ABSTRACT
Acanthamoeba spp. and Balamuthia mandrillaris are causative agents of granulomatous amoebic encephalitis (GAE), while Naegleria fowleri causes primary amoebic meningoencephalitis (PAM). PAM is an acute infection that lasts a few days, while GAE is a chronic to subacute infection that can last up to several months. Here, we present a literature review of 86 case reports from 1968 to 2016, in order to explore the affinity of these amoebae for particular sites of the brain, diagnostic modalities, treatment options, and disease outcomes in a comparative manner.
KEYWORDS: brain, free-living amoebae, meningoencephalitis, central nervous system infections
INTRODUCTION
Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri are pathogenic free-living amoebae (1). They are well known to produce fatal central nervous system (CNS) infections, and pathogenic Acanthamoeba spp. can also produce blinding keratitis, which is often associated with the inappropriate use of contact lenses. All three genera are known as amphizoic amoebae, due to their ability to exist as parasitic organisms and to inhabit natural environments as free-living organisms. In nature, Acanthamoeba seems to be most ubiquitous; it can inhabit a variety of environments and has been isolated from soil, water, and air. B. mandrillaris is rather selective, living in the soil, and it has been rarely isolated from water (1–3). Naegleria fowleri, being a thermophilic protist, prefers warm water such as hot springs in temperate zones and lakes in the tropics (4, 5). Acanthamoeba spp. and B. mandrillaris are known to have two stages in their life cycles, i.e., a vegetative trophozoite stage and a dormant cyst form. N. fowleri exhibits a transient flagellate form in addition to the trophozoite and cyst forms (1–6). These forms are interchangeable, depending on the environmental conditions. Among the various forms, the trophozoite form is often the infectious one. These amoebae cause two distinct clinical entities, namely, granulomatous amoebic encephalitis (GAE), caused by pathogenic Acanthamoeba spp. and B. mandrillaris, and primary amoebic meningoencephalitis (PAM), caused by N. fowleri. GAE and PAM are distinguished by their etiologies, risk factors, duration of illness, clinical features, and laboratory and imaging findings (6). N. fowleri is the only known pathogenic species in the genus Naegleria, which consists of over 40 species, that causes human disease, while B. mandrillaris is the only isolated species in the genus Balamuthia. The genus Acanthamoeba is classified into 20 genotypes (T1 to T20) (1–3, 7, 8). These amoebae and their associated infections have garnered increasing scientific and medical attention in recent years due to their poor prognoses, i.e., less than 5% of patients survive if early intervention is not initiated (1, 6). In addition to poor prognoses, cases of amoebic meningoencephalitis are often underreported, misreported, and underrecognized globally, due to lack of awareness, lack of available diagnostic tools, lack of wide distribution of knowledge regarding public health issues and risk factors, especially in developing countries, and the similarity of symptoms to those of other common CNS infections, such as viral and bacterial meningitis. In addition, the pathogenesis and pathophysiology of CNS infections due to the aforementioned free-living amoebae are incompletely understood. For example, PAM is an acute infection that lasts only a few days, while GAE is a chronic to subacute infection that lasts up to several months. Given the nasal route of entry, N. fowleri is likely to have an intimate correlation with the frontal lobe, due to the anatomical proximity of the olfactory bulb to the frontal lobe; the olfactory bulb is terminal to the olfactory neuroepithelium of the nasal passage, traversing the cribriform plate to the brain (1, 6). Although the intranasal route is the route of infection, current administration of drugs (such as amphotericin B) against PAM is via the intravenous route, which causes significant toxicity to other tissues and requires high doses to reach the site of infection at sufficient concentrations to kill the parasite. In contrast, pathogenic Acanthamoeba and B. mandrillaris spread hematogenously and possibly distribute in the frontal lobe, the temporal lobe, and the parietal lobe, likely through the middle cerebral artery, as these cortices are among the main regions for middle cerebral artery supply (9). By comparing the available reported cases of CNS infections due to free-living amoebae, the aim of the present study was to determine the principle sites of infection within the brain, the diagnostic methods employed (premortem and postmortem), and the available treatment regimens, with examples of successful outcomes, with the goal of increasing awareness for the improved management of amoebic meningoencephalitis.
CASE STUDIES OF AMOEBIC MENINGOENCEPHALITIS
Predilection for sites in the brain.
In this review, we examined cases of brain infections due to free-living amoebae, i.e., Acanthamoeba spp., B. mandrillaris, and Naegleria fowleri. In total, we examined 86 case reports, from 1968 to 2016, that were available at PubMed, in order to explore the affinity of these three types of amoebae for particular sites of the brain. For GAE due to pathogenic Acanthamoeba, a total of 46 cases reported in 35 publications were reviewed. For GAE due to B. mandrillaris, a total of 29 cases reported in 16 publications were reviewed. For PAM due to N. fowleri, a total of 11 cases reported in 10 publications were reviewed. Most cases (up to 90%) were reported from the United States. PAM due to N. fowleri was observed in immunocompetent individuals, while GAE was observed in both immunosuppressed individuals (mostly Acanthamoeba cases) and immunocompetent individuals (mostly B. mandrillaris cases). The cases were stratified based on the year of the report, the patient's age and gender, the place of origin, the chief complaints, relevant positive and negative findings, laboratory findings (cerebrospinal fluid [CSF] and blood profiles, serological data, and culture results), the diagnosis, neuroimaging results, definitive treatments, and the disease outcome. In literature from 1960 to 1970, B. mandrillaris was recognized as Leptomyxid genus when taxonomical categorization was not clear (10); however, those cases were included in this review as B. mandrillaris infections. Cases with imaging studies included magnetic resonance imaging (MRI) (27 cases), computed tomographic (CT) scanning (24 cases), and a combination of CT scanning and MRI (16 cases). Because this was a study of preferential sites, the first imaging studies for the first admission were selected for analyses unless stated otherwise. Moreover, when two imaging modalities were used simultaneously during the first admission, MRI was considered superior to CT scanning in terms of demonstrating focal lesions that were evolving over time. Therefore, we prioritized MRI images and descriptions over CT images and descriptions (11). MRI availability is limited in some parts of the world, however, and CT scanning was used as standard imaging in such instances.
Neuroimaging of GAE typically shows multiple, well-defined, focal, ring-enhancing, space-occupying lesions, with perilesional edema and leptomeningeal enhancement if meninges are involved (12–14). PAM in neuroimaging had a single focus of infection, with diffuse cerebral edema, signs of increased intracranial pressure (midline shift and effacement of ventricles and cisterns), and basilar meningeal enhancement (12–14). For GAE due to pathogenic Acanthamoeba spp., 12 cases (26.1%) were reported to have lesions in the frontal lobe, 11 cases (23.9%) were reported to have lesions in the parietal lobe, 12 cases (26.1%) were reported to have lesions in the temporal lobe, and 9 cases (19.6%) were reported to have lesions in the occipital lobe. For sites beyond the cerebral cortices, the corticomedullary junction and the cerebellum represented most of the cases (17.4% and 8.7%, respectively). In 2 cases (4.3%), the thalamus was also affected. The CSF drainage system was favored in 5 cases (10.9%) (with hydrocephalus), while generalized edema was found in 1 case (2.2%) (Fig. 1; also see Table S1 in the supplemental material). There were possible false-negative findings in 2 cases (4.3%), in which normal findings were observed in early imaging. Other sites made up 8 cases (17.4%) of GAE due to Acanthamoeba. Overall, the frontal lobe, parietal lobe, temporal lobe, and occipital lobe (constituting 56% of the total cases reviewed in this study) were affected most in cases of GAE due to Acanthamoeba.
FIG 1.
Sites of infection of GAE due to Acanthamoeba spp. The majority of cases involved the cerebral cortices, with the frontal lobe and the temporal lobe being most affected, followed by the parietal lobe and the occipital lobe. Among extracortical sites, the cerebellum and the corticomedullary junction were the most favored sites. Hydrocephalus, which results from blockage of CSF drainage, was observed in a few cases. Other affected sites included the thalamus, the caudate nucleus, and the brainstem. Infection can also present as normal findings in early neuroimaging.
For GAE due to B. mandrillaris, 12 cases (41.4%) were reported to have lesions in the frontal lobe, 10 cases (21.7%) were reported to have lesions in the parietal lobe, 15 cases (51.7%) were reported to have lesions in the temporal lobe, and 9 cases (31%) were reported to have lesions in the occipital lobe. Sites beyond the cerebral cortices included the corticomedullary junction, the thalamus, the basal ganglia, and the cerebellum (Fig. 2; also see Table S2). Notably, one case manifested as an aneurysm, while two cases affected the CSF drainage. In one case, coinfection with HIV (advanced infection), Acanthamoeba, and B. mandrillaris with cerebral toxoplasmosis was observed. Overall, the frontal lobe, parietal lobe, temporal lobe, and occipital lobe (constituting 54% of the total cases reviewed in this study) were affected most in cases of GAE due to B. mandrillaris, which appears consistent with the findings for GAE due to Acanthamoeba.
FIG 2.
Sites of infection of GAE due to Balamuthia mandrillaris. Involvement of the temporal lobe was observed in most cases, followed by involvement of the frontal, parietal, and occipital lobes. Among extracortical sites, the thalamus was most affected, followed by the corticomedullary junction, the cerebellum, and the basal ganglia.
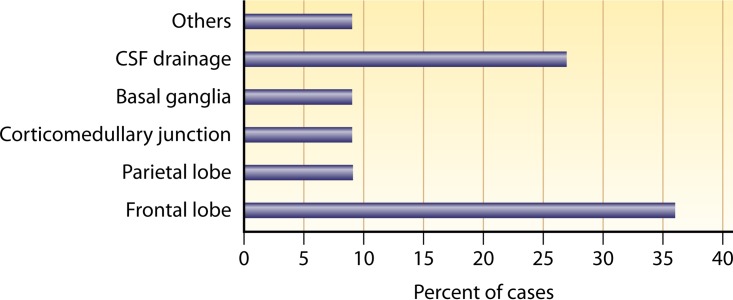
For PAM due to N. fowleri, it was observed that the parasite favors the frontal lobe, followed by the parietal lobe. Among the reported cases of PAM due to N. fowleri, involvement of the frontal lobe was reported for 36% of cases (Fig. 3; also see Table S3). Sites beyond the cerebral cortices included the corticomedullary junction, while the CSF drainage system was targeted in 27% of cases. Three cases (27%) showed signs of hydrocephalus. Notably, one case of PAM showed normal neuroimaging findings. In comparison to GAE due to Acanthamoeba spp. and B. mandrillaris, the frontal lobe was affected most in cases of PAM due to N. fowleri.
FIG 3.
Sites of infection of PAM due to Naegleria fowleri. More cases involved the frontal lobe, followed by the parietal lobe and the corticomedullary junction. Hydrocephalus was observed in 27% of cases.
Diagnosis.
Among cases of GAE due to Acanthamoeba spp., 34.5% were diagnosed postmortem and 65.5% were identified premortem (Table 1). Among the postmortem cases, microscopy was used successfully in 10.9% of cases, immunofluorescence assays (IFAs) were used effectively in 18.2% of cases, and PCR was used positively in 5.4% of cases. Among the premortem cases, CSF observations of amoebae were made in 38.1% of cases (using microscopy [14.5% of cases], culture of parasites [20%], and PCR [3.6%]) and brain biopsy samples were assessed in 30.41% of cases (using microscopy [15.21%], culture [4.34%], PCR [4.34%], and IFAs [6.52%]). Overall, for GAE due to Acanthamoeba spp., observation of parasites in CSF samples using culture or microscopy was the most widely used premortem diagnostic method.
TABLE 1.
Use of various methods for the diagnosis of GAE due to Acanthamoeba spp. or Balamuthia mandrillaris and PAM due to Naegleria fowleri
| Disease (total cases reviewed) | Diagnostic modality | Method of analysis | % of cases (no. of cases)a |
|---|---|---|---|
| GAE due to Acanthamoeba spp. (n = 46) | Brain biopsy | Microscopy | 15.21 (7) |
| PCR | 4.34 (2) | ||
| IFA | 6.52 (3) | ||
| Culture | 4.34 (2) | ||
| CSF | Microscopy | 17.39 (8) | |
| Culture | 23.9 (11) | ||
| PCR | 4.34 (2) | ||
| Postmortem | Microscopy | 13.04 (6) | |
| IFA | 21.7 (10) | ||
| PCR | 6.52 (3) | ||
| Skin biopsy | 2.17 (1) | ||
| GAE due to B. mandrillaris (n = 29) | Brain biopsy | Microscopy | 20.68 (6) |
| PCR | 10.34 (3) | ||
| IFA | 13.79 (4) | ||
| CSF | PCR | 3.44 (1) | |
| Postmortem | Microscopy | 10.34 (3) | |
| IFA | 20.68 (6) | ||
| Skin biopsy | 6.9 (8) | ||
| PAM due to N. fowleri (n = 11) | Postmortem | Microscopy | 36.4 (4) |
| IFA | 18.2 (2) | ||
| PCR | 9.1 (1) | ||
| CSF | Microscopy | 18.2 (2) | |
| Culture | 18.2 (2) |
The data are presented as percentages of cases reviewed in this study. Some cases may involve more than one diagnostic modality.
Among cases of GAE due to B. mandrillaris, 31% were diagnosed postmortem and 68.9% were identified premortem (Table 1). Among the postmortem cases, microscopy was used successfully in 10.34% of cases and IFAs were used effectively in 20.68% of cases. Among the premortem cases, CSF observations of amoebae were made in 3.44% of cases (using PCR) and brain biopsy samples were assessed in 44.81% of cases (using microscopy [20.68%], PCR [10.34%], and IFAs [13.79%]). Overall, for GAE due to B. mandrillaris, observation of parasites in brain biopsy samples using microscopy or IFAs was the most widely used premortem diagnostic method.
Among cases of PAM due to N. fowleri, 63.7% were diagnosed postmortem and 36.3% were identified premortem (Table 1). Among the postmortem cases, microscopy was used successfully in 36.4% of cases, IFAs were used effectively in 18.2% of cases, and PCR was used positively in 9.1% of cases. Among the premortem cases, CSF observations of amoebae were made in 36.4% of cases (using microscopy [18.2%] and culture [18.2%]). Overall, for PAM due to N. fowleri, observation of parasites in CSF samples using microscopy or IFAs was the most widely used premortem diagnostic method.
Treatment.
The findings for the compiled cases indicated that, despite the establishment of clinical guidelines for amoebic meningoencephalitis, the physicians were liberal with combinations of several classes of drugs with different mechanisms of action and individualized the regimens according to age, gender, availability of chemotherapy, and underlying medical conditions that might affect the metabolism of drugs; therefore, we examined the results according to classes of chemotherapeutic agents instead of combinations of agents. Percentages were determined separately for cases of GAE (due to Acanthamoeba or Balamuthia) and cases of PAM. For determination of disease outcomes, cases of survival were deemed successful, while the cases that resulted in death (including brain death) were considered poor outcomes.
When the reported cases of amoebic meningoencephalitis were reviewed, it was clear that no drug was effective against GAE or PAM and, as a result, the majority of cases resulted in death. Various types of drugs and their combinations were tested but the prognoses remained poor. For example, for the cases of GAE due to Acanthamoeba spp. reviewed here, the most commonly used drugs included azole compounds, sulfonamides, amphotericin B, sulfadiazine, macrolides, miltefosine, pentamidine, flucytosine, and rifampin (Table 2). In contrast, azole compounds, sulfadiazine, pentamidine, miltefosine, and amphotericin B were most commonly used for cases of GAE due to B. mandrillaris. For cases of PAM due to N. fowleri, the most commonly used drugs included amphotericin B, azole compounds, sulfadiazine, and rifampin (Table 2). Among cases with successful prognoses, there appeared to be combinations of several compounds (Table 3). For some of those cases, a combination of amphotericin B, sulfamethoxazole-trimethoprim, and rifampin was given for the treatment of GAE due to Acanthamoeba spp. (Table 3). In contrast, a combination of flucytosine, fluconazole, azithromycin, pentamidine, sulfadiazine, azithromycin, and miltefosine was used for the majority of cases of GAE due to B. mandrillaris (Table 3). In recent years, a combination of amphotericin B, fluconazole, rifampin, azithromycin, dexamethasone, and miltefosine was given for PAM (Table 3).
TABLE 2.
Use of various individual drugs for the treatment of GAE due to Acanthamoeba spp. or Balamuthia mandrillaris and PAM due to Naegleria fowleri
| Drug or drug classa | % of cases (no. of cases) |
||
|---|---|---|---|
| GAE due to Acanthamoeba (n = 46) | GAE due to B. mandrillaris (n = 29) | PAM due to N. fowleri (n = 11) | |
| Nonspecific | 19.5 (9) | 20.7 (6) | 18.2 (2) |
| Miltefosine | 15.2 (7) | 13.8 (4) | 0 |
| Pentamidine | 13 (6) | 31 (9) | 0 |
| Sulfadiazine | 19.5 (9) | 34.5 (10) | 18.2 (2) |
| Flucytosine | 13 (6) | 24.1 (7) | 0 |
| Macrolides (azithromycin or clarithromycin) | 17.4 (8) | 31 (9) | 0 |
| Azoles | 41.3 (19) | 48.3 (14) | 18.2 (2) |
| Carbapenems | 4.3 (2) | 3.4 (1) | 0 |
| Sulfonamides (trimethoprim-sulfamethoxazole) | 34.8 (16) | 3.4 (1) | 0 |
| Rifampin | 37 (17) | 6.9 (2) | 18.2 (2) |
| Chloramphenicol | 6.5 (3) | 0 | 9.1 (1) |
| Pyrimethamine | 2.2 (1) | 6.9 (2) | 9.1 (1) |
| Amphotericin B | 30.4 (14) | 10.3 (3) | 27.3 (3) |
| Glycopeptides (vancomycin) | 2.2 (1) | 0 | 0 |
| Tetracyclines | 0 | 3.4 (1) | 0 |
Nonspecific treatment included general measures to reduce intracranial pressure and inflammation (mannitol, decompressive craniotomy, and corticosteroids) and treatment for differential diagnosis (cephalosporins for bacterial meningitis). In cases of combinations of drugs, the therapeutic agents were calculated independently.
TABLE 3.
Selected cases of amoebic meningoencephalitis with successful outcomes
| Year | Patient description | Causative agent | Treatment |
|---|---|---|---|
| 2000 | 33-yr-old man | Acanthamoeba spp. | Sulfadiazine, pyrimethamine, and fluconazole; left homonymous hemianopia (visual field defects) |
| 2002 | 45-yr-old woman | Acanthamoeba spp. | Rifampin, co-trimoxazole, fluconazole, and ceftriaxone for 4 wk; monitored for 1 yr for facial nerve palsy |
| 2006 | 10-yr-old boy | Acanthamoeba spp. | Ketoconazole and rifampin; duration of therapy is unknown |
| 2008 | 25-yr-old man | Acanthamoeba spp. | Miltefosine; seronegative for Acanthamoeba after treatment but neurological deficits did not improve in monitoring for 24 mo |
| 2009 | 63-yr-old man with history of contact with contaminated water | Acanthamoeba spp. | Amphotericin B and rifampin; discharged after 78 days of hospitalization |
| 2011 | 2-yr-old boy with underlying acute lymphoblastic leukemia | Acanthamoeba spp. | Meropenem, teicoplanin, fosfomycin, metronidazole, and liposomal amphotericin B; symptom resolution |
| 2012 | Immunocompetent 38-yr-old man | Acanthamoeba spp. | Voriconazole and miltefosine; radiological and clinical relief after 6 days of treatment, with subsequent monitoring for refractory seizure complications |
| 2012 | 2-yr-old boy | Acanthamoeba spp. | Co-trimoxazole, rifampin, and ketoconazole; improvement after 2 days |
| 2014 | 30-yr-old man | Acanthamoeba spp. | Rifampin, sulfamethoxazole-trimethoprim, and fluconazole for 2 wk; asymptomatic after 2 wk of monitoring |
| 2016 | 2-yr-old boy | Acanthamoeba spp. | Ceftazidime, metronidazole, fluconazole, and rifampin for 3 wk |
| 2016 | 11-yr-old girl | Acanthamoeba spp. | Amphotericin B, sulfamethoxazole-trimethoprim, and rifampin |
| 2016 | 12-yr-old boy | Acanthamoeba spp. | Amphotericin B, sulfamethoxazole-trimethoprim, and rifampin |
| 2016 | 9-mo-old girl | Acanthamoeba spp. | Amphotericin B, sulfamethoxazole-trimethoprim, and rifampin |
| 2003 | 64-yr-old man | Balamuthia mandrillaris | Amphotericin B, flucytosine, fluconazole, and sulfadiazine for 5 yr, clarithromycin for 2 yr, and pentamidine for 18 days |
| 2003 | 5-yr-old girl | Balamuthia mandrillaris | Flucytosine and fluconazole for 2 yr, pentamidine for 34 days, and clarithromycin for 2 yr |
| 2004 | 72-yr-old woman | Balamuthia mandrillaris | Pentamidine, sulfadiazine, fluconazole, and clarithromycin; hospitalized for 13 days |
| 2004 | 72-yr-old man | Balamuthia mandrillaris | Fluconazole, sulfadiazine, clarithromycin, and pentamidine isethionate; duration of therapy is unknown |
| 2006 | 10-yr-old girl | Balamuthia mandrillaris | Albendazole, itraconazole, and sulfamethoxazole-trimethoprim for 6 mo |
| 2006 | 8-yr-old boy | Balamuthia mandrillaris | Albendazole and itraconazole for 14 mo |
| 2010 | 21-yr-old woman | Balamuthia mandrillaris | Albendazole and fluconazole for 7.5 mo and miltefosine for 7 mo |
| 2010 | 2-yr-old boy | Balamuthia mandrillaris | Pentamidine (stopped after 2 mo), sulfadiazine, flucytosine, clarithromycin, and fluconazole |
| 2010 | 27-yr-old man | Balamuthia mandrillaris | Sulfadiazine, azithromycin, and miltefosine for unspecified duration |
| 2011 | 27-yr-old male organ recipient | Balamuthia mandrillaris | Pentamidine, sulfadiazine, flucytosine, fluconazole, azithromycin, and miltefosine |
| 2011 | 80-yr-old woman | Balamuthia mandrillaris | Pentamidine, itraconazole, azithromycin, sulfadiazine, flucytosine, and liposomal amphotericin |
| 2013 | 5-yr-old girl | Balamuthia mandrillaris | Flucytosine, fluconazole, azithromycin, pentamidine, and sulfadiazine, changed to final regimen of azithromycin, fluconazole, and miltefosine |
| 2013 | 4-yr-old immunocompetent girl with history of water contact from floods around her residence | Balamuthia mandrillaris | Flucytosine, fluconazole, azithromycin, pentamidine, and sulfadiazine |
| 2002 | 26-yr-old woman | Naegleria fowleri | Rifampin, amphotericin B, and ornidazole for 2 wk |
| 2008 | 8-mo-old boy | Naegleria fowleri | Amphotericin B, chloramphenicol, and rifampin; afebrile at day 7 of treatment |
| 2013 | 12-yr-old girl and 8-yr-old boy | Naegleria fowleri | Amphotericin B, fluconazole, rifampin, azithromycin, dexamethasone, and miltefosine for both |
CHALLENGES AND OPPORTUNITIES
Free-living pathogenic amoebae are now well-recognized agents of brain infections leading to GAE and PAM. GAE is a chronic infection that can last up to several months, while PAM is an acute fulminant infection that lasts a few days (1, 6). It is intriguing to see the distinctive difference of the chronicity in pathogenicity of these amoebae. For example, Acanthamoeba and B. mandrillaris likely enter the host via the lower respiratory tract and/or skin breaks (1, 6). In contrast, N. fowleri enters the host via the nasal route. Recently, another route of entry has been included, i.e., via organ transplantations, with recipients of organ donations acquiring amoebic meningoencephalitis from the donor diagnosed postmortem with amoebic meningoencephalitis of the same genotype (15–18). This is important because amoebae are ubiquitous and nonresponsive to antibiotics, and organ recipients are already rendered immunosuppressed; therefore, any entry of these pathogenic free-living amoebae may have devastating consequences. Although data on risk factors were not available for all cases reviewed in this study, some factors were observed to dictate the susceptibility of patients to amoebic meningoencephalitis. For GAE due to Acanthamoeba, immunosuppression appeared to be a factor (1, 6, 19, 20), while B. mandrillaris was shown to infect immunocompetent individuals in addition to immunocompromised patients (1, 3). Preceding cutaneous lesions are often liable to GAE caused by both types of amoebae. PAM usually occurred in immunocompetent children and young adults (1, 6, 7). However, all patients had history of activities in or near fresh water sources such as swimming pools or hot springs, including recreational activities and religious practices such as ablution, or health care practices such as the use of neti pots. Eliciting a thorough patient history is absolutely paramount for the accurate diagnosis of PAM, and public health preventive measures such as water treatment should be undertaken for high-risk populations.
Neuroimaging studies revealed the locations of lesions in the frontal, parietal, and temporal lobes in most cases of GAE, but lesions in the frontal lobe were much more frequent for N. fowleri. Neuroimaging modalities can have false-negative results, however, and the specificity of neuroimaging for the diagnosis of amoebic meningoencephalitis has yet to be evaluated. In the absence of accurate diagnoses and effective treatments, both diseases often result in death. N. fowleri was found in the CSF more often than the other two types of amoebae, most likely due to its motile flagellated form. However, diagnosis with biopsy samples may be hindered by the inoculum size and the magnitudes of inflammation and necrosis in the tissue sections. In addition to the aforementioned factors, to untrained eyes the morphology of trophozoites in tissue sections bears a close resemblance to that of macrophages, which are also common in acute inflammatory responses. The other challenges in diagnosis include a wide spectrum of differential diagnoses, e.g., brain tumors, multiple sclerosis, lupus encephalitis, progressive multifocal leukoencephalopathy, stroke, meningitis from other causes (viral, tuberculous, or pyogenic), and cerebral toxoplasmosis (1, 6). A recent case of cerebral toxoplasmosis complicated by GAE caused by both Acanthamoeba and B. mandrillaris highlighted the complex nature of the disease, especially because both types of amoebae are known to act as reservoir hosts for many microorganisms (1, 6, 21–23). It is more intriguing that Acanthamoeba and B. mandrillaris meningoencephalitis cases present as vascular diseases (masquerading as cerebral vascular occlusions or aneurysms). This is most likely due to the ability of amoebae to produce endothelial damage, resulting in cytokine release, crossing of the blood-brain barrier, granulomatous inflammation, thromboembolic events, increased vascular permeability, and ultimately necrosis.
For chemotherapeutic strategies, current available delivery routes include intravenous, oral, and intrathecal administration. Systemic antimicrobial treatment has its limitations, however, due to its adverse effects and reduced delivery, together with delayed diagnosis. Other concerns include poor pharmacodynamic and pharmacokinetic profiles of available drugs, solubility, CNS penetration, drug-drug interactions, patient medical conditions, patient tolerance, and Acanthamoeba susceptibility to amoebicidal agents (24). In the case of PAM, the amphotericin B deoxycholate preparation is preferable to the liposomal formulation against N. fowleri infections, although it has no effect on Acanthamoeba or B. mandrillaris (25, 26). More recently, miltefosine has shown promising results with respect to bioavailability and drug-drug interactions (25). Of note, the major groups of azoles and macrolides are amoebistatic rather than amoebicidal. Additionally, nephrotoxic and hepatotoxic effects due to the use of drugs among patients with compromised renal and liver functions (such as transplant patients) may further complicate treatment. Potential drug delivery systems that directly target the inoculation sites of amoebae by circumventing the need for optimal blood-brain barrier penetration should be the focus of future studies, to increase the odds of survival for patients with PAM while minimizing adverse effects and disease complications. Overall, a complete understanding of the pathogenic mechanisms and the role of the immune system and development of novel chemotherapeutic approaches to drug delivery (27, 28) are important for the rational development of antiamoebic therapy.
CONCLUDING REMARKS
Despite advances in clinical recognition, diagnostic methods, and treatment approaches, the mortality rates associated with CNS infections due to amoebae have remained high. Although neuroimaging findings reveal common areas for lesions, the locations may not be consistent and may vary depending on the causative agent. High levels of clinical suspicion are important, especially for refractory cases of meningoencephalitis, for rapid diagnosis of the infection, which is a prerequisite for successful treatment. The fact that only a few individuals, among all of the hosts exposed to these amoebae, develop infections suggests the possible presence of underlying predisposing factors. Future research is needed to define genetic, immunological, pathogenic, and environmental factors that contribute to deadly amoebic meningoencephalitis. Moreover, the abilities of pathogenic amoebae to host other microbial pathogens as reservoirs and to act as hyperparasites have enhanced their capacity as pathogens of increasing importance to human and animal health.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Sunway University (Malaysia).
We declare no conflicts of interests regarding the publication of this paper.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.02300-16.
REFERENCES
- 1.Visvesvara GS, Moura H, Schuster FL. 2007. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol 50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- 2.Khan NA. 2006. Acanthamoeba: biology and increasing importance in human health. FEMS Microbiol Rev 30:564–595. doi: 10.1111/j.1574-6976.2006.00023.x. [DOI] [PubMed] [Google Scholar]
- 3.Matin A, Siddiqui R, Jayasekera S, Khan NA. 2008. Increasing importance of Balamuthia mandrillaris. Clin Microbiol Rev 21:435–448. doi: 10.1128/CMR.00056-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marciano-Cabral F. 1988. Biology of Naegleria spp. Microbiol Rev 52:114–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Jonckheere JF. 2011. Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri. Infect Genet Evol 11:1520–1528. doi: 10.1016/j.meegid.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Visvesvara GS. 2010. Free-living amebae as opportunistic agents of human disease. J Neuroparasitol 1:N100802. doi: 10.4303/jnp/N100802. [DOI] [Google Scholar]
- 7.Siddiqui R, Khan NA. 2014. Primary amoebic meningoencephalitis caused by Naegleria fowleri: an old enemy presenting new challenges. PLoS Negl Trop Dis 8:e3017. doi: 10.1371/journal.pntd.0003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Jonckheere JF. 2014. What do we know by now about the genus Naegleria? Exp Parasitol 145(Suppl):S2–S9. doi: 10.1016/j.exppara.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Schumacher DJ, Tien RD, Lane K. 1995. Neuroimaging findings in rare amoebic infections of the central nervous system. AJNR Am J Neuroradiol 16:930–935. [PMC free article] [PubMed] [Google Scholar]
- 10.Callicott JH., Jr 1968. Amoebic meningoencephalitis due to free-living amebas of the Hartmannella (Acanthamoeba)-Naegleria group. Am J Clin Pathol 49:84–91. doi: 10.1093/ajcp/49.1.84. [DOI] [PubMed] [Google Scholar]
- 11.Kastrup O, Wanke I, Maschke M. 2005. Neuroimaging of infections. Neurotherapeutics 2:324–332. doi: 10.1602/neurorx.2.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh P, Kochhar R, Vashishta RK, Khandelwal N, Prabhakar S, Mohindra S, Singhi P. 2006. Amoebic meningoencephalitis: spectrum of imaging findings. AJNR Am J Neuroradiol 27:1217–1221. [PMC free article] [PubMed] [Google Scholar]
- 13.Michinaga S, Koyama Y. 2015. Pathogenesis of brain edema and investigation into anti-edema drugs. Int J Mol Sci 16:9949–9975. doi: 10.3390/ijms16059949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guarner J, Bartlett J, Shieh WJ, Paddock CD, Visvesvara GS, Zaki SR. 2007. Histopathologic spectrum and immunohistochemical diagnosis of amoebic meningoencephalitis. Mod Pathol 20:1230–1237. doi: 10.1038/modpathol.3800973. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. 2011. Balamuthia mandrillaris transmitted through organ transplantation—Mississippi, 2009. Am J Transplant 11:173–176. doi: 10.1111/j.1600-6143.2010.03395_1.x. [DOI] [Google Scholar]
- 16.Roy SL, Metzger R, Chen JG, Laham FR, Martin M, Kipper SW, Smith LE, Lyon GM, Haffner J, Ross JE, Rye AK. 2014. Risk for transmission of Naegleria fowleri from solid organ transplantation. Am J Transplant 14:163–171. doi: 10.1111/ajt.12536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basavaraju SV, Kuehnert MJ, Zaki SR, Sejvar JJ. 2014. Encephalitis caused by pathogens transmitted through organ transplants, United States, 2002–2013. Emerg Infect Dis 20:1443. doi: 10.3201/eid2009.131332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orozco L, Hanigan W, Khan M, Fratkin J, Lee M. 2011. Neurosurgical intervention in the diagnosis and treatment of Balamuthia mandrillaris encephalitis: report of 3 cases. J Neurosurg 115:636–640. doi: 10.3171/2011.4.JNS102057. [DOI] [PubMed] [Google Scholar]
- 19.Doan N, Rozansky G, Nguyen HS, Gelsomino M, Shabani S, Mueller W, Johnson V. 2015. Granulomatous amebic encephalitis following hematopoietic stem cell transplantation. Surg Neurol Int 6(Suppl 18):S459–S462. doi: 10.4103/2152-7806.166788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khurana S, Mewara A, Verma S, Totadri SK. 2012. Central nervous system infection with Acanthamoeba in a malnourished child. BMJ Case Rep doi: 10.1136/bcr-2012-007449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietrucha-Dilanchian P, Chan JC, Castellano-Sanchez A, Hirzel A, Laowansiri P, Tuda C, Visvesvara GS, Qvarnstrom Y, Ratzan KR. 2012. Balamuthia mandrillaris and Acanthamoeba amoebic encephalitis with neurotoxoplasmosis coinfection in a patient with advanced HIV infection. J Clin Microbiol 50:1128–1131. doi: 10.1128/JCM.06252-11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Siddiqui R, Khan NA. 2012. Biology and pathogenesis of Acanthamoeba. Parasit Vectors 5:6. doi: 10.1186/1756-3305-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapia JL, Torres BN, Visvesvara GS. 2013. Balamuthia mandrillaris: in vitro interactions with selected protozoa and algae. J Eukaryot Microbiol 60:448–454. doi: 10.1111/jeu.12052. [DOI] [PubMed] [Google Scholar]
- 24.Grace E, Asbill S, Virga K. 2015. Naegleria fowleri: pathogenesis, diagnosis, and treatment options. Antimicrob Agents Chemother 59:6677–6681. doi: 10.1128/AAC.01293-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuster FL, Guglielmo BJ, Visvesvara GS. 2006. In-vitro activity of miltefosine and voriconazole on clinical isolates of free-living amebas: Balamuthia mandrillaris, Acanthamoeba spp. and Naegleria fowleri. J Eukaryot Microbiol 53:121–126. doi: 10.1111/j.1550-7408.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 26.Ferrante A. 1982. Comparative sensitivity of Naegleria fowleri to amphotericin B and amphotericin B methyl ester. Trans R Soc Trop Med Hyg 76:476–478. doi: 10.1016/0035-9203(82)90141-9. [DOI] [PubMed] [Google Scholar]
- 27.Linam WM, Ahmed M, Cope JR, Chu C, Visvesvara GS, da Silva AJ, Qvarnstrom Y, Green J. 2015. Successful treatment of an adolescent with Naegleria fowleri primary amoebic meningoencephalitis. Pediatrics 135:e744–e748. doi: 10.1542/peds.2014-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diestel A, Roessler J, Berger F, Schmitt KR. 2008. Hypothermia downregulates inflammation but enhances IL-6 secretion by stimulated endothelial cells. Cryobiology 57:216–222. doi: 10.1016/j.cryobiol.2008.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.