ABSTRACT
Malaria is a potentially life-threatening disease requiring rapid diagnosis and treatment. Although microscopic examination of thick and thin blood films remains the gold standard for laboratory diagnosis, rapid antigen tests and nucleic acid amplification methods may also play a useful role in detection of acute infection. This review discusses the advantages and disadvantages of the commonly used diagnostic methods and provides important practice points for optimal malaria test utilization.
KEYWORDS: diagnostics, malaria, utilization
CAUSAL AGENTS
Malaria is a potentially life-threatening disease caused by apicomplexan parasites in the genus Plasmodium (1–3). Human infection occurs throughout much of the tropics and subtropics and is caused primarily by four species: P. falciparum, P. vivax, P. ovale, and P. malariae. In parts of Southeast Asia, the zoonotic parasite P. knowlesi also causes a high proportion of locally acquired cases (1). Another nonhuman primate Plasmodium species, P. cynomolgi, was also reported as a rare cause of human malaria in southeast Asia (4). Plasmodium simium and P. brasilianum were documented from human patients in South America, although there is increasing evidence that these species actually represent P. vivax and P. malariae, respectively, which adapted to nonhuman primates after being introduced to South America (4). Plasmodium falciparum is responsible for the majority of malaria-related deaths, with far fewer deaths associated with P. vivax and, rarely, other species (1, 2).
MALARIA TODAY
Malaria remains an important cause of morbidity and mortality worldwide (3, 5). The World Health Organization (WHO) estimates that 212 million cases occurred during 2015 (range, 148 million to 304 million), with 429,000 deaths (range 235,000 to 639,000) (3). The majority of infections occur in Africa (90%), followed by the southeast Asia region (6%), and the eastern Mediterranean region (2%) (3). While there has been a 29% decrease in malaria mortality rates since 2010, significant work is still needed to meet the WHO global targets of reducing malaria case incidence and mortality rates by ≥90% by 2030 (6).
In settings in which malaria is not endemic, such as the United States, infection is seen primarily among individuals who have traveled to, or emigrated from, regions with ongoing malaria transmission (i.e., “imported malaria”) (7). The Centers for Disease Control and Prevention (CDC) received reports of 1,727 malaria cases in 2013 with 10 associated fatalities; this is a 2% increase from the number of cases reported in 2012 and the highest number of malaria-associated deaths since 2001, underscoring the need for considering malaria in the clinical diagnosis and maintaining expertise in malaria diagnosis in the United States (7). Plasmodium falciparum was identified as the causative agent in 60.8% of cases, followed by P. vivax (14.1%), P. ovale (3.7%), and P. malariae (2.5%), and the causes of the remaining cases were undetermined (16.6%) or mixed (2.3%).
DIAGNOSIS
The clinical features of malaria are nonspecific and overlap significantly with those of other febrile illnesses. Therefore, the WHO recommends that all patients have a parasite-specific laboratory test performed to confirm the clinical impression (8). Laboratory testing options vary based on the geographic and clinical setting. While a variety of options may be available in high-income countries, resource-poor settings of malaria endemicity often have limited options, and test results may not be reliable due to a lack of staff training and quality assurance measures. Given the limitations in these settings, this update focuses on testing options that are widely available in middle- to high-income settings and provides guidance on how these different methods may be employed for routine patient care.
Regardless of the method used, testing should be available and performed on a STAT basis 24 hours/day, 7 days/week due to the potentially life-threatening nature of the infection. The most commonly used methods for laboratory diagnosis of malaria are microscopic examination of stained blood films and detection of parasite antigen or nucleic acid (2, 9). Of these, microscopic examination of thick and thin blood films remains the gold standard for malaria diagnosis. Rapid antigen detection methods and molecular amplification tests are also increasingly employed for malaria diagnosis and are useful adjunctive tests. Tests for detection of antiplasmodial antibodies are commercially available but are not recommended for diagnosis of acute disease. The details of these methodologies and their clinical utility are discussed below and summarized in Table 1, while Fig. 1 provides a laboratory testing algorithm. It is important to note that examination of a single set of blood films may be insufficient for malaria diagnosis, particularly with low levels of parasitemia. The Clinical and Laboratory Standards Institute (CLSI) recommends that repeat blood films be obtained and examined every 6 to 8 h for up to 3 days (if clinically indicated) until malaria is definitively excluded from the differential diagnosis (10). Similarly, the CDC recommends that blood smears in nonimmune individuals be repeated every 12 to 24 h for a total of 3 evaluations before ruling out malaria (11). To our knowledge, no similar guidance exists for use of other methodologies.
TABLE 1.
Comparison of microscopy, rapid antigen detection, nucleic acid amplification methods, and serologic tests
| Method | Practical use | Advantage(s) | Disadvantages |
|---|---|---|---|
| Microscopy | Gold standard for detecting and identifying Plasmodium spp. | Allows for detection and identification to the species level of all species Allows for parasite quantification More sensitive than RDTsa Relatively inexpensive Can be used for monitoring treatment success |
Subjective Delays in processing can result in changes in morphology that may hinder reliable identification Challenging to train morphologists and maintain their competency Less sensitive than NAATsb Species-level identification may be difficult at lower parasitemia rates Mixed infections may be missed |
| Antigen RDTs | Rapid screening while other methodologies (e.g., microscopy) are pending Presumptive diagnosis of P. falciparumc |
Rapid (faster than microscopy or NAATs) Presumptive diagnosis of P. falciparum Less subjective than microscopy Low complexity; requires minimal training of personnel |
Not reliable for non-falciparum speciesc Less sensitive than microscopy or NAATs Generally requires confirmation by other methods More expensive than microscopy May not detect HRPII- negative strains of P. falciparum from South America Does not allow for quantification Should not be used to evaluate treatment success |
| NAATs | Detecting mixed infectionsd Detecting cases with low parasitemiad Identifying species when parasite morphology is inadequate for microscopic identification Resolving discrepant results from other methodologies |
More sensitive than microscopy and RDTsd Less subjective than microscopy Allows detection and species-level identification Superior for detecting mixed infectionsd Requires less training of personnel than does microscopy Allows for quantification (real-time PCR) Provides detection of polymorphisms associated with drug resistanced |
Expensive High-complexity method Not usually performed on a STAT basis Limited availability Quantification may not correlate with microscopy-determined percent parasitemia Should not be used to evaluate treatment success |
| Serologic tests | Epidemiologic surveys and research Evaluating febrile patients with recent travel to areas of endemicity who are repeatedly smear negative Supporting the diagnosis of suspected tropical splenomegaly syndrome Screening blood donors Evaluating donors in suspected transfusion-associated cases |
May be positive in cases where parasites are not seen on peripheral blood smears | Not appropriate for detection of acute disease due to time it takes for antibodies to reach detectable levels Cannot differentiate between past and current infections (especially in patients native to areas of endemicity) Not always reliable for species-level identification May be time-consuming and labor intensive Not often available except at specialized reference laboratories |
RDT, rapid diagnostic test.
NAAT, nucleic acid amplification test.
Depends on the kit used; applicable to the BinaxNOW Malaria test.
Method dependent.
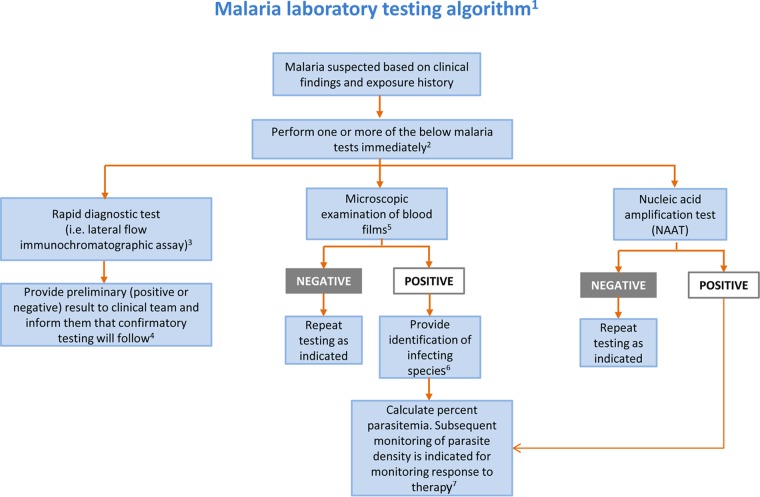
FIG 1.
Malaria laboratory testing algorithm. 1Algorithm includes only commonly used/available laboratory methods. Serology should not be used for detection of acute malaria. 2Malaria can be a rapidly fatal disease, particularly when due to P. falciparum, and less commonly P. vivax and P. knowlesi, and testing must be performed on a STAT basis. If testing is not available at the local laboratory, then arrangements must be made with another nearby laboratory that can provide immediate testing. A single negative test does not rule out malaria. Perform testing every 6 to 8 h for up to 3 days if clinically indicated. Other laboratory tests (e.g., complete blood count with differential, electrolyte panel, blood glucose, bilirubin, urinalysis, blood cultures) may be indicated to assess the severity of malaria and evaluate other potential causes of the patient's illness. 3Rapid screening tests such as lateral flow immunochromatographic assays generally provide sensitive detection of high levels of P. falciparum and P. vivax infection (i.e., 2,000 parasites/μl) but lack sufficient sensitivity for detecting low levels of parasitemia (i.e., ≤200 parasites/μl) and other Plasmodium species. 4Confirmatory testing may be performed by microscopic examination of blood films or NAAT. 5Examination of both thick and thin blood films is the gold standard for malaria diagnosis. 6If necessary, refer for confirmation of species identification by blood film microscopy or NAAT at a reference laboratory. 7Percent parasitemia is calculated using microscopic examination of thick or thin blood films. (Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved.)
Inherent to any laboratory test is the need for a strong quality assurance program, including measures for adequate staff training, competency assessment, quality control, and proficiency testing. Proficiency testing programs are available commercially for both blood film microscopy and antigen detection methods. In the absence of commercial testing programs, laboratories should use an alternative assessment of proficiency.
Morphological diagnosis.
Morphological diagnosis may be accomplished using both light- and fluorescence-based microscopic methods (2, 9, 10). While fluorescent stains such as acridine orange may decrease screening time, they are nonspecific and may be difficult to interpret. Therefore, the discussion below focuses on morphological diagnosis using light microscopy. Regardless of the method, proper collection, smear preparation, and staining are crucial for an accurate and reliable morphological diagnosis.
(i) Blood collection.
Blood specimens should be collected without delay, ideally before antimalarial treatment is initiated. Capillary blood obtained via finger stick and venous blood obtained via venipuncture are both adequate for malaria diagnosis. The finger stick method is generally considered preferable, as anticoagulants in blood collected by venipuncture might alter parasite morphology. However, blood is more commonly obtained via venipuncture in settings in which malaria is not endemic; when this method is used, the preferred anticoagulant is EDTA. Blood films should be made as soon as possible after collection to avoid prolonged exposure to EDTA.
(ii) Smear preparation.
Both thick and thin blood films should be made whenever possible, since the thick film provides the greatest sensitivity for malaria screening while the thin film provides the best detection of morphology for parasite species identification. The thick film consists of 1 to 2 drops of blood spread into a circle of 1.5 to 2.0 cm in diameter. The final film is approximately 20 to 30 cell layers thick and, when dry, should be of a thickness through which newsprint can just barely be read. The erythrocytes are lysed when placed into the relatively hypotonic stain solution, releasing intraerythrocytic stages (i.e., trophozoites, gametocytes, intact schizonts) and thus allowing a large volume of blood to be examined in a single film. In comparison, the thin film is made using a single drop of blood that is spread in a layer such that the thickness decreases progressively toward the feathered edge. The thin film is fixed in absolute methanol and allowed to dry completely prior to staining and therefore the erythrocytes remain intact. In the feathered edge, the cells should be in a monolayer with little to no overlap. Thick and thin films can be made on the same slide or separate slides (11, 12). If thin and thick films are made on the same slide, it is very important not to allow the thick film to be exposed to methanol or methanol vapors. Otherwise, the thick film may become inadvertently fixed and possibly rendered inadequate for reading (11, 12).
It is important to let both the thick and thin smears dry completely before fixing and/or staining. Thick films take longer to dry than thin films, and this could result in a delay of the diagnosis. A scratch method was developed as an alternative method for making thick films that allows for improved adherence and faster turnaround times (13). The process is similar to that of making a normal thick film, but the edge of a glass microscope slide is used for spreading the blood, while simultaneously firm pressure is applied to create small scratches in the underlying slide. The scratches allow for improved adherence of the blood film to the slide without affecting the smear morphology. The smear can then be stained as soon as it is dry, generally within 20 to 30 min of smear preparation (13).
(iii) Staining.
Thick and thin films for malaria diagnosis are typically stained with Giemsa, Wright, or combined Wright-Giemsa stain (10, 12). The Wright-Giemsa stain is commonly used in the United States, as it can be used in automated hematology systems. However, the pH of this method (approximately 6.8) does not adequately highlight cytoplasmic inclusions (e.g., Schüffner's stippling and Maurer's clefts), which are useful for determining the infecting species. Therefore, the recommended stain for species identification is Giemsa with a pH at or around 7.2. Field stains may be helpful for rapid diagnosis of malaria but are not recommended for routine diagnosis in settings in which malaria is not endemic. Various recipes and methodologies for staining are available (9–12, 14). The CDC provides additional guidance for preparing and examining blood films when there is a clinical suspicion of Ebola virus disease (see https://www.cdc.gov/vhf/ebola/healthcare-us/laboratories/safe-specimen-management.html).
(iv) Microscopic examination.
The thick film should be examined first for parasites, and the thin film used to identify organisms to the species level. Both the thick and thin films should be examined at ×100 magnification with oil immersion for a minimum of 100 fields, and up to at least 300 fields for immunologically naive patients (i.e., those without previous Plasmodium exposure), as they may present with symptoms at a lower level of parasitemia. The region of the thin film that provides the best parasite morphology is within the feathered edge, where erythrocytes have minimal overlap and maintain their central pallor. Parasite morphology may be significantly distorted outside this region, which may lead to erroneous species identification.
When examined by knowledgeable microscopists under optimal conditions, the thick film has a reported detection threshold of 10 to 50 parasites/microliter of blood (approximately 0.001% parasitemia, assuming an erythrocyte count of 5 × 106 cells/μl) (15). The sensitivity is generally lower under field conditions, with reported detection thresholds of 100 to 500 parasites/μl of blood (15).
(v) Identification.
Because the different Plasmodium species are clinically managed differently, it is important to identify the causal agent to the species level and rule out morphologically similar organisms, such as Babesia. The morphological specifics are beyond the scope of this work; however, we recommend several publications that may assist the technologist in making an accurate diagnosis (2, 4, 9, 11, 14, 16, 17). The treatise by Coatney et al. (4) is available for free from the CDC on both the DPDx website (11) and as a CD-ROM (see http://www.cdc.gov/dpdx/CDProducts.html).
If species-level identification cannot be performed by the local laboratory, the specimen should be forwarded to a reference laboratory (commercial lab, county or state public health lab, or the CDC) for further study. In addition, the CDC's Division of Parasitic Diseases and Malaria offers a telediagnostic service (http://www.cdc.gov/dpdx/contact.html) that can provide a rapid diagnosis, usually to the species level (11). A cost-savings analysis has showed that the CDC's telediagnostic service is one-twentieth the cost of normal specimen submissions to the CDC and can provide results within 1 to 2 h during normal business hours of operation (18, 19). In a study evaluating data between October 2005 and September 2010, the CDC's telediagnostic service was able to confidently obtain a species-level identification in 70% of the cases (n = 1192) (18). While awaiting confirmatory identification, the clinical team should be notified of the potential diagnosis, and treatment begun if indicated. Given the potentially life-threatening nature of P. falciparum infection, it is important to communicate with the clinical team when P. falciparum cannot be excluded from the differential diagnosis so that appropriate treatment can be started while awaiting the definitive species identification.
(vi) Quantification.
The quantification of malaria parasites can be used to make clinical management decisions as well as for monitoring response to treatment. Quantification can be performed using the thick or thin film (1, 4, 14). For quantification using the thin film, the number of infected erythrocytes among 500 to 2,000 erythrocytes viewed in successive fields is counted and expressed as percent parasitemia according to the equation % parasitemia = (parasitized red blood cells [RBCs]/total RBCs) × 100.
As with species determination, percent parasitemia should be calculated based on observations from the feathered edge monolayer, where there is little to no overlap of cells. When parasitized cells are counted, it is important to count multiply infected RBCs only once. Gametocytes are not counted, as they are a dead-end stage in the human host. Also, since some drugs are not gametocidal, the presence of gametocytes cannot be used to accurately monitor the effectiveness of treatment. For quantification using the thick film, the numbers of parasites and white blood cells (WBCs) are counted in successive fields until 500 parasites or 1,000 WBCs have been tallied (whichever comes first). The results are expressed as the number of parasites per microliter of blood using a predetermined WBC count (if the WBC count is not known, the results can be calculated with an assumption of 8,000 WBCs/μl of blood) according to the equation parasites/ml of blood = (parasites/WBCs) × WBC count (or 8,000 cells)/ml of blood.
(vii) Monitoring response to therapy.
Examination of serial blood smears with parasite quantification is recommended for monitoring response to therapy, looking for decrease in percent parasitemia and eventual parasite clearance (1). The frequency of monitoring is generally based on the clinical severity of the patient. Daily (or more frequent) testing is recommended initially for patients with severe malaria, with the caveat that the degree of parasitemia may rise during the initial 12 to 24 h since commonly used antimalaria drugs do not inhibit release of merozoites from circulating schizonts (20). Increasing parasite loads after 36 to 48 h are indicative of treatment failure due to parasite resistance (20). It may take 6 days or longer for parasite forms (not including gametocytes) to become undetectable on blood films. Therefore, repeat testing is generally recommended, at minimum, on days 7 and 28 after illness onset (20).
Antigen detection.
Several antigen rapid detection tests (RDTs) are commercially available and are increasingly used for malaria diagnosis worldwide (21, 22). These methods are lateral flow assays (cassette, dipstick, or card formats) consisting of a nitrocellulose membrane with bound parasite antigens. Commonly used antigens are P. falciparum histidine-rich protein-II (HRPII), Plasmodium spp. lactate dehydrogenase, and Plasmodium spp. aldolase. Depending on the format and number of antigens present, assays may detect to the Plasmodium genus level only, or they may detect specific species (e.g., P. falciparum, P. vivax).
The WHO, in conjunction with the CDC, the Foundation for Innovative New Diagnostics, and the Special Programme for Research and Training in Tropical Diseases, completed extensive comparisons of commercially available RDTs in 6 separate rounds and found significant disparities in performance characteristics among available methods (21). While many RDTs were capable of detecting P. falciparum at a high level of parasitemia (2,000 or 5,000 parasites/μl of blood), the sensitivities were often significantly lower for non-falciparum species or for low levels of parasitemia (200 parasites/μl). Clinicians and laboratorians must therefore be familiar with the RDT used in their facilities and its associated performance characteristics.
At this time, the BinaxNOW Malaria test (Alere, Waltham, MA) is the only test cleared by the Food and Drug Administration (FDA) for in vitro diagnosis of malaria in the United States. This test targets P. falciparum-specific HRPII and an unspecified pan-malaria antigen common to P. falciparum, P. vivax, P. malariae, and P. ovale. According to the package insert, the BinaxNOW test has sensitivities for the detection of P. falciparum and P. vivax of 100% and 81.6%, respectively, using blood obtained by venous draw (23). The sensitivity of the BinaxNOW test has also been shown to vary by the degree of parasitemia; WHO product testing using wild-type (clinical) samples revealed sensitivities of 100% and 85% for detection of high levels of P. falciparum and P. vivax, respectively, and only 85% and 10% for detection of low levels of P. falciparum and P. vivax, respectively (21). The BinaxNOW test has also been shown to be an insensitive method for detecting other species, with reported sensitivities less than 30% for P. ovale (Western Africa) and P. knowlesi (24, 25).
Several other RDTs that were evaluated in the WHO trials obtained superior sensitivity rates for detection of high and low P. falciparum and P. vivax densities. The reader is referred to the free online results of the WHO trials for a full list of the assays evaluated (21). It is important to note that some of the tests used in the WHO evaluation program have been updated or discontinued since the original data were generated, and not all commercially available RDTs were represented. New RDTs continue to be developed and may provide additional advantages over existing tests.
Given the significantly lower sensitivities for non-falciparum species and for detection of low levels of parasitemia (all species), the BinaxNOW Malaria test should be used for malaria diagnosis only in conjunction with other laboratory tests (e.g., blood film examination) and the clinical findings (23). This holds true for use of many other commercial malaria RDTs as well, particularly for non-falciparum infections. A common use of RDTs in settings in which malaria is not endemic is for preliminary malaria diagnosis when experienced microscopists are not available (e.g., night shift, small laboratories); confirmatory blood film examination is generally performed the following day. In settings of malaria endemicity, RDTs are often used alone without confirmatory blood smear examination, given the lack of resources and reliable microscopy. While not optimal, this strategy is superior to the use of clinical diagnosis alone and is generally sufficient for identifying clinically significant infections with P. falciparum when a suitably sensitive RDT is used.
Some other important caveats to RDT use bear mentioning. First, strains of P. falciparum lacking the pfhrp2 gene were described in Peru and, more recently, in Africa, and therefore tests targeting the HRP2 antigen may produce false-negative results (26). Furthermore, RDT sensitivity for detecting malaria in pregnant women may be decreased, possibly due to sequestration of antigens in the placental circulation (27). Finally, antigens may remain in the bloodstream after successful treatment and therefore RDTs should not be used to evaluate the efficacy of antimalarial therapy (11).
Nucleic acid detection.
Multiple methods have been described for detection of Plasmodium spp. nucleic acid detection, including DNA/RNA hybridization, conventional and real-time PCR, loop-mediated isothermal amplification (LAMP), and nucleic acid sequence-based amplification (NASBA) (2, 28). The preferred specimen type is whole blood (1 to 5 ml) collected in EDTA (2, 22, 23). Another suitable method is collection of fresh blood (e.g., from a finger prick) onto specially designed filter paper. Specimens collected in this manner are used primarily for research and epidemiologic studies and can be archived for future DNA extraction and amplification testing (29). No tests are currently cleared or approved by the FDA, but multiple laboratory-developed tests have been described and several PCR-based kits are commercially available outside the United States. The 18S small subunit rRNA gene is the most commonly used target for amplification and detection (28). Many real-time PCR assays employ different primers and probes to detect each species, using nested, seminested, and single-tube multiplex reactions. These assays take advantage of polymorphisms in the 18S rRNA gene that allow for specific detection of each species without the need for subsequent sequencing. Assays targeting a single conserved region of the 18S rRNA gene can also be used, with species differentiation performed using melting temperature analysis (28). Although there is significant variation in the performance characteristics of reported tests, nucleic acid amplification tests (NAATs) generally provide superior sensitivity over other methods, with reported detection thresholds of <10 parasites/μl. Authors at the CDC have described a nested conventional PCR with a detection threshold of at least 6 parasites/μl blood, while a highly cited real-time multiplex PCR using TaqMan probes has reported sensitivities of 0.7, 1.5, and 4.0 parasites/μl for P. falciparum, P. ovale, and P. vivax, respectively (28). Other assays report sensitivities as low as 0.002 parasites/μl. The reader is referred to a review on this topic for a list of assays described as of 2013 (28). New assays, and refinements to existing assays, continue to be described in this rapidly evolving field, and some have been adapted for use in resource-limited settings.
The advantages and disadvantages of NAATs for diagnosis of acute malaria are listed in Table 1. Of these, important disadvantages include their high cost and high complexity, which hinder the widespread implementation of molecular amplification methods, particularly in low-resource settings. Also, NAAT results are not usually available in a time frame that is conducive for acute patient management. Unless testing can be performed within several hours of specimen collection, the laboratory must have an alternative method (e.g., RDT or blood film examination) for rapid laboratory detection of malaria. Regardless of the method used, blood film examination is still indicated for positive patients to calculate the percentage of parasitemia. For these reasons, the primary roles of NAATs are for confirmation of the infecting species (particularly when the parasite morphology is suboptimal), detection of low levels of parasitemia, and enhanced detection/confirmation of mixed infections. Of note, nucleic acid may remain in the bloodstream after successful treatment and therefore NAATs should not be used to evaluate the efficacy of antimalarial therapy (11). The CDC's Division of Parasitic Diseases and Malaria provides malaria PCR testing at no cost in select situations upon consultation, and testing is also available at select reference laboratories. The CDC also offers molecular characterization using PCR and gene sequencing to detect known mutations associated with resistance to select antimalarials. Testing for drug resistance is recommended by the CDC for all cases diagnosed in the United States; however, the CDC's resistance testing is currently for epidemiologic purposes only and is not used for clinical management of patients (1).
As with other methodologies, regular proficiency testing should be performed for NAATs. Unfortunately, there are currently no commercial options available and so testing laboratories may have to rely on in-house proficiency testing programs.
Antibody detection.
Serologic testing is generally not recommended for routine diagnosis of malaria except for a few scenarios, such as febrile patients with travel to areas of endemicity who are repeatedly smear negative (especially if immunologically naive) and diagnosis of cases of suspected topical splenomegaly syndrome. Serologic testing is also the primary modality for screening blood donors and is commonly used for evaluating donors in cases of suspected transfusion-transmitted malaria. Most available tests are either immunofluorescence assays or enzyme immunoassays and may be performed on serum or plasma derived from blood samples collected in EDTA (11, 30).
REPORTING CRITERIA
Malaria is nationally reportable in the United States. All laboratory-confirmed cases are to be reported to the local state or territorial health department. Epidemiological and clinical data on confirmed malaria cases in the United States are transmitted to the CDC via the National Malaria Surveillance System (NMSS) (see https://www.cdc.gov/malaria/report.html).
SUMMARY
Malaria is a potentially life-threatening disease that requires rapid diagnosis and treatment. Testing should be available continuously throughout the day and night on a STAT basis; laboratories that cannot provide definitive testing (i.e., blood film examination or molecular amplification methods) must provide options for rapid examination elsewhere or offer preliminary testing (e.g., RDT) with confirmatory testing shortly afterward (ideally within 8 h). Microscopic examination of thick and thin blood films, particularly using Giemsa stain (pH 7.2), remains the gold standard for laboratory detection of malaria and identification of the infecting species, although molecular amplification methods are also suitable. Regardless of the method, all positive results should be accompanied by calculation of percent parasitemia using blood film examination. All laboratory-confirmed cases should be reported to the local state or territorial health department.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2017. Malaria. https://www.cdc.gov/malaria/. Accessed 2 April 2017.
- 2.Pritt BS. 2015. Plasmodium and Babesia, p 2338–2356. In Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 11th ed, vol 2 ASM Press, Washington, DC. [Google Scholar]
- 3.World Health Organization. 2016. World malaria report 2016. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world-malaria-report-2016/report/en/ Accessed 2 April 2017. [Google Scholar]
- 4.Coatney GR, Collins WE, Contacos PG. 1971. The primate malarias. U.S. National Institute of Allergy and Infectious Diseases, Bethesda, MD. [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2016. CDC health information for international travel 2016. Oxford University Press, New York, NY. [Google Scholar]
- 6.World Health Organization. 2015. Global technical strategy for malaria 2016-2030. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/9789241564991/en/ Accessed 2 April 2017. [Google Scholar]
- 7.Cullen KA, Mace KE, Arguin PM. 2016. Malaria surveillance—United States, 2013. MMWR Surveill Summ 65:1–22. doi: 10.15585/mmwr.ss6502a1. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. 2015. Guidelines for the treatment of malaria, 3rd ed World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/9789241549127/en/ Accessed 18 March 2017. [Google Scholar]
- 9.Ash LR, Orihel TC. 2007. Ash and Orihel's atlas of human parasitology, 5th ed American Society for Clinical Pathology Press, Chicago, IL. [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2000. Laboratory diagnosis of blood-borne parasitic diseases, vol 20 Approved guideline M15-A. National Committee for Clinical Laboratory Standards, Wayne, PA. [Google Scholar]
- 11.Centers for Disease Prevention and Control. 2013. DPDx. http://www.cdc.gov/dpdx/malaria Accessed 19 December 2016.
- 12.Garcia LS, Isenberg HD. 2007. Clinical microbiology procedures handbook, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 13.Norgan AP, Arguello HE, Sloan LM, Fernholz EC, Pritt BS. 2013. A method for reducing the sloughing of thick blood films for malaria diagnosis. Malar J 12:231. doi: 10.1186/1475-2875-12-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. 2000. Bench aids for the diagnosis of malaria infections. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 15.Ochola LB, Vounatsou P, Smith T, Mabaso ML, Newton CR. 2006. The reliability of diagnostic techniques in the diagnosis and management of malaria in the absence of a gold standard. Lancet Infect Dis 6:582–588. doi: 10.1016/S1473-3099(06)70579-5. [DOI] [PubMed] [Google Scholar]
- 16.Pritt BS. 2014. Parasitology benchtop reference guide: an illustrated guide for commonly encountered parasites. College of American Pathologists, Northfield, IL. [Google Scholar]
- 17.Swierczynski G, Gobbo M. 2008. Atlas of human malaria. Az Color, Sirmione, Italy. [Google Scholar]
- 18.Mathison BA, Bishop H, Johnston SP, Xayavong MV, Arguin PM, Da Silva AJ. 2010. Trends in malaria diagnosis: combining the use of telediagnosis, microscopy and PCR in the identification of Plasmodium spp. 59th Annu Meet Am Soc Trop Med Hyg, Atlanta, GA, 3 to 7 November 2010. [Google Scholar]
- 19.Rhoads DD, Mathison BA, Bishop HS, da Silva AJ, Pantanowitz L. 2016. Review of telemicrobiology. Arch Pathol Lab Med 140:362–370. doi: 10.5858/arpa.2015-0116-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trampuz A, Jereb M, Muzlovic I, Prabhu RM. 2003. Clinical review: severe malaria. Crit Care 7:315–323. doi: 10.1186/cc2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. 2017. WHO-FIND malaria RDT evaluation programme. http://www.who.int/malaria/areas/diagnosis/rapid-diagnostic-tests/rdt-evaluation-programme/en/ Accessed 18 March 2017.
- 22.Wilson ML. 2012. Malaria rapid diagnostic tests. Clin Infect Dis 54:1637–1641. doi: 10.1093/cid/cis228. [DOI] [PubMed] [Google Scholar]
- 23.Alere. 2016. BinaxNOW Malaria Test Kit, http://www.alere.com/en/home/product-details/binaxnow-malaria.html Accessed 2 April 2017.
- 24.Foster D, Cox-Singh J, Mohamad DS, Krishna S, Chin PP, Singh B. 2014. Evaluation of three rapid diagnostic tests for the detection of human infections with Plasmodium knowlesi. Malar J 13:60. doi: 10.1186/1475-2875-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bigaillon C, Fontan E, Cavallo JD, Hernandez E, Spiegel A. 2005. Ineffectiveness of the Binax NOW malaria test for diagnosis of Plasmodium ovale malaria. J Clin Microbiol 43:1011. doi: 10.1128/JCM.43.2.1011.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. 2016. False-negative RDT results and implications of new reports of P. falciparum histidine-rich protein 2/3 gene deletions. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/information-note-hrp2-based-rdt/en/ Accessed 18 March 2017. [Google Scholar]
- 27.Fried M, Muehlenbachs A, Duffy PE. 2012. Diagnosing malaria in pregnancy: an update. Expert Rev Anti Infect Ther 10:1177–1187. doi: 10.1586/eri.12.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasoo S, Pritt BS. 2013. Molecular diagnostics and parasitic disease. Clin Lab Med 33:461–503. doi: 10.1016/j.cll.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Strom GE, Tellevik MG, Hanevik K, Langeland N, Blomberg B. 2014. Comparison of four methods for extracting DNA from dried blood on filter paper for PCR targeting the mitochondrial Plasmodium genome. Trans R Soc Trop Med Hyg 108:488–494. doi: 10.1093/trstmh/tru084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkins P, Nutman TB. 2015. Immunological and molecular approaches for the diagnosis of parasitic infections. In Jorgensen JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 11th ed, vol 2 ASM Press, Washington, DC. [Google Scholar]