ABSTRACT
Achromobacter species are increasingly being detected in cystic fibrosis (CF) patients, with an unclear epidemiology and impact. We studied a cohort of patients attending a Canadian adult CF clinic who had positive sputum cultures for Achromobacter species in the period from 1984 to 2013. Infection was categorized as transient or persistent (≥50% positive cultures for 1 year). Those with persistent infection were matched 2:1 with age-, sex-, and time-matched controls without a history of Achromobacter infection, and mixed-effects models were used to assess pulmonary exacerbation (PEx) frequency and lung function decline. Isolates from a biobank were retrospectively assessed, identified to the species level by nrdA sequencing, and genotyped using pulsed-field gel electrophoresis (PFGE). Thirty-four patients (11% of those in our clinic), with a median age of 24 years (interquartile range [IQR], 20.3 to 29.8 years), developed Achromobacter infection. Ten patients (29%) developed persistent infection. Persistence did not denote permanence, as most patients ultimately cleared infection, often after years. Patients were more likely to experience PEx at incident isolation than at prior or subsequent visits (odds ratio [OR], 2.7 [95% confidence interval {CI}, 1.2 to 6.7]; P = 0.03). Following persistent infection, there was no difference in annual lung function decline (−1.08% [95% CI, −2.73 to 0.57%] versus −2.74% [95% CI, −4.02 to 1.46%]; P = 0.12) or the odds of PEx (OR, 1.21 [95% CI, 0.45 to 3.28]; P = 0.70). Differential virulence among Achromobacter species was not observed, and no cases of transmission occurred. We demonstrated that incident Achromobacter infection was associated with a greater risk of PEx; however, neither transient nor chronic infection was associated with a worsened long-term prognosis. Large, multicenter studies are needed to clarify the clinical impact, natural history, and transmissibility of Achromobacter.
KEYWORDS: Achromobacter xylosoxidans, infection transmission, emerging infections, epidemiology, eradication, infection control, inhaled corticosteroids, multilocus sequence typing, pulsed-field gel electrophoresis, whole-genome sequencing
INTRODUCTION
Chronic inflammation and recurrent/chronic lung infection are the primary contributors to morbidity and mortality in cystic fibrosis (CF) patients (1). The significance of lower airway infection with classical CF pathogens, such as Pseudomonas aeruginosa, Staphylococcus aureus, and Haemophilus influenzae, is well established. Recently, enhanced microbiologic techniques have identified increasing numbers of pathogens infecting the lower airways (2). One such example is Achromobacter spp., in particular Achromobacter xylosoxidans, which is now well recognized in CF populations (2). Although early studies attributed all Achromobacter infections to A. xylosoxidans, a broad range of Achromobacter species exist in CF patients. Several species of Achromobacter have been identified (3), and the prevalences of Achromobacter ruhlandii (4) and Achromobacter insuavis (5, 6) have been observed to rival that of A. xylosoxidans.
Achromobacter spp. are aerobic, Gram-negative, catalase- and oxidase-positive, nonfermenting bacilli that are widely distributed in the environment (7). They are opportunistic pathogens, particularly in older patients, and have been recovered from blood, urine, the respiratory tract, and cerebrospinal fluid (CSF) (2, 8). They possess innate antimicrobial resistance, readily acquire adaptive resistance with antimicrobial exposure (9), and alter the expression of certain genes to promote chronic infection (10).
Whereas single-center European studies have reported prevalence rates of Achromobacter sp. infection in CF patients of 5 to 29% (7, 9, 11–14), national registries suggest rates of 4 to 7% (15, 16). The clinical impact of Achromobacter sp. respiratory infection remains unclear, as the evidence is confined to small cohort studies over short periods, with varied results (11, 12, 14, 17). Whereas some groups have identified clonality among isolates from patients with chronic Achromobacter sp. infections (18, 19), others have not (20, 21). Given the paucity of outcome data for North American CF cohorts, we sought to understand the natural history, impact, and epidemiology of Achromobacter sp. infections in a large Canadian CF center.
RESULTS
Population characteristics.
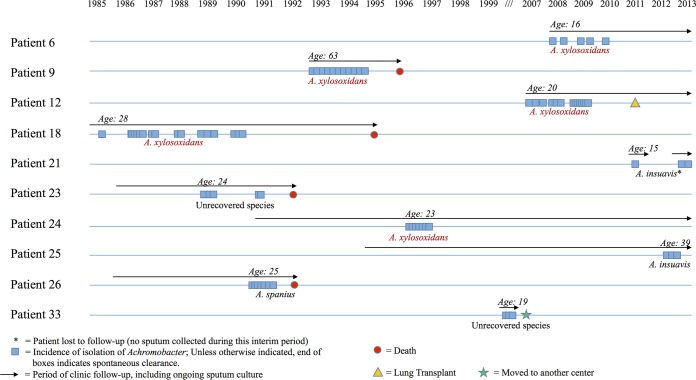
Between 1984 and 2013, Achromobacter spp. were cultured from the sputa of 34 patients from our clinic of 306 CF patients (11%). Twelve patients had Achromobacter spp. cultured more than once, while 10 (29% of the cohort) met our predefined criteria for persistent infection (Fig. 1). Despite persistent infections ranging from 1 to 5 years, all but one patient eventually cleared Achromobacter spp., as determined by culture, for a period of at least 3 months before leaving the cohort (four died, one moved to another center, and five continue to be monitored). Patients had a median of nine negative sputum cultures collected following clearance of Achromobacter. One patient had ongoing infection at study completion, for a period lasting 3 years.
FIG 1.
Natural history of chronic infection with Achromobacter species. The incidence of Achromobacter isolation by time is shown for patients with persistent infection. The age at incident Achromobacter infection is documented. Species were identified as Achromobacter spp. at initial culture and continued with this designation if the isolate was not recoverable (discarded or nonviable) from the biobank for species analysis.
Fifteen patients of the total cohort (44%) were male. The median age at first culture was 24 years (interquartile range [IQR], 20.3 to 29.8 years) (Table 1). The median forced expiratory volume in 1 s (FEV1) predicted at study entry (2 years prior to the first Achromobacter culture) was 51% (IQR, 35.5 to 81.3%), and the forced vital capacity (FVC) predicted was 73% (IQR, 56.3 to 95.8%). Most patients (88%) were not on supplemental home oxygen. The median body mass index (BMI) of the cohort was 19.8 kg/m2 (IQR, 18.3 to 21.4 kg/m2). Eleven patients (32%) were on chronic antibiotics (seven on inhaled tobramycin and four on oral azithromycin) at the time of Achromobacter culture. The median number of visits in the 2 years prior to incident isolation was 8 (IQR, 5 to 12), and that in the 2 years following was 10 (IQR, 7 to 14). The median number of sputum samples collected in the 2 years following “transient” infection was 6 (IQR, 4 to 10). In patients with persistent Achromobacter infection (during which they were defined as being “persistent”), the percentage of positive sputum cultures was 79% (IQR, 70 to 88%). Thirty patients (88%) were chronically coinfected with P. aeruginosa, while 26/34 (76%) patients had coinfection with S. aureus.
TABLE 1.
Demographics of patients with Achromobacter sp. infectiona
| Parameter | Value for group |
P valued |
||||
|---|---|---|---|---|---|---|
| Total (n = 34) | Transient infection (n = 24) | Persistent infection (n = 10) | Controls (n = 18) | Transient infection vs persistent infection | Persistent infection vs controls | |
| Median age (IQR) | 24 (20.3–29.8) | 25 (21.0–30.3) | 24 (19.3–27.3) | 22 (18.0–25.8) | 0.80 | 0.45 |
| No. (%) of males | 15 (44.1) | 9 (37.5) | 6 (60.0) | 11 (61.1) | 0.23 | 0.95 |
| Median BMI (IQR) | 20 (18.3–21.4) | 20 (18.4–21.1) | 19 (18.2–21.8) | 21 (19.2–23.1) | 0.76 | 0.50 |
| No. (%) of patients with pancreatic insufficiency | 29 (85.3) | 20 (83.3) | 9 (90.0) | 13 (72.2) | 0.62 | 0.27 |
| Median FVC% (IQR) | 73 (56.3–95.8) | 69 (56.8–102.3) | 75 (54.8–85.5) | 86 (64.8–104.8) | 0.25 | 0.11 |
| Median FEV1% (IQR) | 51 (35.5–81.3) | 47 (34.5–87.3) | 57 (38.3–75.5) | 66 (43.3–88.5) | 0.63 | 0.22 |
| No. (%) of patients with home O2 | 4 (11.8) | 3 (12.5) | 1 (10.0) | 0 (0.0) | 0.84 | 0.17 |
| No. (%) of patients who received: | ||||||
| Inhaled tobramycinb | 7 (20.6) | 5 (20.8) | 2 (20.0) | 4 (22.2) | 0.96 | 0.89 |
| Azithromycinb | 4 (11.8) | 3 (12.5) | 1 (10.0) | 4 (22.2) | 0.84 | 0.42 |
| Inhaled corticosteroid | 8 (23.5) | 3 (12.5) | 5 (50.0) | 3 (16.7) | 0.02 | 0.06 |
| No. (%) of patients with chronic coinfection | ||||||
| P. aeruginosa | 28 (82.4) | 20 (83.3) | 8 (80.0) | 12 (66.6) | 0.82 | 0.45 |
| S. aureus | 25 (73.5) | 17 (70.8) | 8 (80.0) | 7 (38.8) | 0.58 | 0.04 |
| No. (%) of patients with comorbidityc | ||||||
| CFRD | 5 (14.7) | 3 (12.5) | 2 (20.0) | 3 (16.7) | 0.57 | 0.83 |
| Sinus disease | 10 (29.4) | 6 (25.0) | 4 (40.0) | 10 (55.6) | 0.38 | 0.43 |
| Bone disease | 9 (26.5) | 7 (29.2) | 2 (20.0) | 4 (22.2) | 0.58 | 0.89 |
| CFLD | 8 (23.5) | 6 (25.0) | 2 (20.0) | 2 (11.1) | 0.75 | 0.52 |
| DIOS | 3 (8.8) | 3 (12.5) | 0 (0.0) | 2 (11.1) | 0.24 | 0.27 |
Baseline data are from the 2 years prior to incident Achromobacter culture. Note that the “persistent” cohort included a 10th patient who was unable to be compared to control patients for clinical outcomes.
Azithromycin or tobramycin use at time of Achromobacter sp. culture or control study entry.
CFRD, CF-related diabetes; CFLD, CF liver disease; DIOS, distal intestinal obstruction syndrome.
Values in bold indicate those which were statistically significant.
Pulmonary exacerbation (PEx) at the first isolation of Achromobacter occurred in 14 patients (42%; 95% confidence interval [CI], 25 to 58%). Relative to the visits immediately before and after, patients were more likely to experience PEx at incident culture, i.e., 14/33 (42%) visits versus 14/66 (21%) visits (odds ratio [OR], 2.7 [95% CI, 1.1 to 6.7]; P = 0.03). Of these exacerbations at first isolation, 4 (29%) were severe, requiring intravenous therapies and/or hospitalization.
The mean age at first isolation was not significantly different between those who experienced exacerbation (23.8 years) and those who did not (28.2 years) (difference, 4.4 years; 95% CI, −2.1 to 10.9 years). There was also no difference in baseline FEV1 (as recorded at the visit prior to first isolation) in those with exacerbation (FEV1, 57.5%) and those without exacerbation (FEV1, 50.9%) (difference, −6.6%; 95% CI, −28.5 to 15.3%). Further, patients on chronic antibacterials were not more likely to experience exacerbation than those not on chronic antibacterials (4/14 patients [28.6%] versus 5/20 patients [25%]) (difference, 3.57%; 95% CI, −24% to 33%). An increasing bioburden as measured in CFU per milliliter of sputum did not predict exacerbation risk (data not shown). The 10 patients who eventually developed persistent infection were no more likely to experience PEx at the time of incident Achromobacter infection than the patients with transient infections (4/10 patients [40%] versus 10/24 patients [42%]; risk ratio [RR], 1.0 [95% CI, 0.3 to 2.8]).
Epidemiology of CF patients with persistent infection.
Nine of the 10 patients with persistent infection were matched to age- and cohort-matched patients (we were unable to obtain suitable control patients against the 10th patient, a 64-year-old female). Patient characteristics were not different between those with persistent infection and age-matched controls. The control group trended toward improved pulmonary and nutritional outcomes (Table 1). Medications did not significantly differ in the control populations, apart from inhaled corticosteroids (ICS), which were prescribed more commonly in those who developed persistent Achromobacter sp. infection. In a comparison of the persistent and matched cohorts for baseline bacterial coinfection, 8/10 patients (80.0%) versus 12/18 patients (66.6%) (P = 0.45) were coinfected with P. aeruginosa, while 8/10 patients (80.0%) versus 7/18 patients (38.8%) (P = 0.04) were coinfected with S. aureus.
Association of Achromobacter sp. infections with long-term outcomes in CF patients.
In all patients who experienced Achromobacter infection, there was no significant difference in the rate of annual lung function decline (as measured by the FEV1 percent predicted) preceding compared to following Achromobacter infection (−0.79%/year [95% CI, −1.60% to 0.01%/year] versus −0.22%/year [95% CI, −1.01% to 0.57%/year]). Similarly, no significant difference in the odds of PEx was noted in the pre- and postinfection periods (OR, 0.74 [95% CI, 0.50 to 1.11]; P = 0.15).
There was no difference in annual lung function decline in those transiently versus persistently infected with Achromobacter (0.59% [95% CI, −0.73% to 1.91%] versus −0.39% [95% CI, −1.44% to 0.67%]; P = 0.26). The odds of experiencing a PEx following infection in these cohorts were not significantly different (OR, 1.02 [95% CI, 0.42 to 2.46]; P = 0.96).
Comparing persistently infected patients to matched controls demonstrated no difference in annual lung function decline (−1.08% [95% CI, −2.73% to 0.57%] versus −2.74% [95% CI, −4.02% to −1.46%]; P = 0.12). Further, there was no significantly increased risk in PEx occurrence in the persistently infected cohort relative to matched controls (OR, 1.21 [95% CI, 0.45 to 3.28]; P = 0.70).
Microbiological characteristics of Achromobacter.
Within our biobank, 115 Achromobacter isolates were found, spanning 29 years. We sought only incident isolates, the last available isolate for each patient in the biobank, and isolates at 2-year intervals for individuals with prolonged carriage. Some isolates were either not found or not recovered from the frozen state despite multiple attempts. We were able to characterize 31 available isolates (19 incident, 3 intermediate, and 9 follow-up isolates) from 18/34 patients. Whereas isolates were exclusively identified as A. xylosoxidans by the clinical microbiology laboratory in real time, retrospective analysis of samples from the biobank revealed a broader distribution of isolates, as follows: A. xylosoxidans, 9 isolates (50%); A. insuavis, 5 isolates (28%); Achromobacter dolens, 2 isolates (11%); Achromobacter spanius, 1 isolate (6%); and A. ruhlandii, 1 isolate (6%). Species establishing persistent infections were as follows: A. xylosoxidans, 5/8 isolates (62.5%); A. insuavis, 2/8 isolates (25%); and A. spanius, 1/8 isolates (12.5%). A. xylosoxidans was not more likely to culminate in persistent infection than in transient infection (5/8 versus 4/10 isolates; RR, 1.6 [95% CI, 0.6 to 4.0]; P = 0.63).
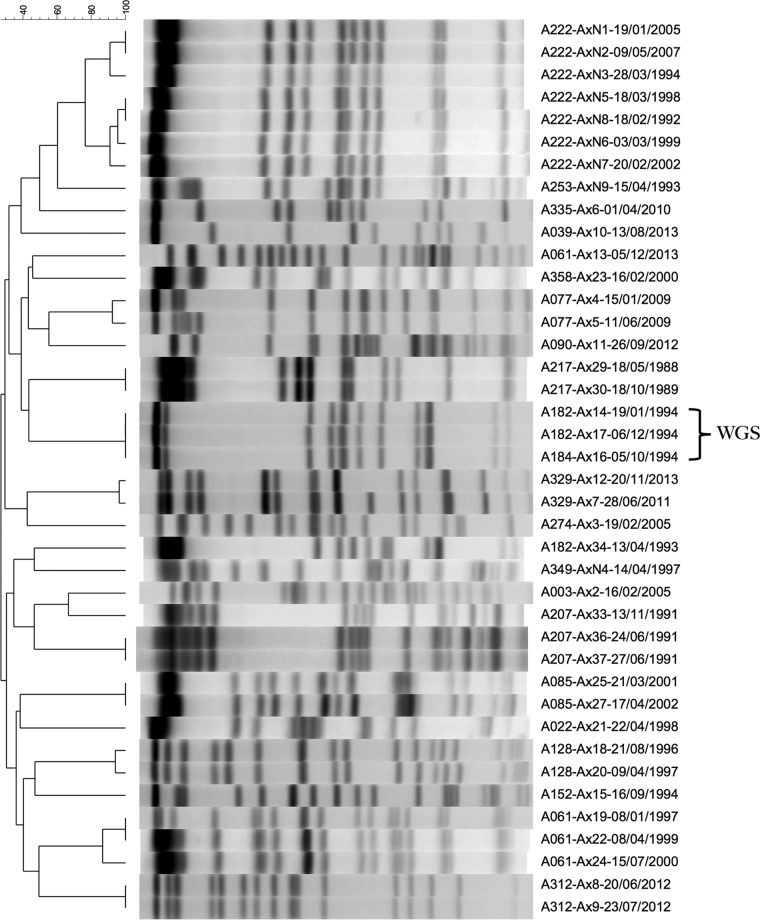
Pulsed-field gel electrophoresis (PFGE) was used to genotype the 31 available strains. A total of 89% (16/18) of patients were initially identified to be infected by unique Achromobacter sp. pulsotypes (Fig. 2). The results suggested one possible shared strain between two patients in 1994 (a strain chronically infecting a 64-year-old female was transiently identified in a 31-year-old female). Clonality between the isolates was confirmed by whole-genome sequencing (WGS) (the isolates differed by 15 single nucleotide polymorphisms [SNPs]) (10, 22; data not shown). Upon confirmation testing of other pathogens within the suspect sputum sample, we identified that in addition to the A. xylosoxidans strain of patient A182 being identified in the sputum of patient A184, the P. aeruginosa PFGE pulsotypes of these patients were discordant (each representing nonclonal strains), such that patient A182's isolate at that particular time point showed patient A184's chronic pulsotype, and vice versa (data not shown). A review of clinical records over the prior 2 years identified no period of <48 h in which the patients shared the same space, other than the one clinic appointment during which the sputum in question was collected. Rather than transmission of infection, this was thought to represent mislabeling of sputum prior to submission to the clinical microbiology laboratory. Patients with persistent infections demonstrated stable pulsotypes over time, save for one patient (A061) whose sputum grew a second unique strain 16 years after an initial transient infection.
FIG 2.
Dendrogram for pulsed-field gel electrophoresis of Achromobacter isolates recovered from the Calgary Adult CF Clinic biobank and assessed for natural history and epidemiology. Designations show patient-strain-date (day/month/year).
There was no difference in biofilm biomass between isolates causing persistent versus transient infections (P = 0.89) (see Table S2 in the supplemental material). We also compared the biofilm production capacity of recovered Achromobacter species. A. xylosoxidans isolates produced significantly more biofilm than all included non-A. xylosoxidans isolates (P = 0.0001) as well as just those present at incident infection (P = 0.0070).
Achromobacter clearance.
As afforded by a multidecade observational cohort study, we assessed the durability of the “persistent” definition defined a priori based on those available in the literature. Over a median 3.1 (IQR, 2.6 to 6.15) years of microbiologic follow-up after incident infection, only one patient remained infected with Achromobacter. Three persistently infected patients were treated with oral antimicrobials that the Achromobacter isolate was sensitive to at the time of final culture (trimethoprim-sulfamethoxazole for PEx with known S. aureus/Achromobacter, ciprofloxacin for PEx with known P. aeruginosa/Achromobacter, and doxycycline/colistin for attempted Achromobacter eradication), and they cleared the infection following therapy completion. The remaining 60% (6/10 patients) of patients appeared to spontaneously clear infection without any antimicrobial treatment. Before the time of clearance, two of these patients developed infection with a new pathogen (S. aureus or Burkholderia cepacia complex) that may have assumed a dominant pathogenic role.
DISCUSSION
Achromobacter species derived from the sputa of CF patients are garnering increasing attention. Indeed, improvements in diagnostic techniques enabling the correct distinction of these species from other CF pathogens, such as P. aeruginosa, and selective antimicrobial pressures may have contributed to their initial recognition (19, 23), although their rates did not appear to be changing over the last decade (7). Our study is the first to report epidemiological and clinical outcome data for CF patients infected with Achromobacter species in a North American cohort.
The cumulative prevalence of Achromobacter isolation of 11% in our center is comparable to those previously reported for small centers, which ranged from 5 to 29% (11, 12), although this spans 3 decades, making the incidence of infection very low. While others have reported that older patients and patients with greater lung disease burden appear to be predisposed to infection with Achromobacter species (11, 18), we did not observe any correlation between age, nutritional status, CF comorbidities, baseline lung status, or home oxygen use and incident infection.
Rates of chronic infection have typically ranged from 3 to 12% of incident Achromobacter infections (5, 24, 25), again with the risk factors of older age and the burden of lung disease (11, 26). Coinfection with P. aeruginosa (2, 17, 19, 27) is common due to increased exposure to antibiotics for chronic infection (26). The rate of persistent infection in our population was high, at 29%, likely due to a less stringent definition (25). We found no baseline characteristics, including age and lower FEV1 scores, which were predictive of strain persistence. No microbial factors influenced the risk of progression to persistence. The significance of the larger proportion of persistently infected patients with chronic S. aureus infection, which was also observed previously (26), is unclear. Uniquely, we observed that ICS were associated with the risk of persistence, in contrast to transient infections or controls. Indeed, the use of ICS has been identified as a detrimental factor in other microbiological outcomes, including risk of infection with specific pathogens (Aspergillus fumigatus) (28), time to PEx (29), and reduced bacterial killing during PEx (30). This is potentially concerning given the overused status of ICS in CF patients (31).
Studies have attributed persistent Achromobacter infections predominantly to A. xylosoxidans (2, 11, 12, 18), with a few exceptions, including A. insuavis and A. dolens (5, 6). We now know that this may represent a broader species distribution. Nonetheless, our results reiterate these findings, with A. xylosoxidans accounting for 62% of persistent infections and, additionally, with a persistent infection by A. spanius, which has not previously been reported. The infrequent recovery of A. ruhlandii in our center differs from previous data (4, 32, 33) and may reflect variance in the environmental distribution of species in North America.
The virulence factors enabling certain Achromobacter species to persist in airways are undetermined. Biofilm production in A. xylosoxidans was previously found to be significantly associated with CF patient infections (9). It may also facilitate horizontal gene transfer between bacteria, promoting the spread of antimicrobial resistance (9, 34), which may provide a differential adaptive stability in the environment and airways to establish chronic infection (5, 24, 35). We found that A. xylosoxidans demonstrated increased biofilm biomass production compared to that of other species (A. insuavis and A. dolens). There was no evidence that this promoted persistence. We had a limited number of non-A. xylosoxidans species with which to compare biofilm production levels. Given the now understood diverse spectrum of Achromobacter species distribution, future studies should focus on differential behaviors, including biofilm production, in these species.
Ours represents the longest assessment of the epidemiology and impact of Achromobacter sp. infections. This afforded the observation that a large number of patients with persistent infection may eventually clear the Achromobacter infection, which was not previously apparent. The reason for spontaneous clearance of infection after prolonged carriage is not immediately clear and may simply reflect the natural pattern of infection for Achromobacter, the organism being overtaken by another pathogen, or immune clearance by the patient. Regardless, this highlights how the natural history of novel infections in CF airways may differ from that of P. aeruginosa, suggesting a need for unique terminology to account for these fundamental differences (7, 36). Additionally, it demonstrates the difficulty of short-term studies and the value of long-term follow-up.
Despite the increasingly frequent isolation of Achromobacter from CF patients, its pathogenicity is unclear (11, 12, 26), though the growing body of evidence seems to support the significance of its isolation (17, 37, 38). Although PEx in CF patients are common, we hypothesized a discernible clinical impact with subsequent lung function decline after Achromobacter detection. We observed that the first isolation of Achromobacter was associated with PEx and that patients were almost three times more likely to experience exacerbation than at the visit before or after incident isolation, which has not previously been noted. Factors predicting who might experience exacerbation at first isolation, including age, lung function, or bioburden, were not obvious in this small study. Patients on chronic suppressive antimicrobial therapy were just as likely to experience exacerbation at the first isolation with Achromobacter. We did not assess viral pathogens or nonpathogenic contributors to exacerbation risk (environment, pollution, or therapy compliance); furthermore, there may be alternative, patient-specific factors that are not yet clear but that increase the risk of exacerbation.
Predicting the clinical course following persistent Achromobacter isolation is challenging. Studies of small European and South American cohorts have found no evidence of decline in FEV1 (11, 12, 39), while others dispute this (14, 17, 37). An increased need for antimicrobials (11) and hospitalization (26) have also been reported. We found no evidence of clinical decline (lung function loss or risk of PEx) following infection, nor was there a significant change in nutritional status (BMI) over the course of follow-up, similar to the results of a previous study (12). It may be valuable to follow-up with patients for a longer duration to see if such correlations become evident. Treating patients who showed exacerbation at first isolation with oral antibiotics did not reduce the subsequent PEx frequency over the next 2 years.
Given the negative impact associated with chronic P. aeruginosa infection, the practice of early eradication has become applied universally, with multiple regimens being compared for efficacy (40). Due to concerns about the adverse impact and antibiotic resistance of Achromobacter spp., some groups, understandably, have adopted a similar practice (41). In the present study, we did not note that changing therapy at the time of incident Achromobacter culture reduced the risk of progression to persistent infection. Given the global importance of these organisms, it seems that a multicenter study similar to STAR-2 (studying methicillin-resistant S. aureus [MRSA] eradication versus placebo) is in order (42).
The transmissibility of Achromobacter is controversial. Two small single-center studies found that all patients were infected with unique Achromobacter strains (20, 21). Conversely, several studies (both single and multicenter) have suggested that shared strains do exist (2, 12, 18, 19, 24, 25, 27, 33, 43–48), with common strains accounting for 5 to 50% of total Achromobacter infections. Additionally, chronic infection was most commonly due to persistence of the original infecting strain (14, 19, 45). Extreme variations in the prevalence of shared clones of other organisms known to be transmissible (e.g., P. aeruginosa) exist in different clinics, reflecting some combination of organism fitness, patient population, and historical infection control practices. In our center, where an epidemic P. aeruginosa strain accounts for >1/3 of all chronic infections, we confirmed that no true shared infections occurred (49). Uniquely relative to other studies of infection transmission in CF patients, we sought to confirm potential patient-patient spread not only through WGS but also by assessing other organisms in the same sputum. In doing so, we refuted a potential case of infection transmission and identified an important step relevant to future studies of infection transmission in CF patients. Specimen mislabeling is among the most common preanalytic errors identified in clinical laboratories and is a particular risk in environments, such as CF clinics, where the same sample type is collected from multiple patients (50). Our data support the argument that transmission of Achromobacter among CF patients appears to be an uncommon event.
A primary strength of our study is that we are one of the first to describe the epidemiology and outcomes of Achromobacter infection, including by species, but several limitations must be considered. These include the retrospective study design, selection bias, and information bias, including misclassification of infection status and missing data (including the inability to determine species type for 41% of our cohort). We also must be cognizant of the potential bias of effect modification and the difference in CF management given the length of time spanned by our study. Patients had serial cultures enabling us to study incident cases of Achromobacter infection, but it is possible that some were prevalent cases based on sampling frequency. It is possible that despite serial cultures, the association of increased risk of exacerbation occurrence with the first isolation of Achromobacter was coincidental and that incident infection occurred prior to exacerbation. Our ability to assess clinical outcomes was also limited by the study sample size, and we suggest that multicenter and/or registry-based studies of acute and chronic Achromobacter sp. infections in North American cohorts are warranted. Furthermore, given the lag time after samples were collected, a proportion of isolates were not recoverable from our biobank, thereby limiting our ability to determine species and to assess biofilm formation. In selecting controls without predetermined limitations (i.e., only those with chronic P. aeruginosa infection), we were unable to compare the relative pathogenic potentials of Achromobacter spp. versus other organisms. Such a consideration may be valuable in future studies drawing from a larger control patient population. Changes in PEx frequency or lung function decline may have been evident in a larger cohort or with longer follow-up. Finally, there is currently no uniform definition of “chronic” or “persistent” Achromobacter infection. While our definition is similar to those applied previously to chronic Pseudomonas infection (51, 52) and there is value in developing uniform definitions for epidemiologic purposes, we have demonstrated that a definition derived through a detailed understanding of organism-specific natural history acquired from long-term studies supersedes uniform standardized periods, as other organisms may lack the long-term tenacity of P. aeruginosa.
Conclusions.
In this retrospective cohort study spanning a period of almost 30 years, we studied, for the first time, the epidemiology, clinical impact, and transmissibility of Achromobacter infections in CF patients in a North American population. The prevalence and species distribution of Achromobacter in this cohort were similar to those in other small center studies. Baseline characteristics of patients persistently infected with Achromobacter, including age, lung function, antibiotic use, and CF comorbidities, were not different. There was no evidence of lung function decline or risk of PEx following persistent infection with Achromobacter. Notably, patients were at increased risk of experiencing PEx at the time of first isolation of Achromobacter. We found that patients with persistent infection maintained the same strain for prolonged periods, with no evidence of transmission.
MATERIALS AND METHODS
Population.
The Calgary Adult CF Clinic monitors all patients with CF residing in Southern Alberta, Canada. Upon clinic enrollment, patients provide consent for prospective collection, storage, and analysis of respiratory secretions and sputum-derived organisms. Patient follow-up is intended to be quarterly. Patients were included if Achromobacter spp. were cultured from routine assessments of sputum from January 1984 to December 2013. Patients were classified as having transient infection (defined as having ≥1 positive culture but not meeting the definition for persistent infection) or persistent infection (defined as having ≥50% of all cultures in a 12-month period that grew Achromobacter [with ≥3 cultures collected]). For patients who were persistently infected, we collected data on control CF patients (age [±2 years], birth cohort, and sex matched at a 2:1 ratio) without a history of Achromobacter sputum infection.
Clinical data.
We collected data through chart review for 2 years preceding and following the initial Achromobacter sp. culture. For the CF controls, we collected data for two consecutive years matched to those of our test patients. For all patients, baseline demographic data (age, sex, BMI, and CF mutations), medications, pulmonary function as measured by forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) at each visit, comorbid sputum pathogens, exacerbations, and medical comorbidities were recorded.
The primary outcome of interest was the proportion of patients with pulmonary exacerbation (PEx) at the time of first isolation of Achromobacter relative to those for the visits immediately preceding and following isolation. A PEx was defined as symptoms consistent with acute infection for which new oral or parenteral antimicrobial therapy was started by the CF physician in either the clinic or hospital setting (2, 53). Prior work from our group demonstrated concordance between clinically diagnosed PEx and Fuchs criteria (30). Next, we assessed the impacts of transient and persistent Achromobacter infections, including (i) rate and risk factors for progression to persistent Achromobacter infection, (ii) differential decline in FEV1 in patients following Achromobacter infection, and (iii) the risk of PEx after initial Achromobacter infection. Although we collected clinical data for 2 years pre- and postinfection, we monitored patients longer to assess the pattern of infection. This study was approved by the conjoint health research ethics board at the University of Calgary (approvals REB-15-0854 and REB-15-2744).
Characterization of Achromobacter species.
All Achromobacter species were identified as part of routine care by use of standard methodologies (54). In real time, isolates were frozen in skim milk and stored at −80°C. From our prospectively maintained biobank, we retrospectively confirmed the genus identification and characterized viable isolates. All first and last available isolates, as well as isolates at 2-year intervals (where available), were retrospectively assessed. PCR sequencing of the 16S rRNA gene was used to confirm that the isolates were from the genus Achromobacter, using single-colony preparations and primers 8F and 926R and running the results through NCBI's GenBank. Isolates with >99% sequence identity to Achromobacter spp. were considered to be Achromobacter. Species identification was determined by nrdA locus sequencing, one of the multilocus sequence typing (MLST) loci for Achromobacter spp. (18, 54).
To investigate the presence of clonality, strains underwent pulsed-field gel electrophoresis (PFGE) according to established protocols adapted from the work of Parkins et al. (36). SpeI (New England BioLabs)-digested samples were run in 1% SeaKem Gold agarose. Dendrograms were generated with a 1.0% position tolerance, using the unweighted-pair group method using average linkages (UPGMA) and the Sørensen-Dice similarity coefficient. Strains with banding patterns that were ≥80% identical (≤3 band differences) were considered related, conforming to the Tenover criteria (55). To investigate if strains with the same PFGE pulsotype were acquired independently from the natural environment or related more directly via patient-patient spread, isolates underwent whole-genome sequencing (WGS) and single nucleotide polymorphism (SNP) analysis (56). If a suspected case of transmission was identified, we sought to perform genotyping on other relevant organisms from the same sputum sample to ensure that a true event had occurred.
To assess if biofilm formation played a role in Achromobacter sp. airway persistence, a modification of the protocol of Tomlin et al. (57) was performed. Isolates were grown overnight in tryptic soy broth, normalized to an optical density at 600 nm (OD600) of 0.01, and plated in Nunclon Delta Surface 96-well plates with Nunc-Immuno TSP lids with pins (Thermo Scientific, Kamstrupvej, Roskilde, Denmark). Plates were then incubated at 37°C overnight on a rocker table. The biofilms that formed on lid pins were stained with 0.1% crystal violet, subsequently washed with water, and destained with 95% ethanol. Finally, a plate reader was used to quantify crystal violet staining at 550 nm.
Statistical analysis.
Symmetrical and asymmetrical variables were described as means with standard deviations (SD) and medians with interquartile ranges (IQR), respectively. Pairwise comparisons were conducted using the Wilcoxon rank sum test for continuous variables and the Fisher exact test for proportions. Unadjusted risk ratios were calculated to determine the PEx risk at initial acquisition compared to that at preceding or subsequent clinical encounters. Mixed-effects linear regression models with an exchangeable correlation structure were conducted to assess the rate of lung function decline. Mixed-effects logistic regression models with a Poisson distribution were constructed to assess the odds of PEx. The mixed-effects models were utilized to compare pre- and post-Achromobacter infection variables within patients, between the transient and persistent groups, and between patients with persistent infection and matched controls. All hypotheses were evaluated with a two-sided α value of 0.05, and analyses were conducted with STATA V14.2 (StataCorp, College Station, TX).
Supplementary Material
ACKNOWLEDGMENTS
B.D.E., J.G.-W., R.S., B.W., F.J.W., and D.G.S. have no conflicts to report. M.D.P., H.R.R., and M.G.S. have received research support from Gilead Sciences. M.D.P. and H.R.R. have performed advisory board work for Gilead, Novartis, Roche, and Vertex. No conflicts are relevant to the work discussed herein.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.02556-16.
REFERENCES
- 1.Stoltz DA, Meyerholz DK, Welsh MJ. 2015. Origins of cystic fibrosis lung disease. N Engl J Med 372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambiase A, Catania MR, Del Pezzo M, Rossano F, Terlizzi V, Sepe A, Raia V. 2011. Achromobacter xylosoxidans respiratory tract infection in cystic fibrosis patients. Eur J Clin Microbiol Infect Dis 30:973–980. doi: 10.1007/s10096-011-1182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandamme P, Moore ERB, Cnockaert M, De Brandt E, Svensson-Stadler L, Houf K, Spilker T, LiPuma JJ. 2013. Achromobacter animicus sp. nov., Achromobacter mucicolens sp. nov., Achromobacter pulmonis sp. nov. and Achromobacter spiritinus sp. nov., from human clinical samples. Syst Appl Microbiol 36:1–10. doi: 10.1016/j.syapm.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Spilker T, Vandamme P, Lipuma JJ. 2013. Identification and distribution of Achromobacter species in cystic fibrosis. J Cyst Fibros 12:298–301. doi: 10.1016/j.jcf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Amoureux L, Bador J, Zouak FB, Chapuis A, de Curraize C, Neuwirth C. 2016. Distribution of the species of Achromobacter in a French cystic fibrosis centre and multilocus sequence typing analysis reveal the predominance of A. xylosoxidans and clonal relationships between some clinical and environmental isolates. J Cyst Fibros 15:486–494. doi: 10.1016/j.jcf.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 6.Coward A, Kenna DTD, Perry C, Martin K, Doumith M, Turton JF. 2016. Use of nrdA gene sequence clustering to estimate the prevalence of different Achromobacter species among cystic fibrosis patients in the UK. J Cyst Fibros 15:479–485. doi: 10.1016/j.jcf.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Parkins MD, Floto RA. 2016. Emerging bacterial pathogens and changing concepts of bacterial pathogenesis in cystic fibrosis. J Cyst Fibros 14:293–304. doi: 10.1016/j.jcf.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Ridderberg W, Bendstrup KEM, Olesen HV, Jensen-Fangel S, Nørskov-Lauritsen N. 2011. Marked increase in incidence of Achromobacter xylosoxidans infections caused by sporadic acquisition from the environment. J Cyst Fibros 10:466–469. doi: 10.1016/j.jcf.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Trancassini M, Iebba V, Citera N, Tuccio V, Magni A, Varesi P, Biase RV, De Totino V, Santangelo F, Gagliardi A, Schippa S. 2014. Outbreak of Achromobacter xylosoxidans in an Italian cystic fibrosis center: genome variability, biofilm production, antibiotic resistance, and motility in isolated strains. Front Microbiol 5:138. doi: 10.3389/fmicb.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridderberg W, Nielsen SM, Norskov-Lauritsen N. 2015. Genetic adaptation of Achromobacter sp. during persistence in the lungs of cystic fibrosis patients. PLoS One 10:1–14. doi: 10.1371/journal.pone.0136790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Baets F, Schelstraete P, Van Daele S, Haerynck F, Vaneechoutte M. 2007. Achromobacter xylosoxidans in cystic fibrosis: prevalence and clinical relevance. J Cyst Fibros 6:75–78. doi: 10.1016/j.jcf.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Raso T, Bianco O, Grosso B, Zucca M, Savoia D. 2008. Achromobacter xylosoxidans respiratory tract infections in cystic fibrosis patients. APMIS 116:837–841. doi: 10.1111/j.1600-0463.2008.00995.x. [DOI] [PubMed] [Google Scholar]
- 13.Amoureux L, Bador J, Siebor E, Taillefumier N, Fanton A, Neuwirth C. 2013. Epidemiology and resistance of Achromobacter xylosoxidans from cystic fibrosis patients in Dijon, Burgundy: first French data. J Cyst Fibros 12:170–176. doi: 10.1016/j.jcf.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Ronne Hansen C, Pressler T, Høiby N, Gormsen M. 2006. Chronic infection with Achromobacter xylosoxidans in cystic fibrosis patients; a retrospective case control study. J Cyst Fibros 5:245–251. doi: 10.1016/j.jcf.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 15.LiPuma JJ. 2010. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev 23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cystic Fibrosis Foundation. 2010. Cystic Fibrosis Foundation patient registry annual data report. Cystic Fibrosis Foundation, Bethesda, MD. [Google Scholar]
- 17.Llorca Otero L, Girón Moreno R, Buendía Moreno B, Valenzuela C, Guiu Martínez A, Alarcón Cavero T. 2016. Achromobacter xylosoxidans infection in an adult cystic fibrosis unit in Madrid. Enferm Infecc Microbiol Clin 34:184–187. doi: 10.1016/j.eimc.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Barrado L, Brañas P, Orellana MÁ, Martínez MT, García G, Otero JR, Chaves F. 2013. Molecular characterization of Achromobacter isolates from cystic fibrosis and non-cystic fibrosis patients in Madrid, Spain. J Clin Microbiol 51:1927–1930. doi: 10.1128/JCM.00494-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cools P, Ho E, Vranckx K, Schelstraete P, Wurth B, Franckx H, Ieven G, Van Simaey L, Van Daele S, Verhulst S, De Baets F, Vaneechoutte M. 2016. Epidemic Achromobacter xylosoxidans strain among Belgian cystic fibrosis patients and review of literature. BMC Microbiol 16:122. doi: 10.1186/s12866-016-0736-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vu-Thien H, Moissenet D, Valcin M, Dulot C, Tournier G, Garbarg-Chenon A. 1996. Molecular epidemiology of Burkholderia cepacia, Stenotrophomonas maltophilia, and Alcaligenes xylosoxidans in a cystic fibrosis center. Eur J Clin Microbiol Infect Dis 15:876–879. doi: 10.1007/BF01691221. [DOI] [PubMed] [Google Scholar]
- 21.Dunne WM, Maisch S. 1995. Epidemiological investigation of infections due to Alcaligenes species in children and patients with cystic fibrosis: use of repetitive-element-sequence polymerase chain reaction. Clin Infect Dis 20:836–841. doi: 10.1093/clinids/20.4.836. [DOI] [PubMed] [Google Scholar]
- 22.Ormerod KL, George NM, Fraser JA, Wainwright C, Hugenholtz P. 2015. Comparative genomics of non-pseudomonal bacterial species colonising paediatric cystic fibrosis patients. PeerJ 3:1–27. doi: 10.7717/peerj.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igra-Siegman Y, Chmel H, Cobbs C. 1980. Clinical and laboratory characteristics of Achromobacter xylosoxidans infection. J Clin Microbiol 11:141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dupont C, Michon AL, Jumas-Bilak E, Norskov-Lauritsen N, Chiron R, Marchandin H. 2015. Intrapatient diversity of Achromobacter spp. involved in chronic colonization of cystic fibrosis airways. Infect Genet Evol 32:214–223. doi: 10.1016/j.meegid.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Pereira RHV, Carvalho-Assef AP, Albano M, Folescu TW, Jones MCMF, Leão S, Marques EA. 2011. Achromobacter xylosoxidans: characterization of strains in Brazilian cystic fibrosis patients. J Clin Microbiol 49:3649–3651. doi: 10.1128/JCM.05283-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Firmida MC, Pereira RHV, Silva EASR, Marques EA, Lopes AJ. 2016. Clinical impact of Achromobacter xylosoxidans colonization/infection in patients with cystic fibrosis. Braz J Med Biol Res 49:11–15. doi: 10.1590/1414-431X20155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Daele S, Verhelst R, Claeys G, Verschraegen G, Franckx H, Van Simaey L, de Ganck C, De Baets F, Vaneechoutte M. 2005. Shared genotypes of Achromobacter xylosoxidans strains isolated from patients at a cystic fibrosis rehabilitation center. J Clin Microbiol 43:2998–3002. doi: 10.1128/JCM.43.6.2998-3002.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noni M, Katelari A, Dimopoulos G, Kourlaba G, Spoulou V, Athanassoulis HA, Doudounakis SE, Bakoula CT. 2014. Inhaled corticosteroids and Aspergillus fumigatus isolation in cystic fibrosis. Med Mycol 52:715–722. doi: 10.1093/mmy/myu038. [DOI] [PubMed] [Google Scholar]
- 29.Block JK, Vandemheen KL, Tullis E, Fergusson D, Doucette S, Haase D, Berthiaume Y, Brown N, Wilcox P, Bye P, Bell S, Noseworthy M, Pedder L, Freitag A, Paterson N, Aaron SD. 2006. Predictors of pulmonary exacerbations in patients with cystic fibrosis infected with multi-resistant bacteria. Thorax 61:969–974. doi: 10.1136/thx.2006.061366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam JC, Somayaji R, Surette MG, Rabin HR, Parkins MD. 2015. Reduction in Pseudomonas aeruginosa sputum density during a cystic fibrosis pulmonary exacerbation does not predict clinical response. BMC Infect Dis 15:145. doi: 10.1186/s12879-015-0856-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balfour-Lynn IM, Welch K. 2012. Inhaled corticosteroids for cystic fibrosis. Cochrane Database Syst Rev 11:CD001915. doi: 10.1002/14651858.CD001915.pub5. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues ER, Ferreira AG, Leao RS, Leite CC, Carvalho-Assef AP, Albano RM, Marques EA. 2015. Characterization of Achromobacter species in cystic fibrosis patients: comparison of blaOXA-114 PCR amplification, multilocus sequence typing, and matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 53:3894–3896. doi: 10.1128/JCM.02197-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridderberg W, Wang M. 2012. Multilocus sequence analysis of isolates of Achromobacter from patients with cystic fibrosis reveals infecting species other than Achromobacter xylosoxidans. J Clin Microbiol 50:2688–2694. doi: 10.1128/JCM.00728-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eberl L, Tümmler B. 2004. Pseudomonas aeruginosa and Burkholderia cepacia in cystic fibrosis: genome evolution, interactions and adaptation. Int J Med Microbiol 294:123–131. doi: 10.1016/j.ijmm.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Amoureux L, Bador J, Fardeheb S, Mabille C, Couchot C, Massip C, Salignon A. 2013. Detection of Achromobacter xylosoxidans in hospital, domestic, and outdoor environmental samples and comparison with human clinical isolates. Appl Environ Microbiol 79:7142–7149. doi: 10.1128/AEM.02293-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parkins MD, Glezerson BA, Sibley CD, Sibley KA, Duong J, Purighalla S, Mody CH, Workentine ML, Storey DG, Surette MG, Rabin HR. 2014. Twenty-five-year outbreak of Pseudomonas aeruginosa infecting individuals with cystic fibrosis: identification of the prairie epidemic strain. J Clin Microbiol 52:1127–1135. doi: 10.1128/JCM.03218-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen CR, Pressler T, Nielsen KG, Jensen P, Bjarnsholt T, Høiby N. 2010. Inflammation in Achromobacter xylosoxidans infected cystic fibrosis patients. J Cyst Fibros 9:51–58. doi: 10.1016/j.jcf.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 38.De Baets F, Schelstraete P, Haerynck F, Van Biervliet S, De Bruyne R, Franckx H, Van Daele S. 2014. Achromobacter xylosoxidans induced bronchiolitis obliterans in cystic fibrosis. Pediatr Pulmonol 49:414–416. doi: 10.1002/ppul.22864. [DOI] [PubMed] [Google Scholar]
- 39.Tan K, Conway SP, Brownlee KG, Etherington C, Peckham DG. 2002. Alcaligenes infection in cystic fibrosis. Pediatr Pulmonol 34:101–104. doi: 10.1002/ppul.10143. [DOI] [PubMed] [Google Scholar]
- 40.Mogayzel PJ, Naureckas ET, Robinson KA, Brady C, Guill M, Lahiri T, Lubsch L, Matsui J, Oermann CM, Ratjen F, Rosenfeld M, Simon RH, Hazle L, Sabadosa K, Marshall BC, Mueller G, Hadjiliadis D, Hoag JB. 2014. Cystic Fibrosis Foundation pulmonary guideline pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Ann Am Thorac Soc 11:1640–1650. doi: 10.1513/AnnalsATS.201404-166OC. [DOI] [PubMed] [Google Scholar]
- 41.Wang M, Ridderberg W, Hansen CR, Høiby N, Jensen-Fangel S, Olesen HV. 2013. Early treatment with inhaled antibiotics postpones next occurrence of Achromobacter in cystic fibrosis. J Cyst Fibros 12:638–643. doi: 10.1016/j.jcf.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Goss C, Thompson V, Popowitch E, Howe D, Baines A, Mayer-Hamblett N, Jill V, Muhlebach M. 2015. Efficacy of a protocol for eradication of newly acquired MRSA: results of the STAR-too trial. J Cyst Fibros 14:S3. [Google Scholar]
- 43.Moissenet D, Baculard A, Valcin M, Marchand V, Tournier G, Garbarg-Chenon A, Vu-Thien H. 1997. Colonization by Alcaligenes xylosoxidans in children with cystic fibrosis: a retrospective clinical study conducted by means of molecular epidemiological investigation. Clin Infect Dis 24:274–275. doi: 10.1093/clinids/24.2.274. [DOI] [PubMed] [Google Scholar]
- 44.Hansen CR, Pressler T, Ridderberg W, Johansen HK, Skov M. 2013. Achromobacter species in cystic fibrosis: cross-infection caused by indirect patient-to-patient contact. J Cyst Fibros 12:609–615. doi: 10.1016/j.jcf.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Peltroche-Llacsahuanga H, Haase G, Kentrup H. 1998. Persistent airway colonization with Alcaligenes xylosoxidans in two brothers with cystic fibrosis. Eur J Clin Microbiol Infect Dis 17:132–134. [DOI] [PubMed] [Google Scholar]
- 46.Krzewinski JW, Nguyen CD, Foster JM, Burns JL. 2001. Use of random amplified polymorphic DNA PCR to examine epidemiology of Stenotrophomonas maltophilia and Achromobacter (Alcaligenes) xylosoxidans from patients with cystic fibrosis. J Clin Microbiol 39:3597–3602. doi: 10.1128/JCM.39.10.3597-3602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turton JF, Mustafa N, Shah J, Hampton CV, Pike R, Kenna DT. 2011. Identification of Achromobacter xylosoxidans by detection of the blaOXA-114-like gene intrinsic in this species. Diagn Microbiol Infect Dis 70:408–411. doi: 10.1016/j.diagmicrobio.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 48.Kanellopoulou M, Pournaras S, Iglezos H, Skarmoutsou N, Papafrangas E, Maniatis AN. 2004. Persistent colonization of nine cystic fibrosis patients with an Achromobacter (Alcaligenes) xylosoxidans clone. Eur J Clin Microbiol Infect Dis 23:336–339. doi: 10.1007/s10096-004-1105-9. [DOI] [PubMed] [Google Scholar]
- 49.Somayaji R, Waddell B, Workentine ML, Surette MG, Brager NP, Rabin HR, Parkins MD. 2015. Infection control knowledge, beliefs and behaviours amongst cystic fibrosis patients with epidemic Pseudomonas aeruginosa. BMC Pulm Med 15:138. doi: 10.1186/s12890-015-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snydman LK, Harubin B, Kumar S, Chen J, Lopez RE, Salem DN. 2012. Voluntary electronic reporting of laboratory errors: an analysis of 37,532 laboratory event reports from 30 health care organizations. Am J Med Qual 27:147–153. doi: 10.1177/1062860611413567. [DOI] [PubMed] [Google Scholar]
- 51.Lee TWR, Brownlee KG, Conway SP, Denton M, Littlewood JM. 2003. Evaluation of a new definition for chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Cyst Fibros 2:29–34. doi: 10.1016/S1569-1993(02)00141-8. [DOI] [PubMed] [Google Scholar]
- 52.Ramsay KA, Sandhu H, Geake JB, Ballard E, Rourke PO, Wainwright CE, Reid DW, Kidd TJ, Bell SC. 2017. The changing prevalence of pulmonary infection in adults with cystic fibrosis: a longitudinal analysis. J Cyst Fibros 16:70–77. doi: 10.1016/j.jcf.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstein BJ, Smith AL, Wohl ME. 1994. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med 331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 54.Spilker T, Vandamme P, LiPuma JJ. 2012. A multilocus sequence typing scheme implies population structure and reveals several putative novel Achromobacter species. J Clin Microbiol 50:3010–3015. doi: 10.1128/JCM.00814-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams D, Evans B, Haldenby S, Walshaw MJ, Brockhurst MA, Winstanley C, Paterson S. 2015. Divergent, coexisting Pseudomonas aeruginosa lineages in chronic cystic fibrosis lung infections. Am J Respir Crit Care Med 191:775–785. doi: 10.1164/rccm.201409-1646OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomlin KL, Malott RJ, Ramage G, Storey DG, Sokol PA, Ceri H. 2005. Quorum-sensing mutations affect attachment and stability of Burkholderia cenocepacia biofilms. Appl Environ Microbiol 71:5208–5218. doi: 10.1128/AEM.71.9.5208-5218.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.