Abstract
Introduction
This article discusses the process of defining competencies and development of a best practices training course for investigators and clinical research coordinators who conduct social and behavioral research.
Methods
The first project phase established recommendations for training in Good Clinical Practice (GCP) and was done in conjunction with representatives from 62 Clinical and Translational Science Award (CTSA) hubs. Diversity in behavioral clinical trials and differences in regulation of behavioral trials compared with clinical trials involving drugs, devices, or biologics necessitated a separate Social and Behavioral Work Group. This group worked with CTSA representatives to tailor competencies and fundamental GCP principles into best practices for social and behavioral research.
Results
Although concepts underlying GCP were deemed similar across all clinical trials, not all areas were equally applicable and the ways in which GCP would be enacted differ for behavioral trials. It was determined that suitable training in best practices for social and behavioral research was lacking.
Discussion
Based on the training need, an e-learning course for best practices is available to all CTSA sites. Each institution is able to track outcomes for its employees to help achieve standardized competency-based best practices for social and behavioral investigators and staff.
Key words: Education, behavioral research, social sciences
Introduction
Clinical trials yield the vast majority of discoveries that are translated into practice. The importance of the rigor of the research and scientific integrity of data collected necessitate regulatory processes and a highly trained workforce to conduct research in accordance with these processes. Although these processes are clearly laid out for drug, device, and biologic clinical trials, the same is not always true for behavioral intervention studies. Behavioral intervention studies have not typically been held to the same intensity of regulation as drug, device, or biologic trials and thus the processes to conduct high-quality behavioral research have not been well specified. This may be because behavioral intervention studies tend to involve low risk to participants. In addition, any deliverables that result from these behavioral studies, such as specific behavioral intervention protocols, usually do not require regulation by the Food and Drug Administration (FDA), which is required for drugs and certain devices.
Recently, the National Institutes of Health (NIH) revised their definition of clinical trials to include behavioral research. Clinical trials are defined as “a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes” [1]. This new definition in which behavioral intervention studies are being called “clinical trials” is a paradigm shift in social and behavioral research. This shift demands competence in new professional domains for research teams. Thus, the need for competency-based training for investigators and clinical research coordinators (hereafter referred to as coordinators) of behavioral clinical trials is urgent. When engaging in drug, device, or biologic clinical trials, investigators and coordinators require training in Good Clinical Practice (GCP). This type of training is based on an international standard to ensure participant safety and data integrity, as well as to ensure consistency in reporting and regulation. Investigators and coordinators of behavioral clinical trials have not typically been required to undergo this training, as evidenced by a recent review from the GCP Training Project by the Clinical Trials Transformation Initiative [2]. Because GCP training was designed for drug, device, and biologic trials, a need was identified to examine how GCP training relates to social and behavioral research and particularly in the conduct of behavioral trials.
Other papers in this issue have discussed the background of the Enhancing Clinical Research Professionals’ Training and Qualifications (ECRPTQ) project, recommendations for training, and the process for defining competencies for study investigators and coordinators. This paper will present the process the Social and Behavioral Work Group undertook to determine how GCP relates to the conduct of behavioral clinical trials. This was done for the purposes of developing an e-learning course for best practices training in social and behavioral interventional research.
Materials and Methods
Formation of Social and Behavioral Work Group
The initial ECRPTQ meeting was held in Chicago to bring representatives of all 62 Clinical Translational Science Award (CTSA) hubs and key stakeholders in the human subject clinical research area together to adopt a standardized approach to GCP training for clinical trials. The attendees determined that an equivalent training for GCP in behavioral clinical trials was vital. The Social and Behavioral Work Group was formed with the primary purpose of ensuring that a core set of competencies and training in GCP would be relevant to behavioral trials. The work group was composed of 25 members including an administrative and faculty lead (online Supplementary Material). The members represented many CTSA-funded hubs nationwide and included both study investigators and coordinators who had experience in behavioral trials. The primary mode of interaction of this work group was through biweekly conference calls. A central web storage site was used to share materials. The group co-leads reported, advised, and determined strategic direction from the ECRPTQ leadership through direct participation on the project steering committee.
Preliminary Discussions and Consensus Building
After the Social and Behavioral Work Group was formed, it was first necessary to orient the group to the NIH’s revised definition of clinical trials and to discuss how the definition related to behavioral interventions. Some group members had not conceptualized their intervention studies as clinical trials, and there was a need to operationalize what was considered a “behavioral” intervention. The group members and leads provided examples from their own work as well as relevant materials from the literature which were uploaded onto a central web storage site. Consequently, the group discussed various types of interventions that could be defined as behavioral in nature and tested through clinical trials. This discussion was important for the group to begin thinking about key differences between design and conduct of behavioral clinical trials compared with drug, device, or biologic trials. Examples of behavioral trials discussed are provided in Table 1. Differences between behavioral trials compared with drug and device trials included variety of research settings (such as clinics, schools, home), the tendency of these trials to be of lower risk to participants, and the increased complexity of these trials through the use of people who provide interventions (referred to as interventionists) and the fidelity tracking necessary to ensure integrity of the delivery and receipt of the intervention.
Table 1.
Examples of behavioral clinical trials
| Trial example | Setting | Factors that may impact risk level | Use of interventionists | Fidelity tracking |
|---|---|---|---|---|
| Intervention to prevent childhood behavior problems | School based | Urban, high poverty sample | Trained teachers | Tracking of teacher delivery of intervention |
| Parenting intervention to reduce childhood obesity | Clinic setting | High-risk mothers who have experienced trauma | Social worker supervised by a psychologist | Sessions are videotaped |
| Brief intervention due to elevated alcohol consumption at their annual physical | Interactive voice response (phone) intervention | People followed by physician | No | No |
| Incentive programs to promote cigarette-smoking cessation (or cessation of other substance use) and reduce relapse rates | Smokers and/or substance abusers Assessments typically include urine samples that are analyzed as measures of recent smoking | No | No | |
| Physical therapy for sepsis patients | Hospital, inpatient | Medically unstable patients, intervention may be risky for them to participate | Trained physical therapists | Yes |
| Rehabilitation for the reduction of tremors in multiple sclerosis (MS) | Clinic setting | Use of a mobile app to measure the degree of tremor before and after test | Trained occupational therapists | Yes |
Once the group came to consensus regarding what was considered a behavioral clinical trial, they were oriented to the concept of GCP. The term GCP was not familiar to many members in the work group as most social and behavioral researchers do not undergo this training unless they are involved in a drug or device clinical trial. Relevant materials about GCP, its origins, and rationale for training were uploaded to the central web storage site for members to read and discuss on initial conference calls. The group reviewed the Minimum Criteria for GCP training of investigators and site personnel from TransCelerate BioPharma (based upon the document from the International Committee on Harmonization [ICH]) [3] and its criteria for relevancy to behavioral trials are listed in Table 2. This was done by sending an electronic survey to group members and asking them if they felt that each criterion was relevant, maybe relevant, or not relevant to behavioral clinical trials. The survey responses were tabulated and discussed in the group.
Table 2.
TransCelerate criteria: results of survey and group discussion regarding behavioral trial differences
| TransCelerate criteria | n | Report of “yes it applies” [% (n)] | Comments from group on how behavioral trials are different | |
|---|---|---|---|---|
| 1 | Investigator qualifications and agreements | |||
| Investigator qualification (education, training, experience) Demonstrate evidence of adequate training (provide up-to-date CV) Awareness of and compliance with GCP and regulatory requirements IP familiarity Allow for monitoring/auditing/inspection to enable sponsor/regulatory oversight Introduce definitions of monitoring (1.38), audit (1.6), and inspection (1.29) Use of qualified support staff Document delegation of duties to appropriately qualified persons | 15 | 93 (14) 93 (14) 67 (10) 7 (1) 40 (6) 47 (7) 100 (15) 87 (13) | No IP No requirement to comply to GCP Different methods of monitoring, audit, and inspection (may be different within behavioral trials depending on risk level of trial) Sponsor/regulatory oversight is different | |
| 2 | Adequate resources | |||
| Potential to recruit suitable subjects Sufficient time to conduct trial Sufficient qualified staff and adequate facilities to conduct trial Staff are adequately informed about protocol, IP, and tasks related to the protocol | 15 | 93 (14) 93 (14) 93 (14) 80 (12) | ||
| 3 | Medical care of trial subjects | |||
| Qualified physician or dentist who is an investigator or sub-investigator should be responsible for all trial-related medical decisions During and following the trial, the investigator/institution should ensure appropriate medical care for AEs and clinically significant lab deviations related to trial and inform subjects if medical care is needed for intercurrent illness Physician to make a reasonable effort to ascertain the reasons for subject’s premature withdrawal from the trial | 15 | 27 (4) 40 (6) 33 (5) | Trial dependent May not need a qualified physician, dentist, etc. for trial-related medical decisions Ensuring appropriate care for AEs or lab deviations Ascertain reasons for a subject’s premature withdrawal from a trial Good for tracking purposes in behavioral trial, but maybe not for “medical care” reasons | |
| 4 | Communication with IRB/IEC | |||
| Definition of IRB (1.31) and IEC (1.27) Before trial begins, obtain written, dated approval/favorable opinion for protocol and all documents provided to subjects (eg, ICF, advertisements) Provide a copy of investigator’s brochure/updated IB Before and during the trial, provide all documents required by IRB/IEC for review and appropriate approval/favorable opinion | 15 | 93 (14) 80 (12) 7 (1) 80 (12) | No investigational brochure required May be different level of communication depending on risk level of behavioral trial | |
| 5 | Compliance with protocol | |||
| Conduct trial according to approved protocol, GCP, and applicable regulatory requirements, for example, sufficient documentation to support subject meeting inclusion/exclusion criteria Document the acceptance to follow protocol in a protocol signature page or contract Protocol deviation process—no deviations or changes before sponsor and IRB/IEC approval/favorable opinion | 15 | 93 (14) 60 (9) 60 (9) | Do not conduct trial according to regular GCP Documenting acceptance to follow protocol in a protocol signature page (trial specific, depends on study sponsor) Protocol deviation may not need same level of reporting (ie, documented and rationale submitted to sponsor) | |
| 6 | IPs | |||
| Responsibility for IP (refer to 1.33) accountability and delegation of activities and supervision of an appropriately qualified person Documentation of delivery, inventory, dispensation, usage, disposal or return, and reconciliation of all IP and other study medication Stored per requirement Explanation of correct use of IP to subjects and periodic check for understanding/compliance | 15 | 33 (5) 20 (3) 20 (3) 20 (3) | Not applicable | |
| 7 | Randomization procedures and unblinding | |||
| Follow the trial’s randomization procedures Blinded trials: promptly document and report to sponsor any premature unblinding | 16 | 88 (14) 38 (6) | May not need to unblind in a behavioral trial | |
| 8 | Informed consent of trial subjects | |||
| Definition of informed consent (1.28) Explain the informed consent process and ICF IRB/IEC written approval in advance of use for written consent and other written information to be provided to subjects Subject to be fully informed of all pertinent aspects of the trial before participation The informed consent discussion and form needs to include all relevant explanations. Refer or link to ICH 4.8.10 Language used in oral and written information (ICF) should be understandable to subject or legal representative and impartial witness (where applicable) Subject should have ample time to review the ICF and to ask any questions and receive answers before decision is made Subject should not be unduly influenced to participate ICF should be obtained/signed before a subject’s participation in a trial (before any study procedures are performed) Subject should be aware that withdrawal is possible at any time Subject should not be asked to waive legal rights or release investigator or sponsor from liability for negligence Written ICF must be updated/approved when new information is available that may be relevant to subject’s consent | 16 | 82 (13) 94 (15) 94 (15) 94 (15) 94 (15) 94 (15) 94 (15) 94 (15) 94 (15) 94 (15) 88 (14) | Informed consent process not often feasible to be documented in medical record as these trials may occur outside a clinical setting | |
| Informed consent of special population Refer to or add definition of vulnerable subjects (1.61) When a subject (eg, minor, incapacitated) can only be enrolled with the consent of the legal representative, the subject must be informed to the level of their understanding, provide assent (where this is feasible), and personally sign and date the consent form In emergency situations, where the subject and legal representative are unable to consent, enrollment requires protective measures to be described in protocol or other IRB/IEC-approved documents. Subject or legal representative should be informed as soon as possible and consent to continue and other consent as appropriate If the subject/legal representative are unable to read, an impartial witness must be present during the consent discussion and sign and date the consent form Informed consent documentation The ICF should be signed and personally dated by the subject and/or the legal representative and by the person who conducted the consent A signed and dated copy of the ICF should be given to the subject or the legal representative (including any other written information provided to the subject) The informed consent process should be documented in the medical record/source file (as well as documentation regarding communication of new information) | 16 | 88 (14) 94 (15) 75 (12) 94 (15) 82 (13) 82 (13) 50 (8) | ||
| 9 | Records and reports | |||
| Definition of source documents: the actual documents (originals), GCP glossary 1.52 (brief) Refer to or add definition of source data (1.51) Definition of essential documents (section 8) The need to maintain essential documents. Refer/link to section 8 Retention of essential documents CRFs and all required reports (written or electronic) Corrections are dated and initialed, do not obscure original entry and explained if necessary (applies to written and electronic changes/updates). Retain records of changes and corrections Financial aspects documented in an agreement between sponsor and investigator/institution Direct access to all trial-related documents by the monitor, the auditor, the IRB/IEC, or regulatory authority | 16 | 69 (11) 56 (9) 69 (11) 75 (12) 81 (13) 75 (12) 75 (12) 75 (12) 75 (12) | Source documents and essential documents may have specific meaning for drug trials not applicable/known to behavioral trials Financial aspects in sponsor agreement appears to refer to drug trials only | |
| 10/13 | Progress reporting/final reports | |||
| Investigator submits written summaries of progress to IRB/IEC at least annually or as required Provide written reports to sponsor and IRB/IEC (and institution where required) of any significant changes affecting the study or increased risk to subjects Upon completion of trial, provide sponsor with all required reports Final report with a summary of trials and outcomes submitted to IRB/IEC and regulatory authorities as required | 16 | 88 (14) 81 (13) 81 (13) 75 (12) | Final report submitted to IRB/regulatory authorities may be trial specific | |
| 11 | Safety reporting | |||
| AE definition (1.2) Refer to or add definition of ADR (1.1) and unexpected ADR (1.60) AE reporting—all AEs and/or laboratory abnormalities should be reported to the sponsor within the time period defined in protocol All SAEs should be reported immediately to the sponsor except for those SAEs that the protocol or other document (eg, investigator’s brochure) identifies as not needing immediate reporting | 6 (1) 56 (9) 56 (9) | ADR is not applicable Reporting of SAEs is likely to be on different timeline than in drug trials | ||
| 12 | Premature termination or suspension of trial | |||
| Responsibility to promptly inform the trial subjects and ensure appropriate therapy and follow-up. Inform regulatory authorities when required Responsibility for communication of study termination or suspension of study to sponsor, IRB/IEC, and institution as applicable, including a detailed written explanation | 16 | 63 (10) 75 (12) | May be trial specific |
GCP, Good Clinical Practice; IP, investigational product; AE, adverse event; ICF, informed consent form; SAE, serious adverse event; ADR, adverse drug reaction.
Building on these foundational activities, the group was asked to review existing training for GCP and to identify gaps specific to behavioral research. Pairs of reviewers were assigned to submit a written review of each of the programs. Individually they accessed the training and completed the course. Reviewers were asked to evaluate the effectiveness of the content for best practices training for social and behavioral researchers. They also reviewed the courses for design features and were asked to describe what they liked and did not like about the experience of taking the course. During conference calls, reviews were discussed, compared, and synthesized. Pairs of group members were assigned different GCP training courses and their ratings were compared and discussed in conference calls.
Participation in Research Competency Adaptation for Social and Behavioral Trials
Representatives of the Social and Behavioral Work Group participated in the face-to-face meeting at the ECRPTQ conference in Dallas in February 2015 as described in the paper by Calvin-Naylor et al. [5]. These work group members helped to evaluate and refine research competencies that were part of a larger framework developed by the Joint Task Force for Clinical Trial Competency [4]. The work group offered recommendations which resulted in some adaptations to competencies shown in the appendix of that paper. The competencies served as a foundation for conceptualizing GCP training for social and behavioral researchers.
Results
Table 2 shows the results of the relevance of the GCP criteria to behavioral clinical trials. There were 15–16 responses for each criterion and the results were presented and discussed on a conference call with the group for clarity. The areas thought to be least relevant to behavioral trials included investigational products, medical care, safety reporting, and premature suspension of trials. Investigational products is an area clearly not relevant to behavioral trials, as having an investigational product would classify the trial as a drug, device, or biologic for regulation through the FDA. As behavioral trials typically pose minimal risks to participants and may not be conducted in a clinical setting, medical care of a qualified physician was considered to be only necessary in specific situations, such as behavioral trials with ill or medically unstable participants. This same line of reasoning applied to safety reporting as the reporting would be different for minimal risk trials. In addition, the group felt that unblinding or premature suspension of a trial would be a rare occurrence in behavioral trials.
Review of Existing GCP Training
Four commercial e-learning courses as well as one course through the NIH were reviewed. Across all courses reviewed, significant gaps in training were identified. The topics, with the exception of the focus on regulatory processes related to GCP, were felt to be largely relevant. However, the content was not specific to behavioral trials and lacked examples or scenarios that arise in social and behavioral research. In courses that were geared toward social and behavioral researchers, there was discussion about the breadth and depth of content presented. Most of the training was thought to be either too general or too specific for a best practices course. Some course content seemed redundant with basic training in human subject protections that investigators and coordinators need to take at their institutions when engaging in research. Other course content was deemed to be appropriate but highly specialized, such as modules about working with prisoners or international research studies. With regard to the user experience with each course, aspects that reviewers mentioned as promoting their engagement included high visual appeal, good narration, and interactive features. One course included job aids and reference links that were deemed especially useful. Although some reviewers felt that quizzes or knowledge checks throughout the course were engaging, others liked the ability to test out of a course altogether by taking a quiz separate from the course itself.
Discussion
The activities of the Social and Behavioral Work Group identified a clear need for specifying the construct of GCP for social and behavioral researchers. The term GCP was largely unfamiliar to the content experts and required group discussion and consensus building to relate GCP to the design and conduct social and behavioral research. After thorough review, it was determined that existing GCP training was not applicable for researchers conducting behavioral trials. Therefore, the Social and Behavioral Work Group recommended to ECRPTQ leadership that a new training program be created, focusing on the specific needs and unique research processes of social and behavioral research teams.
Best Practices Training in Social and Behavioral Research
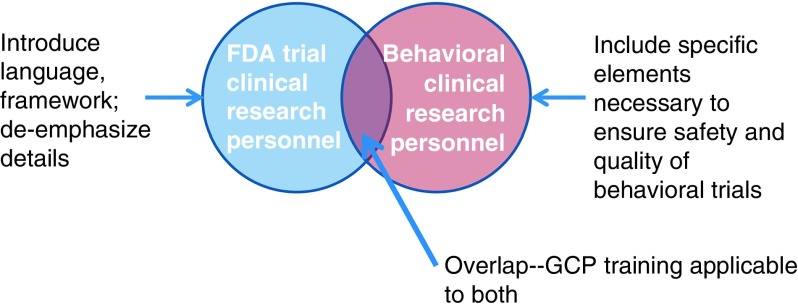
In order to develop appropriate training, it was necessary to better define the construct of GCP for social and behavioral researchers. Fig. 1 shows the conceptual model used. We felt that we should rename GCP to “best practices” as GCP in the traditional sense is not a term typically used in the context of social and behavioral research and has specific ties to regulation from FDA that is usually not applicable. Despite differences, there is an overlap in the competencies required for research personnel of FDA-regulated trials and behavioral trials. In order to create best practices training, it was necessary to address the overlap of what all research personnel need to know. Thus, the training requires an introduction to the context of GCP and definition of terms specific for behavioral research personnel. However, it is also important to de-emphasize areas not relevant to social and behavioral research. In addition, because behavioral trials often have additional complexity in design and implementation, specific content needed to be addressed, such as treatment fidelity. Content for best practices needed tailoring from GCP and needed to focus on specific job skills for both investigators and clinical research coordinators.
Fig. 1.
Conceptual framework of how Good Clinical Practice (GCP) relates to behavioral clinical trials. FDA, Food and Drug Administration.
After receiving approval to move forward, a subset of volunteers from the Social and Behavioral Work Group were recruited to define content areas for a social and behavioral best practices training program. A mapping process was employed to identify potential topics and link them to competency domains defined by the ECRPTQ project. To do this, the work group team leads at the University of Michigan researched existing GCP and social and behavioral research training and provided information to the subgroup members. The Work Group leads reviewed several books on implementing GCP, examined educational materials on the principles of GCP from various sources, examined the results of the TransCelerate survey (shown in Table 2), and reviewed the current human subject training University of Michigan offers for social and behavioral research. Through a series of discussions via conference call, 8 topic areas were selected for inclusion as modules in a training program (see Table 3).
Table 3.
Training modules for social and behavioral best practices course
| Module topics | Learning objectives By the end of this module, the participant will: |
|---|---|
| Introduction | Be familiar with the role of ICH in providing guidelines for regulations Be able to define GCP and the goals of GCP Be able to articulate how GCP relates to regulations of clinical trials in social and behavioral research |
| Research protocol | Describe elements of a research protocol Articulate the importance of standard operating procedures Gain knowledge in aspects of treatment fidelity as they apply to behavioral trials Be familiar with protocol violations and how to handle them |
| Roles and responsibilities | Understand roles and responsibilities of the sponsor, IRB, research investigator, and clinical research coordinator |
| Informed consent | Identify key aspects of the consent process that ensure participants rights, safety, and well-being are prioritized Analyze an informed consent process between study team member and participants to determine areas needing improvement |
| Confidentiality/privacy | Differentiate concepts of confidentiality and privacy Select strategies to ensure confidentiality and privacy Identify instances when confidentiality or privacy are compromised and when to report to IRB |
| Recruitment/retention | Identify best practices for recruitment including appropriate wording on flyers and in advertisements and ensuring adequate representation from women and diverse populations Identify strategies to retain participants |
| Participant safety/adverse event reporting | Be familiar with aspects of seriousness and relatedness as they relate to an adverse event Choose appropriate strategies to detect adverse events Define the role of a data safety and monitoring board |
| Quality control/assurance | Articulate the importance of quality assurance in a clinical trial Select strategies that can help systematically track participant progress through a study Define sources of bias that can affect data quality in a behavioral trial Assess how different biases can affect data quality from a case-based example |
| Research misconduct | Define research misconduct and who this applies to Describe the process for reporting an instance of misconduct Name consequences of research misconduct |
GCP, Good Clinical Practice.
Content experts were identified for each training module topic. Working with an instructional designer, these experts defined learning objectives and outlined content for inclusion in the training modules. Learning objectives and content were thoroughly discussed by the entire work group and consensus was reached regarding the overall structure and learning objectives for a new social and behavioral training program. At this time, the course has been completed and available for download by interested institutions [6].
Conclusion
The goal of this project was to tailor competencies and the fundamental principles of GCP into what are deemed as best practices for social behavioral research and to translate those competencies into a framework for a series of e-learning modules. This project involved a high level of collaboration across CTSA hubs and codevelopment with benchmarking organizations such as The Clinical Trials Transformation Initiative that allowed for the opportunity to create a translational bridge between more traditional clinical trials and behavioral interventions. The training program is currently tailored to the specific needs and unique research processes of social and behavioral researchers and consequently will fill a critical training gap identified through the ECRPTQ process.
Acknowledgments
This research was supported by the National Center for Advancing Translational Research grant no. 3UL1TR000433-08S1 (Thomas Shanley, M.D.). The authors gratefully acknowledge the members of the Social and Behavioral Work Group as well as the numerous staff and faculty members from academic institutions nationally who graciously donated their time and expertise to this project.
Declaration of Interest
The authors have no conflicts of interest.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/cts.2016.3.
click here to view supplementary material
References
- 1. Notice of Revised NIH Definition of “Clinical Trial” [Internet] [cited March 23, 2016]. (https://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-015.html).
- 2. Arango J, et al. Good clinical practice training: identifying key elements and strategies for increasing training efficiency. Therapeutic Innovation & Regulatory Science 2016; 50: 480–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Minimum Criteria for ICH E6 GCP Investigator Site Personnel Training. [Internet] [Cited March 3, 2016]. (http://www.transceleratebiopharmainc.com/wp-content/uploads/2013/10/TransCelerate-GCP-Training-Minimum-Criteria-in-Operating-Principles_0.pdf).
- 4. Sonstein SA, et al. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. Clinical Researcher 2014; 28: 17–23. [Google Scholar]
- 5. Calvin-Naylor NA, et al. Education and training of clinical and translational study investigators and research coordinators: A competency-based approach. Journal of Clinical and Translational Science 2017; doi:10.1017/cts.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Good Clinical Practice for Social and Behavioral Research – eLearning Course. Office of Behavioral and Social Sciences Research. [Internet] [cited December 20, 2016]. (https://obssr.od.nih.gov/training/web-based-learning/good-clinical-practice-for-social-and-behavioral-research-elearning-course/).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/cts.2016.3.
click here to view supplementary material