ABSTRACT
During the last decade, many investigators have studied matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) for identification of mycobacteria. Diverse and contradictory results indicated that optimal level for routine testing has not been reached yet. This work aimed to assess Vitek MS through two distinct versions, Saramis v4.12 RUO and the IVD v3.0, under conditions close to routine laboratory practice. Overall, 111 mycobacterial isolates were subjected to protein extraction and same spectra were matched against both databases. The IVD v3.0 database proved to be superior to Saramis v4.12 and its identification rates remarkably increased, from 67% to 94% for isolates grown on Middlebrook 7H10 solid medium and from 62% to 91% for isolates grown on mycobacterial growth indicator tube (MGIT) liquid medium. With this new version, IVD v3.0, MALDI-TOF MS might be integrated into routine clinical diagnostics, although molecular techniques remain mandatory in some cases.
KEYWORDS: MALDI-TOF mass spectrometry, Mycobacterium spp., genotypic identification, phenotypic identification
INTRODUCTION
The genus Mycobacterium consists of 177 species whose taxonomy has constantly been evolving over the past few decades. The major causes of morbidity and mortality are due to mycobacteria belonging to the Mycobacterium tuberculosis complex (MTC), with 9.6 million people newly infected and 1.5 million people dying from tuberculosis every year (1). Nontuberculous mycobacteria (NTM) comprise a large group of organisms widely present in the environment and considered opportunistic pathogens causing pulmonary, soft tissue, lymphatic, and disseminated infections (2). Species such as M. abcsessus, M. xenopi, and M. chimaera have also been involved in nosocomial outbreaks (3–6). An accurate identification (ID) of NTM is, then, important for epidemiological and public health and for therapeutic reasons. Species identification is especially essential in diagnosis of M. avium complex (MAC) lung disease to differentiate transient colonization by different species from chronic pulmonary infection (2). Identification of mycobacterial isolates has traditionally been based on phenotypic characteristics and conventional biochemical tests, which were complex and required long incubation times. More recently, molecular methods such as DNA sequencing and DNA hybridization have become the new “gold standards” for mycobacterial identification. Although these techniques are fast and specific ways of identifying main Mycobacterium spp., they remain expensive, are available for only a limited number of common species, and require a high level of technical expertise. The limitations encountered with currently available methods led several investigators to suggest the use of matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) as an alternative strategy. MALDI-TOF MS is now predominant in an increasing number of clinical laboratories (7). This technique is quite simple and cost-effective (8) and allows rapid identification of organisms on the basis of spectral fingerprints produced by extracted proteins. It was initially evaluated with mycobacterial intact cells (9–12), but several investigators worked on improving inactivation and protein extraction (13–20). Most published studies assessed the performance of MALDI-TOF BioTyper (Bruker Daltonics, Bremen, Germany) (15, 17, 19–31). Few of them evaluated Vitek MS (bioMérieux, Marcy l'Etoile, France) (16, 17, 20, 24, 25, 32). Performances ranged widely, from 0% (24) to 94.4% (17) correct identifications depending on database versions, algorithms, and media (liquid or solid) used. We aimed to assess identification of Mycobacterium spp. from both liquid and solid media through two distinct databases: the Saramis v4.12 Vitek MS-Plus RUO (research use only) open database and the v3.0 CE-marked In Vitro Diagnostic (IVD). To our knowledge, IVD v3.0 has been evaluated only for mycobacteria growing from solid media (20). We aimed to establish the performance of this technique in the routine clinical microbiology laboratory and to highlight its limits.
(The data in this article were partially presented in poster format at the 55th International Conference on Antimicrobial Agents and Chemotherapy, 18 to 21 September 2015, San Diego, CA.)
RESULTS
Nonviability assays.
None of the 5 isolates tested to confirm organism inactivation grew. Fifteen minutes of cell disruption in alcoholic suspension and 10 min of inactivation time rendered Mycobacterium tuberculosis nonviable. We therefore confirm the data provided by bioMérieux through their certificate of inactivation.
Comparison of the Saramis v4.12 and IVD v3.0 databases for mycobacterial identification.
We assessed the performance of the two Vitek MS databases, Saramis v4.12 and IVD v3.0, for the identification of 111 mycobacterial isolates cultivated on Middlebrook 7H10 solid medium (Table 1) and 108 isolates cultivated in mycobacterial growth indicator tube (MGIT) liquid medium (Table 2). The Saramis database correctly identified 67% and 62% of isolates from 7H10 and MGIT, respectively: 39% and 40% to the species level and 28% and 22% to the complex level. Among these correct identifications, 66% and 54% reached a high level of confidence (>90% of homology with superspectra [SS; see Materials and Methods]), 20% and 26% a medium to low level of confidence (75 to 90% of homology with SS), and 14% and 20% matched only with reference spectra (<75% of homology with SS). Despite three distinct extractions performed on different days, 33% and 37% of isolates remained unidentified from solid and liquid media, respectively, although the corresponding species are present in the library of the Saramis database. Among these failures, 75% were due to a lack of a sufficient number of peaks from liquid medium and the remaining 25% indicated “no match” with the database. Using the IVD v3.0 database, 94% and 91% of isolates were accurately identified with a high level of confidence (>99%) from 7H10 and MGIT, respectively; 50% and 52% were identified to the species level and 44% and 39% to the complex level. However, 6% and 7% of isolates did not match with any spectrum after three protein extractions, mainly due to an insufficient amount of peaks. In both databases, no difference was observed when considering discrimination between MTC, slowly growing mycobacteria (SGM), and rapidly growing mycobacteria (RGM). Neither Saramis v4.12 nor IVD v3.0 was able to distinguish species from MTC, M. fortuitum complex, and some species from M. avium complex (MAC) such as M. chimaera, M. arosiense, and M. colombiense. Lastly, two discrepant results were detected for two isolates of M. interjectum, wrongly identified with a high level of confidence as M. szulgai and as Achromobacter xylosoxidans.
TABLE 1.
Identification of Mycobacterium spp. grown on solid medium 7H10 by Vitek MS Saramis v4.12 and IVD v3.0a
| Organism | Total no. of isolates (HCC/NRC)b | No. (%) of isolates identified by Saramis v4.12 RUO |
No. (%) of isolates identified by IVD v3.0 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Species level ID | Complex level ID | No ID | Incorrect ID | Species level ID | Complex level ID | No ID | Incorrect ID | ||
| SGM | |||||||||
| M. tuberculosis complex | |||||||||
| M. tuberculosis | 16 (16/0) | 0 | 11 | 5 | 0 | 0 | 16 | 0 | 0 |
| M. bovis BCG | 1 (0/1) | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| M. avium complex | |||||||||
| M. avium | 10 (8/2) | 7 | 0 | 3 | 0 | 10 | 0 | 0 | 0 |
| M. intracellulare | 8 (8/0) | 4 | 0 | 4 | 0 | 6 | 2 | 0 | 0 |
| M. chimaera | 22 (19/3) | 0 | 16 | 6 | 0 | 0 | 22 | 0 | 0 |
| M. arosiense | 1 (1/0) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| M. colombiense | 2 (2/0) | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 0 |
| M. scrofulaceum | 1 (0/1) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| M. marinum | 1 (0/1) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| M. kansasii | 2 (1/1) | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| M. szulgai | 1 (0/1) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| M. simiae | 1 (0/1) | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| M. gordonae | 12 (12/0) | 8 | 0 | 4 | 0 | 12 | 0 | 0 | 0 |
| M. celatum | 1 (1/0) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| M. cookii | 1 (1/0) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| M. interjectum | 2 (2/0) | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 0 |
| M. nebraskense | 2 (2/0) | 0 | 0 | 2 | 0 | 1 | 0 | 1 | 0 |
| M. xenopi | 3 (1/2) | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| M. malmoense | 1 (1/0) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Total (%) for SGM | 88 (100) | 28 (32) | 28 (32) | 32 (36) | 0 | 39 (44) | 42 (48) | 7 (8) | 0 |
| RGM | |||||||||
| M. chelonae/M. abscessus complex | |||||||||
| M. chelonae | 4 (1/3) | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| M. abscessus subspeciesc | 1 (1/0) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| “M. abscessus subsp. abscessus” | 3 (0/3) | 2 | 0 | 1 | 0 | 3 | 0 | 0 | 0 |
| “M. abscessus subsp. bolletii” | 3 (0/3) | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| “M. abscessus subsp. massiliense” | 3 (0/3) | 1 | 0 | 2 | 0 | 3 | 0 | 0 | 0 |
| M. fortuitum complex | 7 (7/0) | 2 | 3 | 2 | 0 | 0 | 7 | 0 | 0 |
| M. mucogenicum | 1 (1/0) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| M. smegmatis | 1 (1/0) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Total (%) for RGM | 23 (100) | 15 (65) | 3 (13) | 5 (22) | 0 | 16 (70) | 7 (30) | 0 | 0 |
| Total (%) for all isolates (SGM and RGM) | 111 (100) | 43 (39) | 31 (28) | 37 (33) | 0 | 55 (50) | 49 (44) | 7 (6) | 0 |
ID, identification; SGM, slowly growing mycobacteria; RGM, rapidly growing mycobacteria.
HCC, strains isolated in Hôpitaux Civils de Colmar; NRC, strains provided by the National Reference Center for Mycobacteria, Paris, France.
Species not characterized to the subspecies level.
TABLE 2.
Results of Mycobacterium spp. grown in liquid medium MGIT identification by Vitek MS Saramis v4.12 and IVD v3.0a
| Organism | Total no. of isolates (HCC/NRC)b | No. (%) of isolates identified by Saramis v4.12 RUO |
No. (%) of isolates identified by IVD v3.0 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Species level ID | Complex level ID | No ID | Incorrect ID | Species level ID | Complex level ID | No ID | Incorrect ID | ||
| SGM | |||||||||
| M. tuberculosis complex | |||||||||
| M. tuberculosis | 16 (16/0) | 0 | 12 | 4 | 0 | 0 | 15 | 1 | 0 |
| M. avium complex | |||||||||
| M. avium | 9 (7/2) | 6 | 0 | 3 | 0 | 8 | 0 | 1 | 0 |
| M. intracellulare | 7 (7/0) | 4 | 0 | 3 | 0 | 7 | 0 | 0 | 0 |
| M. chimaera | 22 (19/3) | 0 | 7 | 15 | 0 | 0 | 17 | 5 | 0 |
| M. arosiense | 1 (1/0) | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| M. colombiense | 2 (2/0) | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| M. scrofulaceum | 1 (0/1) | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| M. marinum | 1 (0/1) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| M. kansasii | 2 (1/1) | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| M. szulgai | 1 (0/1) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| M. simiae | 1 (0/1) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| M. gordonae | 12 (12/0) | 10 | 0 | 2 | 0 | 12 | 0 | 0 | 0 |
| M. celatum | 1 (1/0) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| M. cookii | 1 (1/0) | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| M. interjectum | 2 (2/0) | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 |
| M. nebraskense | 2 (2/0) | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 |
| M. xenopi | 3 (1/2) | 2 | 0 | 1 | 0 | 3 | 0 | 0 | 0 |
| M. malmoense | 1 (1/0) | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Total (%) for SGM | 85 (100) | 28 (33) | 19 (22) | 37 (44) | 1 (1) | 40 (47) | 35 (41) | 8 (9) | 2 (2) |
| RGM | |||||||||
| M. chelonae/M. abscessus complex | |||||||||
| M. chelonae | 4 (1/3) | 3 | 0 | 1 | 0 | 4 | 0 | 0 | 0 |
| M. abscessus subspeciesc | 1 (1/0) | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| “M. abscessus subsp. abscessus” | 3 (3/0) | 2 | 0 | 1 | 0 | 3 | 0 | 0 | 0 |
| “M. abscessus subsp. bolletii” | 3 (3/0) | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| “M. abscessus subsp. massiliense” | 3 (3/0) | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| M. fortuitum complex | 7 (7/0) | 2 | 5 | 0 | 0 | 0 | 7 | 0 | 0 |
| M. mucogenicum | 1 (1/0) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| M. smegmatis | 1 (1/0) | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Total (%) for RGM | 23 (100) | 15 (65) | 5 (22) | 3 (13) | 0 | 16 (70) | 7 (30) | 0 | 0 |
| Total (%) for isolates (SGM and RGM) | 108 (100) | 43 (40) | 24 (22) | 40 (37) | 1 (1) | 56 (52) | 42 (39) | 8 (7) | 2 (2) |
ID, identification; SGM, slowly growing mycobacteria; RGM, rapidly growing mycobacteria.
HCC, strains isolated in Hôpitaux Civils de Colmar; NRC, strains provided by the National Reference Center for Mycobacteria, Paris, France.
Species not characterized to the subspecies level.
Comparison between Vitek MS IVD v3.0 and hsp65 and rpoB sequencing.
In order to compare MS data with a reference method for identification of Mycobacterium spp., sequencing of the hsp65 and rpoB genes was used for the 58 strains of NTM (Table 3). For approximately half of these isolates (28 isolates belonging to 12 distinct species), the sequencing showed results equivalent to those obtained with MALDI-TOF MS. The sequencing of hsp65 and rpoB was more discriminant than MS for all strains of M. chimaera, M. colombiense, and M. arosiense, all identified as M. intracellulare by the IVD v3.0 system. Two strains of M. interjectum and one strain of M. cookii were misidentified because they are not included in the bioMérieux database. Conversely, MS provided more accurate identification for two strains: one strain of M. marinum isolated from skin infection in an aquarium owner, not correctly discriminated between M. marinum and M. ulcerans by sequencing, and one mucoid strain of M. mucogenicum, identified as M. fortuitum by hsp65 and rpoB sequencing. M. mucogenicum exhibits a high genetic heterogeneity within clinical isolates, which could explain some sequence divergences (33).
TABLE 3.
Comparison between hsp65 or rpoB sequencing and MALDI-TOF MS IVD v3.0 for NTM identification
| Comparisona | No. of strains | Species |
|---|---|---|
| MS = SEQ | 28 | M. intracellulare, M. avium, M. celatum, M. gordonae, M. kansasii, M. malmoense, M. nebraskense, M. chelonae, M. abscessus, M. fortuitum, M. peregrinum, M. smegmatis |
| SEQ > MS | 28 | M. chimaera,b M. colombiense,b M. arosienseb |
| M. interjectum,c M. cookiic | ||
| MS > SEQ | 2 | M. marinum, M. mucogenicum |
MS, mass spectrometry; SEQ, sequencing.
Species identified as M. intracellulare by MS.
Species not included in the database.
Assessment of the quality of protein extraction.
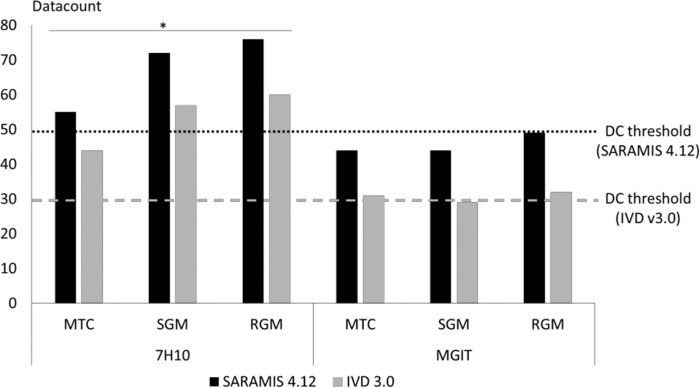
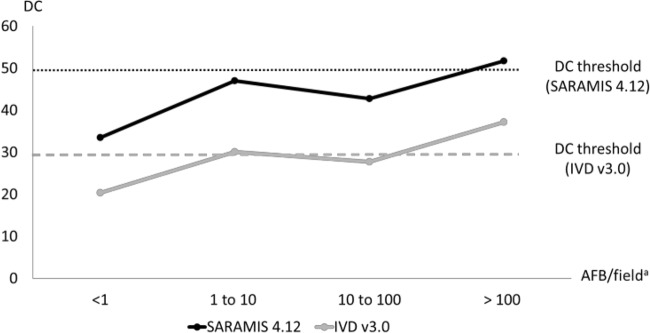
Protein extraction was assessed with data counts (DC) rated by the two distinct algorithms, Saramis v4.12 and IVD v3.0, and was compared between MTC, SGM, and RGM, each grown on solid and liquid media (Fig. 1). Regardless of the algorithm used, DC obtained from mycobacteria cultivated on solid medium were significantly higher than those obtained from liquid medium (P < 0.05). They were over the threshold defined by the manufacturer as the minimum amount of peaks required to obtain identification. No significant difference was reported between DC averages obtained with MTC, SGM, and RGM. Because of low DC averages observed from mycobacteria grown on liquid medium, we assessed the link between DC values and number of acid-fast bacilli (AFB) (Fig. 2). DC appeared over the threshold in both the Saramis and IVD versions when MGIT medium showed more than 100 AFB/field. For lower AFB values, DC and identification rates were uncertain and repeated extractions, i.e., 1.3 from solid medium and 1.5 from liquid medium, were needed.
FIG 1.
Data count (DC) means depending on mycobacteria and media used. MTC, Mycobacterium tuberculosis complex; SGM, slowly growing mycobacteria; RGM, rapidly growing mycobacteria.
FIG 2.
Data count means depending on the AFB wealth assessed from MGIT medium. a, each positive MGIT was subjected to auramine staining at a magnification of ×1,000 prior to MS extraction. AFB, acid-fast bacilli.
DISCUSSION
During the last decade, the development of MALDI-TOF MS has highly increased rapid identification of bacterial isolates in many laboratories. Identification of mycobacteria has not reached an optimal level of routine testing yet and published studies showed discrepant performances, depending on spectrometer, extraction method, algorithm, library, media, and biomass used for the assays (15–17, 19–32). In this study, we compared two distinct databases of Vitek-MS, the Saramis v4.12 open database and the new IVD v3.0 closed database. Performances were assessed under conditions as close as possible to routine laboratory practice; i.e., protein extraction started from 48 h to 72 h after growth was detected on 7H10 medium or the MGIT tube was flagged positive, and biomass was harvested for further tests such as molecular identification or antimicrobial susceptibility testing.
Unlike the previous extraction procedure developed by the manufacturer (25), the tested protocol was safe as shown by the subsequent lack of growth of MTC species. The protein extraction method also proved its ability to disrupt cords formed by M. tuberculosis complex, and identifications were accurate and reliable (94% to 100% of accurate identifications with the IVD v3.0 depending on the medium used). Rapid and safe alternatives, e.g., immunochromatographic assay using anti-MPT64 antibody or molecular probes, could be easily performed from both liquid and solid media (34) in a biosafety level 3 (BSL3) lab and led us to exclude MALDI-TOF MS for a rapid diagnosis of tuberculosis.
In our study, MALDI-TOF MS Saramis v4.12 correctly identified 67% and 62% of mycobacterial isolates grown on solid and liquid media, respectively, with approximately half of isolates identified with a confidence score of >90% homology with SS. Performances observed were less promising than suggested in previous studies, which showed between 77% and 94% correct identifications (17, 20, 32). IVD v3.0 highly increased identification rates, with 94% and 91% of reliable identifications from colonies or positive MGIT, respectively, all with high-level confidence (>99%). These performances were similar to those published by Wilen et al., who reported 89.2% correct identifications for mycobacterial isolates grown on 7H10 medium (20). By using IVD v3.0, half of the strains were identified to the species level. As widely reported, Vitek MS is able to accurately identify M. avium, but neither Saramis v4.12 nor IVD v3.0 appeared to be able to differentiate M. intracellulare, M. chimaera, M. arosiense, and M. colombiense (35). Previous identification methods used in our laboratory, the AccuProbe test and 16S rRNA gene sequencing, in case of failure of the AccuProbe assay, also misidentified M. chimaera, M. arosiense, and M. colombiense as M. intracellulare. Sequencing of hsp65 and rpoB revealed that the prevalence of M. chimaera was highly underestimated in our lab and actually represented >75% of strains identified as M. intracellulare (data not shown). These results are in agreement with the conclusions of Boyle et al., who showed that M. chimaera is more prevalent than M. intracellulare in pulmonary infections (35). They also suggested that specific MAC species have various degrees of virulence and that patients treated for pulmonary infection with M. avium or M. chimaera were more likely to have clinical relapse or reinfection than those with M. intracellulare (36). Current American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) guidelines state that “clinically significant NTM isolates should be routinely identified to the species level” (2). Then, in cases of proved infections, MALDI-TOF MS would not be reliable enough and hsp65 or rpoB sequencing is required to reach the expected level of accuracy. The same findings were obtained with M. fortuitum complex and M. abscessus subspecies. Strains of “Mycobacterium abscessus subsp. abscessus” (n = 3), “M. abscessus subsp. massiliense” (n = 3), and “M. abscessus subsp. bollettii” (n = 3) were all identified as M. abscessus by IVD v3.0, from both liquid and solid media. Since these subspecies differ in antibiotic susceptibility and clinical relevance (37, 38), resistance detection and subspecies identification are required. Panagea et al. revealed peaks potentially able to differentiate between subspecies by using both Vitek MS Saramis v4.12 and IVD v3.0 (39). Both versions failed to identify M. interjectum (n = 2) and M. cookii (n = 1) due to gaps in the databases.
DC were higher when spectra were submitted to the Saramis v4.12 than when submitted to the IVD v3.0 algorithm. The narrower mass spectra analyzed (3,000 to 17,000 Da in IVD v3.0 instead of 2,000 to 20,000 Da in Saramis) may contribute to this difference. However, even if fewer peaks were included, the IVD v3.0 algorithm was associated with significantly better identification rates. This improvement was not due to any database update between the two versions, as discrepancies concerned species belonging to the two databases. The algorithm based on the principle of “mass binning” revealed a greater robustness than the principle of SS matching.
Nevertheless, even with this more robust algorithm, the critical point remains the current need to repeat testing. While Wilen et al. show that Vitek MS v3.0 requires slightly fewer repeats of analyses than the Biotyper and Saramis methods (20), our results indicated that extraction had to be repeated averages of 1.3 and 1.5 times to obtain correct identification from solid and liquid media, respectively, which may have implications for laboratory workflow. So we suggest performing each extraction in duplicate or in triplicate. As observed by van Eck et al. (31) in a real-life setting from primary cultures of respiratory samples, MALDI-TOF MS identification yields insufficient results when protein extraction is performed from low-AFB-wealth liquid medium. Comparison with DC based on number of AFB in this work yielded the same conclusion. Indeed, at the time of detection in the liquid culture system, the mycobacterial biomass is usually insufficient for analysis by MALDI-TOF MS. Some authors performed their assays on MGIT after cultivation extended at room temperature by 1 to 2 weeks (40). A reincubation time of a few days (2 to 3 days as performed in this work) at 35 to 37°C and a sample of 3 ml instead of 1.8 ml might be suggested after elimination of MTC species by other rapid assays. A less satisfying alternative would be the identification from subculture of MGIT on solid medium. Then, the time-saving aspect of the method would be highly questionable.
Like most of the published studies, this work used isolates of Mycobacterium spp. stored in a strain collection and subcultured in MGIT or 7H10 medium. Under these conditions, we did not assess potential interference with proteins from patient and oropharyngeal flora killed during decontamination. As recently shown by van Eck et al. with MALDI Biotyper (Bruker Daltonics), identification rates drastically dropped, from 91% to 22%, between colonies subcultured and primary culture of respiratory samples. Although two distinct media were compared (Middlebrook 7H10 plates for subcultures and MGIT medium for primary cultures), these data highlighted the problem of mixed-protein patterns and possible interference with proteins from patients (31). A similar prospective study on Vitek MS would be helpful to define real performances of IVD v3.0 from primary cultures of respiratory samples. Several authors recently described new extraction protocols (12, 18, 40, 41), which could increase the ratio of mycobacterial proteins to patients' proteins and probably improve the identification rates at the time of detection.
Two other limits of MALDI-TOF MS for mycobacterial identification have to be considered: possible contaminations by common bacteria or yeasts and coinfections with distinct species of mycobacteria. Depending on the laboratories, contamination rates ranged from 5% to 10% of positive liquid media. Molecular techniques such as line probe assay could bypass this issue. Otherwise, a decontamination process is required and identification time is extended. In the rare event of coinfection by two mycobacterial isolates, careful examination of positive cultures from solid media is still suggested. Lastly, it appears that operator experience impacts significantly the rate of good extraction (31), and a few experienced technicians would be beneficial to optimize the method.
In summary, the current Saramis v4.12 RUO appears under our laboratory conditions neither accurate nor reliable enough to allow routine implementation. Conversely, the new IVD v3.0 seems promising, with a more robust algorithm and reliable identification rates. Although these results need to be confirmed both with a larger number of strains and by a prospective study in a clinical setting, this new version of the database might be a trusted first-line approach for the routine identification of most NTM. However, the identification algorithm should recommend a molecular approach such as hps65 or rpoB gene sequencing to reach the expected level of accuracy, especially in cases of documented infection due to M. intracellulare.
MATERIALS AND METHODS
Isolates and identification.
A total of 111 clinical strains of mycobacteria belonging to 21 species and 3 subspecies were included. Eighty-six strains were isolated in the clinical microbiology laboratory of Hôpital Pasteur (Hôpitaux Civils de Colmar, France) from 2011 to 2014 and were stored in Bactec mycobacterial growth indicator tube (MGIT) liquid medium (Becton Dickinson, Le Pont-de-Claix, France) at room temperature until analysis. These isolates were routinely identified by the AccuProbe test (GenProbe, San Diego, CA) for M. tuberculosis, M. intracellulare complex, M. avium sensu stricto, and M. gordonae species, according to the instructions of the manufacturer. For remaining species or in case of failure of the AccuProbe test, identification was performed by sequencing a 500-bp fragment of the 16S rRNA gene. Although it is not a reference technique, short 16S product analysis was sufficient in terms of clinical relevance in most cases. In addition, 25 clinical strains were provided by the Associated National Reference Center for Mycobacteria (CNR-Myc, Hôpital Lariboisière, Paris, France) and were identified by DNA hybridization GenoType Mycobacterium MTBC, CM/AS (Hain Lifescience GmbH, Nehren, Germany) or hsp65 or rpoB gene sequencing.
Confirmation of organism inactivation.
As members of the M. tuberculosis complex are BSL3 pathogens, it was necessary to control the total absence of viability after the protein extraction protocol. For 5 isolates of Mycobacterium tuberculosis, protein extracts obtained after inactivation were added to an MGIT tube and onto Middlebrook 7H10 plates, both incubated for 56 days at 35°C.
Culture conditions.
All 111 strains were inoculated onto Middlebrook 7H10 agar (Becton Dickinson, Le Pont-de-Claix, France) without supplemental CO2 at 35°C or 30°C according to the optimal temperature of each species. In parallel, 108 isolates (3 failed to subcultivate after inoculation) were grown in fresh liquid MGIT medium and incubated in the Bactec MGIT 960 mycobacterial detection system (Becton Dickinson, Le Pont-de-Claix, France). Each liquid culture was subjected to auramine staining to assess acid-fast bacillus (AFB) wealth (magnification, ×1000) when detected as positive by the system. Gram staining was also performed to ensure lack of any microbial contamination.
Mycobacterial preparation protocol.
Protein extraction was performed in a period ranging from 48 h to 72 h following macroscopic or automated growth detection for solid or liquid medium, respectively. In both cases, culture media were reincubated at 35°C during this period. The extraction protocol was performed according to the MS supplier (bioMérieux). From colonies grown on 7H10 medium, a 1-μl disposable inoculation loopful of colonies was harvested and added to 500 μl of 70% ethanol. From liquid medium, MGIT culture was first strongly vortexed. Then 1.8 ml of liquid was pipetted into a centrifuge vial and centrifuged at 14,000 × g for 10 min. The supernatant was discarded, and the pellet was resuspended in 500 μl of 70% ethanol. At this step, mycobacteria from both solid and liquid media were at the same stage of the protocol. The alcoholic suspension was transferred into a centrifuge vial containing 200 μl of 0.5-mm glass beads. The cells were disrupted with a vortex for 15 min and then incubated at room temperature for 10 min to ensure inactivation. The suspension was transferred into a sterile tube and centrifuged for 4 min at 14,000 × g. The pellet was resuspended with 10 μl of 70% formic acid. After addition of 10 μl of 100% acetonitrile, the suspension was briefly vortexed and then centrifuged at 14,000 × g for 4 min. The next steps were performed under standard BSL2 conditions. One microliter of the supernatant was pipetted onto a MALDI-TOF target slide. The samples were dried at room temperature, and 1 μl of α-cyano-4-hydroxy cinnamic acid (CHCA) matrix solution (bioMérieux, Marcy l'Etoile, France) was added. Samples were analyzed in duplicate after drying at room temperature.
Vitek MS databases assessed.
All spectra were generated with the Vitek MS Plus System, which records mass spectra from 2,000 to 20,000 Da by shooting 100 times per spot. Acquired spectra were analyzed by both the Saramis v4.12 and v3.0 databases. With the Saramis v4.12 system, identification match was compared with superspectra (SS), defined by a “consensus spectrum” constructed with 8 strains and containing approximately the 40 most specific peaks for each considered species. Confidence levels range from high (homology > 98%) to medium (85 to 98% homology) to low (75 to 85% homology). When homology is below 75%, the algorithm compares spectra with reference spectra instead of SS. In another way, IVD v3.0 sums each generated spectrum into a single spectrum according to the principle of mass binning. In this algorithm, the confidence levels are divided into a high level of confidence (>99%), a medium level of confidence (90 to 99%), and a low level of confidence (<90%). Then, each software has its own acquisition system and its own algorithm and identification rules. Databases are also different when considering number of species. For both systems, quality of protein extraction can be assessed by the data count (DC), defined by the interpretable number of peaks considered in the algorithm. For DC lower than 50 peaks in Saramis v4.12 or lower than 30 peaks in IVD v3.0, no identification is assigned and a new protein extraction must be performed starting from the first step of the protocol. If identification fails after 3 distinct extractions, the sample is labeled “No ID” and no identification is assigned. The comparison of DC obtained from each medium for each species was carried out by a Student t test.
Gene sequencing.
As identification tests routinely performed (AccuProbe test and partial 16S rRNA gene sequencing) are not reference methods, strains were subjected to DNA sequencing of both the hsp65 and rpoB genes, according to previously described methods (33, 42). Strains belonging to M. tuberculosis complex (n = 16), easily identified by rapid molecular methods, and M. gordonae (n = 12), for which clinical implication is scarce, as well as the 25 strains identified by the Associated National Reference Center for Mycobacteria were excluded from this analysis. Gene sequencing of the 58 remaining strains was carried out on an ABI 3500 genetic analyzer (Thermo Fisher, Courtabeuf, France).
Sequence data were analyzed on the peer-reviewed BIBIQBPP by using a high degree of stringency (comparison with sequences of type strains). Taxonomic assignment was made by using an approximate maximum likelihood approach (43).
Criteria used to define the species of unknown isolates were defined as follows: >97% hsp65 sequence similarity with the reference strain for all isolates (44), >98.3% rpoB sequence similarity with the reference strain for rapidly growing mycobacteria (RGM) (33), and >99.3% rpoB sequence similarity with the reference strain for slowly growing mycobacteria (SGM) (45, 46).
ACKNOWLEDGMENTS
We thank Olivier Lafrique from bioMérieux for technical assistance.
No financial support was provided for performing this study.
REFERENCES
- 1.World Health Organization. 2015. Global tuberculosis control: WHO report 2015. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 3.Song JY, Sohn JW, Jeong HW, Cheong HJ, Kim WJ, Kim MJ. 2006. An outbreak of post-acupuncture cutaneous infection due to Mycobacterium abscessus. BMC Infect Dis 6:6. doi: 10.1186/1471-2334-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song JY, Son JB, Lee MK, Gwack J, Lee KS, Park JY. 2012. Case series of Mycobacterium abscessus infections associated with a trigger point injection and epidural block at a rural clinic. Epidemiol Health 34:e2012001. doi: 10.4178/epih/e2012001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Achermann Y, Rossle M, Hoffmann M, Deggim V, Kuster S, Zimmermann DR, Bloemberg G, Hombach M, Hasse B. 2013. Prosthetic valve endocarditis and bloodstream infection due to Mycobacterium chimaera. J Clin Microbiol 51:1769–1773. doi: 10.1128/JCM.00435-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salmon JH, Direz G, Ziza JM, Desplaces N, Brochot P, Eschard JP. 2012. Discitis and sacroiliitis diagnosed 15 years after iatrogenic Mycobacterium xenopi inoculation. Joint Bone Spine 79:409–411. doi: 10.1016/j.jbspin.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Clark AE, Kaleta EJ, Arora A, Wolk DM. 2013. Matrix-assisted laser desorption ionization–time of flight mass spectrometry: a fundamental shift in the routine practice of clinical microbiology. Clin Microbiol Rev 26:547–603. doi: 10.1128/CMR.00072-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran A, Alby K, Kerr A, Jones M, Gilligan PH. 2015. Cost savings realized by implementation of routine microbiological identification by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 53:2473–2479. doi: 10.1128/JCM.00833-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hettick JM, Kashon ML, Simpson JP, Siegel PD, Mazurek GH, Weissman DN. 2004. Proteomic profiling of intact mycobacteria by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Chem 76:5769–5776. doi: 10.1021/ac049410m. [DOI] [PubMed] [Google Scholar]
- 10.Lotz A, Ferroni A, Beretti JL, Dauphin B, Carbonnelle E, Guet-Revillet H, Veziris N, Heym B, Jarlier V, Gaillard JL, Pierre-Audigier C, Frapy E, Berche P, Nassif X, Bille E. 2010. Rapid identification of mycobacterial whole cells in solid and liquid culture media by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 48:4481–4486. doi: 10.1128/JCM.01397-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pignone M, Greth KM, Cooper J, Emerson D, Tang J. 2006. Identification of mycobacteria by matrix-assisted laser desorption ionization–time-of-flight mass spectrometry. J Clin Microbiol 44:1963–1970. doi: 10.1128/JCM.01959-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zingue D, Flaudrops C, Drancourt M. 2016. Direct matrix-assisted laser desorption ionisation time-of-flight mass spectrometry identification of mycobacteria from colonies. Eur J Clin Microbiol Infect Dis 35:1983–1987. doi: 10.1007/s10096-016-2750-5. [DOI] [PubMed] [Google Scholar]
- 13.Adams LL, Salee P, Dionne K, Carroll K, Parrish N. 2015. A novel protein extraction method for identification of mycobacteria using MALDI-ToF MS. J Microbiol Methods 119:1–3. doi: 10.1016/j.mimet.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Dunne WM Jr, Doing K, Miller E, Moreno E, Baghli M, Mailler S, Girard V, van Belkum A, Deol P. 2014. Rapid inactivation of Mycobacterium and Nocardia species before identification using matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 52:3654–3659. doi: 10.1128/JCM.01728-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Khéchine A, Couderc C, Flaudrops C, Raoult D, Drancourt M. 2011. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry identification of mycobacteria in routine clinical practice. PLoS One 6:e24720. doi: 10.1371/journal.pone.0024720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Machen A, Kobayashi M, Connelly MR, Wang YF. 2013. Comparison of heat inactivation and cell disruption protocols for identification of mycobacteria from solid culture media by use of Vitek matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 51:4226–4229. doi: 10.1128/JCM.02612-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mather CA, Rivera SF, Butler-Wu SM. 2014. Comparison of the Bruker Biotyper and Vitek MS matrix-assisted laser desorption ionization–time of flight mass spectrometry systems for identification of mycobacteria using simplified protein extraction protocols. J Clin Microbiol 52:130–138. doi: 10.1128/JCM.01996-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Connor JA, Lynch-Healy M, Corcoran D, O'Reilly B, O'Mahony J, Lucey B. 2016. Improved matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS)-based identification of Mycobacterium spp. by use of a novel two-step cell disruption preparatory technique. J Clin Microbiol 54:495–496. doi: 10.1128/JCM.02998-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tudó G, Monte MR, Vergara A, Lopez A, Hurtado JC, Ferrer-Navarro M, Vila J, Gonzalez-Martin J. 2015. Implementation of MALDI-TOF MS technology for the identification of clinical isolates of Mycobacterium spp. in mycobacterial diagnosis. Eur J Clin Microbiol Infect Dis 34:1527–1532. doi: 10.1007/s10096-015-2381-2. [DOI] [PubMed] [Google Scholar]
- 20.Wilen CB, McMullen AR, Burnham CA. 2015. Comparison of sample preparation methods, instrumentation platforms, and contemporary commercial databases for identification of clinically relevant mycobacteria by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 53:2308–2315. doi: 10.1128/JCM.00567-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amlerová J, Studentova V, Hrabak J. 2014. Identification of Mycobacterium spp. isolates using matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS). Epidemiol Mikrobiol Imunol 63:196–199. (In Czech.) [PubMed] [Google Scholar]
- 22.Balada-Llasat JM, Kamboj K, Pancholi P. 2013. Identification of mycobacteria from solid and liquid media by matrix-assisted laser desorption ionization–time of flight mass spectrometry in the clinical laboratory. J Clin Microbiol 51:2875–2879. doi: 10.1128/JCM.00819-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckwalter SP, Olson SL, Connelly BJ, Lucas BC, Rodning AA, Walchak RC, Deml SM, Wohlfiel SL, Wengenack NL. 2016. Evaluation of matrix-assisted laser desorption ionization–time of flight mass spectrometry for identification of Mycobacterium species, Nocardia species, and other aerobic actinomycetes. J Clin Microbiol 54:376–384. doi: 10.1128/JCM.02128-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JH, Yam WC, Ngan AH, Fung AM, Woo WL, Yan MK, Choi GK, Ho PL, Cheng VC, Yuen KY. 2013. Advantages of using matrix-assisted laser desorption ionization–time of flight mass spectrometry as a rapid diagnostic tool for identification of yeasts and mycobacteria in the clinical microbiological laboratory. J Clin Microbiol 51:3981–3987. doi: 10.1128/JCM.01437-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lévesque S, Dufresne PJ, Soualhine H, Domingo MC, Bekal S, Lefebvre B, Tremblay C. 2015. A side by side comparison of Bruker Biotyper and VITEK MS: utility of MALDI-TOF MS technology for microorganism identification in a public health reference laboratory. PLoS One 10:e0144878. doi: 10.1371/journal.pone.0144878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mediavilla-Gradolph MC, De Toro-Peinado I, Bermudez-Ruiz MP, Garcia-Martinez Mde L, Ortega-Torres M, Montiel Quezel-Guerraz N, Palop-Borras B. 2015. Use of MALDI-TOF MS for identification of nontuberculous Mycobacterium species isolated from clinical specimens. Biomed Res Int 2015:854078. doi: 10.1155/2015/854078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinlan P, Phelan E, Doyle M. 2015. Matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry (MS) for the identification of mycobacteria from MBBacT ALERT 3D liquid cultures and Lowenstein-Jensen (LJ) solid cultures. J Clin Pathol 68:229–235. doi: 10.1136/jclinpath-2014-202374. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-Sánchez B, Ruiz-Serrano MJ, Marin M, Lopez Roa P, Rodriguez-Creixems M, Bouza E. 2015. Evaluation of matrix-assisted laser desorption ionization–time of flight mass spectrometry for identification of nontuberculous mycobacteria from clinical isolates. J Clin Microbiol 53:2737–2740. doi: 10.1128/JCM.01380-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez-Sánchez B, Ruiz-Serrano MJ, Ruiz A, Timke M, Kostrzewa M, Bouza E. 2016. Evaluation of MALDI Biotyper Mycobacteria Library v3.0 for identification of nontuberculous mycobacteria. J Clin Microbiol 54:1144–1147. doi: 10.1128/JCM.02760-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saleeb PG, Drake SK, Murray PR, Zelazny AM. 2011. Identification of mycobacteria in solid-culture media by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 49:1790–1794. doi: 10.1128/JCM.02135-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Eck K, Faro D, Wattenberg M, de Jong A, Kuipers S, van Ingen J. 2016. Matrix-assisted laser desorption ionization–time of flight mass spectrometry fails to identify nontuberculous mycobacteria from primary cultures of respiratory samples. J Clin Microbiol 54:1915–1917. doi: 10.1128/JCM.00304-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kehrmann J, Schoerding AK, Murali R, Wessel S, Koehling HL, Mosel F, Buer J. 2016. Performance of Vitek MS in identifying nontuberculous mycobacteria from MGIT liquid medium and Lowenstein-Jensen solid medium. Diagn Microbiol Infect Dis 84:43–47. doi: 10.1016/j.diagmicrobio.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Adékambi T, Colson P, Drancourt M. 2003. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J Clin Microbiol 41:5699–5708. doi: 10.1128/JCM.41.12.5699-5708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park MY, Kim YJ, Hwang SH, Kim HH, Lee EY, Jeong SH, Chang CL. 2009. Evaluation of an immunochromatographic assay kit for rapid identification of Mycobacterium tuberculosis complex in clinical isolates. J Clin Microbiol 47:481–484. doi: 10.1128/JCM.01253-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyle DP, Zembower TR, Qi C. 2015. Evaluation of Vitek MS for rapid classification of clinical isolates belonging to Mycobacterium avium complex. Diagn Microbiol Infect Dis 81:41–43. doi: 10.1016/j.diagmicrobio.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 36.Boyle DP, Zembower TR, Reddy S, Qi C. 2015. Comparison of clinical features, virulence, and relapse among Mycobacterium avium complex species. Am J Respir Crit Care Med 191:1310–1317. doi: 10.1164/rccm.201501-0067OC. [DOI] [PubMed] [Google Scholar]
- 37.Bastian S, Veziris N, Roux AL, Brossier F, Gaillard JL, Jarlier V, Cambau E. 2011. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob Agents Chemother 55:775–781. doi: 10.1128/AAC.00861-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sassi M, Drancourt M. 2014. Genome analysis reveals three genomospecies in Mycobacterium abscessus. BMC Genomics 15:359. doi: 10.1186/1471-2164-15-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panagea T, Pincus DH, Grogono D, Jones M, Bryant J, Parkhill J, Floto RA, Gilligan P. 2015. Mycobacterium abscessus complex identification with matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 53:2355–2358. doi: 10.1128/JCM.00494-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park JS, Choi SH, Hwang SM, Hong YJ, Kim TS, Park KU, Song J, Kim EC. 2016. The impact of protein extraction protocols on the performance of currently available MALDI-TOF mass spectrometry for identification of mycobacterial clinical isolates cultured in liquid media. Clin Chim Acta 460:190–195. doi: 10.1016/j.cca.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 41.Adams LL, Dionne K, Fisher S, Parrish N. 2016. A rapid, standardized protein extraction method using adaptive focused acoustics for identification of mycobacteria by MALDI-ToF MS. Diagn Microbiol Infect Dis 86:284–288. doi: 10.1016/j.diagmicrobio.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 42.Telenti A, Marchesi F, Balz M, Bally F, Bottger EC, Bodmer T. 1993. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol 31:175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flandrois JP, Perriere G, Gouy M. 2015. leBIBIQBPP: a set of databases and a webtool for automatic phylogenetic analysis of prokaryotic sequences. BMC Bioinformatics 16:251. doi: 10.1186/s12859-015-0692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNabb A, Eisler D, Adie K, Amos M, Rodrigues M, Stephens G, Black WA, Isaac-Renton J. 2004. Assessment of partial sequencing of the 65-kilodalton heat shock protein gene (hsp65) for routine identification of Mycobacterium species isolated from clinical sources. J Clin Microbiol 42:3000–3011. doi: 10.1128/JCM.42.7.3000-3011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ben Salah I, Adekambi T, Raoult D, Drancourt M. 2008. rpoB sequence-based identification of Mycobacterium avium complex species. Microbiology 154:3715–3723. doi: 10.1099/mic.0.2008/020164-0. [DOI] [PubMed] [Google Scholar]
- 46.de Zwaan R, van Ingen J, van Soolingen D. 2014. Utility of rpoB gene sequencing for identification of nontuberculous mycobacteria in the Netherlands. J Clin Microbiol 52:2544–2551. doi: 10.1128/JCM.00233-14. [DOI] [PMC free article] [PubMed] [Google Scholar]