent J.O., Miller D.M. Extended phase I study of AS1411 in renal and non-small cell lung cancers. J. Clin. Oncol. 2006;24:13098. [Google Scholar]
- 217.Rosenberg J.E., Bambury R., Drabkin H.A., Lara P.N., Harzstark A.L., Figlin R.A., Smith G.W., Choueiri T., Erlandsson F., Laber D.A. A phase II trial of the nucleolin-targeted DNA aptamer AS1411 in metastatic refractory renal cell carcinoma. Investig. New Drugs. 2014;32:178–187. doi: 10.1007/s10637-013-0045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stuart R.K., Stockerl-Goldstein K., Cooper M., Devetten M., Herzig R., Medeiros B., Schiller G., Wei A., Acton G., Rizzieri D. Randomized phase II trial of the nucleolin targeting aptamer AS1411 combined with high-dose cytarabine in relapsed/refractory acute myeloid leukemia (AML) J. Clin. Oncol. 2009;27:15s. [Google Scholar]
- 219.Vater A., Sahlmann J., Kröger N., Zöllner S., Lioznov M., Maasch C., Buchner K., Vossmeyer D., Schwoebel F., Purschke W.G., et al. Hematopoietic Stem and Progenitor Cell Mobilization in Mice and Humans by a First-in-Class Mirror-Image Oligonucleotide Inhibitor of CXCL12. Clin. Pharmacol. Ther. 2013;94:150–157. doi: 10.1038/clpt.2013.58. [DOI] [PubMed] [Google Scholar]
- 220.Liu S.-C., Alomran R., Chernikova S.B., Lartey F., Stafford J., Jang T., Merchant M., Zboralski D., Zöllner S., Kruschinski A., et al. Blockade of SDF-1 after irradiation inhibits tumor recurrences of autochthonous brain tumors in rats. Neuro-Oncol. 2014;16:21–28. doi: 10.1093/neuonc/not149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hoellenriegel J., Zboralski D., Maasch C., Rosin N.Y., Wierda W.G., Keating M.J., Kruschinski A., Burger J.A. The Spiegelmer NOX-A12, a novel CXCL12 inhibitor, interferes with chronic lymphocytic leukemia cell motility and causes chemosensitization. Blood. 2014;123:1032–1039. doi: 10.1182/blood-2013-03-493924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Steurer M., Montillo M., Scarfò L., Mauro F.R., Andel J., Wildner S., Trentin L., Janssens A., Burgstaller S., Kruschinski A., et al. Results from a Phase IIa Study of the Anti-CXCL12 Spiegelmer Olaptesed Pegol (NOX-A12) in Combination with Bendamustine/Rituximab in Patients with Chronic Lymphocytic Leukemia. Blood. 2014;124:1996. [Google Scholar]
- 223.Ludwig H., Weisel K., Petrucci M.T., Leleu X., Cafro A.M., Garderet L., Leitgeb C., Foa R., Greil R., Yakoub-Agha I., et al. Olaptesed pegol, an anti-CXCL12/SDF-1 Spiegelmer, alone and with bortezomib–dexamethasone in relapsed/refractory multiple myeloma: A Phase IIa Study. Leukemia. 2017;31:997–1000. doi: 10.1038/leu.2017.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Li S., Xu H., Ding H., Huang Y., Cao X., Yang G., Li J., Xie Z., Meng Y., Li X., Zhao Q., Shen B., Shao N. Identification of an aptamer targeting hnRNP A1 by tissue slide-based SELEX. J. Pathol. 2009;218:327–336. doi: 10.1002/path.2543. [DOI] [PubMed] [Google Scholar]
- 225.Zhang J., Li S., Liu F., Zhou L., Shao N., Zhao X. SELEX aptamer used as a probe to detect circulating tumor cells in peripheral blood of pancreatic cancer patients. PLoS ONE. 2015;10:e0121920. doi: 10.1371/journal.pone.0121920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wang H., Li X., Volk D.E., Lokesh G.L.-R., Elizondo-Riojas M.-A., Li L., Nick A.M., Sood A.K., Rosenblatt K.P., Gorenstein D.G. Morph-X-Select: Morphology-based tissue aptamer selection for ovarian cancer biomarker discovery. BioTechniques. 2016;61:249–259. doi: 10.2144/000114473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mi J., Liu Y., Rabbani Z.N., Yang Z., Urban J.H., Sullenger B.A., Clary B.M. In vivo selection of tumor-targeting RNA motifs. Nat. Chem. Biol. 2010;6:22–24. doi: 10.1038/nchembio.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cheng C., Chen Y.H., Lennox K.A., Behlke M.A., Davidson B.L. In vivo SELEX for Identification of Brain-penetrating Aptamers. Mol. Ther. Nucleic Acids. 2013;2:e67. doi: 10.1038/mtna.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]