ABSTRACT
Fecal samples submitted to our clinical microbiology laboratory from patients in the Philadelphia region were prospectively analyzed for Campylobacter species other than C. jejuni and C. coli using a filtration method and microaerobic conditions with increased H2 concentrations. Of 225 samples tested, 13 (5.8%) yielded Campylobacter species, with frequent isolation of C. concisus. The majority of Campylobacter species were not clinically significant. Additional studies in U.S. populations are warranted.
KEYWORDS: Campylobacter jejuni, campylobacter, campylobacteriosis, culture methods, gastrointestinal infection
INTRODUCTION
Campylobacter species other than C. jejuni and C. coli, such as C. concisus, C. upsaliensis, C. ureolyticus, C. sputorum, and others, are not isolated commonly from routine stool cultures due to the nonthermophilic nature of the species and/or inhibition by antimicrobial agents in commonly used selective medium (1). Except for C. upsaliensis, these species may not be detected in recently developed molecular multiplex stool pathogen test kits. Little is known about the occurrence of these species in U.S. patients. While species such as C. upsaliensis are known pathogens (2), the pathogenicity of many other species, such as C. concisus, is controversial (3). Recovery of Campylobacter spp. from stool cultures requires the addition of a filtration method and sufficient H2 in the microaerobic environment (1). The purpose of this study was to determine the frequency and clinical relevance of Campylobacter species from stool cultures in a United States-based clinical laboratory.
RESULTS AND DISCUSSION
We processed 225 fecal samples submitted to the Hospital of the University of Pennsylvania (HUP) Clinical Microbiology Laboratory for routine testing for gastrointestinal pathogens from September to December 2016 for the presence of Campylobacter spp. using the filtration method. Campylobacter spp., including C. jejuni, C. coli, C. lari (n = 10), and other species (n = 13), were recovered from 23 samples processed by this method. All of the samples from which C. jejuni, C. coli, or C. lari isolates were recovered using the filtration method were also positive in the Verigene enteric panel multiplex molecular assay (Table 1). Of the 13 isolates other than C. jejuni, C. coli, and C. lari, 4 were recovered using 0.45-μm nitrocellulose filters (30.7%), 8 (61.5%) were recovered on 0.65-μm nitrocellulose filters, and 13 (100%) were recovered from 0.6-μm polycarbonate filters, suggesting the superiority of polycarbonate filters for recovery of Campylobacter spp. (Fig. 1). These results are consistent with those reported by Nielsen et al. (4), who also found that polycarbonate filters were superior to cellulose acetate filters for recovery of C. concisus from stool samples.
TABLE 1.
Campylobacter species isolated by the filtration method
| Organism | No. positive by filtration | No. recovered on 0.45-μm nc/0.65-μm nc/0.6-μm pca | No. positive for Campylobacter spp. by Verigene multiplex testing | Hospital status (no. inpatient/no. outpatient) | Other pathogens detectedb |
|---|---|---|---|---|---|
| C. jejuni, C. coli, C. lari | 10 | 9/10/10 | 10 | 2/8 | None |
| C. concisus | 8 | 4/5/8 | 0 | 4/4 | Salmonella (V), Norovirus (V), Cryptosporidium (I) |
| C. ureolyticus | 3 | 0/3/3 | 0 | 2/1 | None |
| C. sputorum | 1 | 0/0/1 | 0 | 0/1 | None |
| C. showae | 1 | 0/0/1 | 0 | 1/0 | None |
nc, nitrocellulose; pc, polycarbonate.
Detection was by Verigene system (V) or immunoassay (I).
FIG 1.
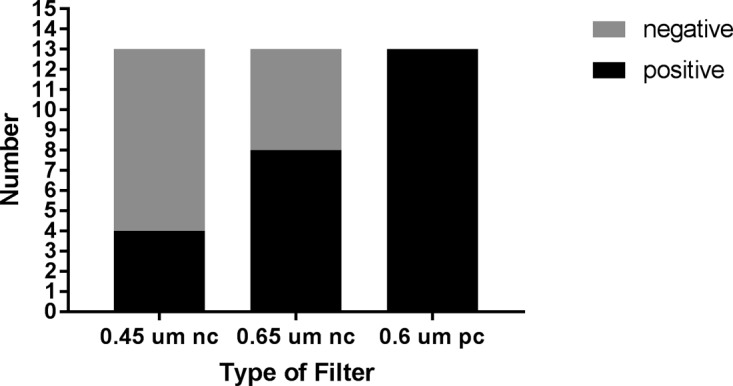
Recovery of Campylobacter species other than C. jejuni, C. coli, or C. lari using different types of filters. nc, nitrocellulose; pc, polycarbonate.
Of the 13 non-C. jejuni, -C. coli, -C. lari isolates, 4 (30.8%) were categorized as being clinically significant. C. concisus was recovered from stool samples of 8 of the 13 patients, equally distributed between male (age range, 37 to 57 years) and female (age range, 27 to 77 years) patients. Six of the 8 isolates were categorized as not clinically significant. In 3 of the 8 patients with C. concisus, well-recognized conventional pathogens were detected (Salmonella in 1, Cryptosporidium in 1, and Norovirus in 1), and 3 patients had other, noninfectious etiologies (1 patient had an inflammatory bowel disease [IBD] flare, 1 had a stroke [unclear why a sample was submitted], and 1 had bile salt-induced diarrhea). Two isolates were considered to be clinically significant based on available information in the medical record. One isolate was from a patient who had traveler's diarrhea and in whom no other routine enteric pathogens were detected. The patient had not been treated with antimicrobial agents, and the illness was self-limited. The other patient had HIV infection and 2 weeks of diarrhea that resolved without specific antimicrobial therapy.
There were 3 patients who had C. ureolyticus isolated from stool samples, and all three were female patients (aged 53, 82, and 86 years). Isolates from 2 of the 3 patients with C. ureolyticus were categorized as not clinically significant. Two isolates were from patients with gastrointestinal (GI) malignancies and were thought to be the cause of GI symptoms. One isolate was categorized as clinically significant and was from a patient with traveler's diarrhea; no other conventional pathogens were detected and the diarrhea was self-limited, resolving without specific antimicrobial treatment. There was one isolate of C. sputorum categorized as clinically significant from a male patient aged 36 years who had traveled to Mexico several months prior (>30 days), had no other enteric pathogens detected during that time, and had slowly resolving intermittent diarrhea that did not require antimicrobial therapy. C. showae isolated from one patient (a female aged 52 years) was categorized as not clinically significant (the patient had familial polyposis).
The HUP Clinical Microbiology Laboratory uses a reflex culture method for Campylobacter spp. on any stool sample positive by the Verigene Enteric Panel multiplex assay. There were 12 samples positive by Verigene that were processed for filtration where C. jejuni, C. coli, or C. lari was recovered from routine reflex culture in the clinical microbiology laboratory. Ten were positive by filtration for 83.3% sensitivity for filtration for these organisms. There was no instance where the Verigene multiplex assay was positive for Campylobacter spp., the culture was negative by reflex culture, and Campylobacter species isolates were recovered by filtration.
The filtration method was first described as a method for isolating C. jejuni from stool samples (5). Subsequently, a number of studies performed outside the United States recognized the importance of a filtration method for isolating non-C. jejuni, non-C. coli Campylobacter species from stool samples (6–8). C. concisus was the most frequently isolated Campylobacter species in our survey. In a recent study by Nielsen et al. (9), C. concisus was the Campylobacter species most frequently isolated from fecal samples using filtration, and C. ureolyticus was also detected, but they did not report on the clinical significance of these isolates. Similarly, Vandenberg et al. (7) showed that C. concisus and other species were frequently isolated from fecal samples using the filtration method in a Belgium microbiology laboratory; however, clinical details were not reported. We are not aware of any study from a U.S. laboratory on the use of the filtration method for isolating Campylobacter species from fecal samples.
The role of C. concisus as a cause of gastrointestinal infection has been the subject of debate for many years. There are no case-control studies to help delineate whether C. concisus is a significant enteropathogen; however, some studies suggest an etiologic role in certain patient populations (10). A recent study by Nielsen et al. (11) did not show, however, a difference in azithromycin therapy versus placebo in a small group of patients with C. concisus-associated diarrhea. In a questionnaire survey of patients with C. concisus isolated from fecal samples, Nielsen et al. concluded that the patients had a milder course of infection compared with patients who had C. jejuni/C. coli isolated from stool samples but were more likely to have prolonged symptoms (12). The role of other species in gastrointestinal infection such as C. ureolyticus isolated from 3 patients in our survey is less certain (13).
Campylobacter species other than C. jejuni, C. coli, or C. lari were isolated in 5.7% of fecal samples in a survey of patients from the Philadelphia region. Our study suggests that Campylobacter species other than C. jejuni, C. coli, and C. lari can be isolated frequently from U.S. patients with a filtration system and increased H2 microaerobic conditions. In most circumstances, we did not find that the isolates were clinically significant; however, several patients did not have other reasons for their diarrheal illness, which suggested that these species may be clinically relevant in certain patients. Further studies in U.S. populations are warranted.
MATERIALS AND METHODS
We prospectively cultured fecal samples submitted to the Hospital of the University of Pennsylvania (HUP) Clinical Microbiology Laboratory from September to December 2016 (∼10 weeks) using a filtration method (1). Stool samples, primarily from outpatients, were submitted in Cary-Blair transport medium and refrigerated if not processed the day of collection. The filtration method used was as follows. Three brucella blood agar plates (Becton Dickinson BBL brucella agar with 5% sheep blood, hemin, and vitamin K; Becton, Dickinson, Sparks, MD) were used as the nonselective medium. For comparison, three different filter types were used, 47-mm cellulose acetate filters, (0.45 μm and 0.65 μm; Sartorius, Goettingen, Germany) and polycarbonate (0.6 μm; EMD Millipore Corp., Billerica, MA) filters. A single filter was placed onto the surface of the plate, 10 drops of fecal material from the Cary-Blair transport vial, gently mixed prior to dispensing of the drops, were placed on each filter, each drop in a separate location on the filter, and plates were incubated for 1 h at 37°C in ambient air. Filters were then removed and plates placed into anaerobic jars, processed to create microaerobic conditions (6% O2, 7% CO2, 7% H2, 80% N2) using an evacuation-replacement protocol, (Anoxomat System, Advanced Instruments, Inc., Norwood, MA) and incubated at 37°C. Plates were examined on day 2 and day 3 for colonies resembling Campylobacter spp., Gram stained, and subjected to matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) identification (Vitek MS, bioMérieux, Durham, NC) and 16S rRNA gene sequence analysis. All stool samples were tested routinely with a multiplex GI panel that included Campylobacter spp. (C. jejuni, C. coli, C. lari), Salmonella spp., Shigella spp., Vibrio spp., Yersinia spp., Stx1, Stx2, rotavirus, and norovirus (Verigene enteric pathogens test, Luminex Corp., Austin, TX). Samples positive for one of the bacterial panel targets were routinely cultured (reflex culture) to provide an isolate for antimicrobial susceptibility testing and to submit to the Pennsylvania State Bureau of Laboratories. For isolation of Campylobacter spp., fecal material from the original sample in Cary-Blair transport medium was plated onto Campy CVA agar medium (BBL Campy CVA Agar, Becton, Dickinson, Sparks, MD) and incubated at 42°C in microaerobic conditions for 72 h. Some samples, if ordered by the medical provider, were tested for intestinal parasites by antigen immunoassay for detection of Giardia and Cryptosporidium spp. (Giardia/Cryptosporidium Quik Chek, Alere, Waltham, MA) and C. difficile glutamate dehydrogenase (GDH) antigen and toxin A/B (Cdiff Quik Chek Complete, Techlab, Blacksburg, VA) with indeterminate samples (antigen positive/toxin negative) tested for tcdB (BD Max Cdiff, BD Diagnostics, Sparks, MD).
We categorized isolates as clinically significant, not significant, or of unclear significance. To determine the clinical significance of isolates, patient medical charts were retrospectively reviewed for relevant clinical data, including the date of culture, patient age and gender, hospital status (inpatient/outpatient), primary diagnosis at the time of culture, onset of gastrointestinal symptoms, indications for submitting the culture, presence of fever, chills, nausea, vomiting, and/or abdominal pain, description of diarrhea (i.e., watery, bloody, other), other underlying conditions (e.g., IBD, gastrointestinal malignancy), travel history (within the past 30 days or previously), treatment with antimicrobial agents for GI illness or other illnesses in the past 30 days, and resolution of symptoms. A Campylobacter isolate was considered clinically significant if (i) the clinical presentation described in the chart was noted by the provider as consistent with a gastrointestinal infection or (ii) charted notes recorded a strong suspicion or high likelihood of infectious gastrointestinal infection and (iii) no other recognized caused of infectious gastroenteritis was detected or documented in the medical record. Isolates were considered not significant if (i) documented reasons for the current gastrointestinal findings were attributed to noninfectious causes such as postsurgical complications or gastrointestinal malignancy or (ii) other recognized causes of infectious gastrointestinal infection were detected in the sample by other laboratory tests. All other cases were categorized as being of unclear significance.
This study was reviewed and approved by the institutional review board of the University of Pennsylvania.
ACKNOWLEDGMENTS
We thank Jill Wadlin and Mei Yu in the clinical microbiology laboratory for assistance during this study.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
REFERENCES
- 1.Fitzgerald C, Nachamkin I. 2015. Campylobacter and Arcobacter. p 998–1012. In Jorgensen, JH, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW (ed), Manual of clinical microbiology, 11th ed ASM Press, Washington, DC. [Google Scholar]
- 2.Goossens H, Giesendorf BA, Vandamme P, Vlaes L, Van den Borre C, Koeken A, Quint WG, Blomme W, Hanicq P, Koster DS. 1995. Investigation of an outbreak of Campylobacter upsaliensis in day care centers in Brussels: analysis of relationships among isolates by phenotypic and genotypic typing methods. J Infect Dis 172:1298–1305. doi: 10.1093/infdis/172.5.1298. [DOI] [PubMed] [Google Scholar]
- 3.Man SM. 2011. The clinical importance of emerging Campylobacter species. Nat Rev Gastroenterol Hepatol 8:669–685. doi: 10.1038/nrgastro.2011.191. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen HL, Engberg J, Ejlertsen T, Nielsen H. 2013. Comparison of polycarbonate and cellulose acetate membrane filters for isolation of Campylobacter concisus from stool samples. Diagn Microbiol Infect Dis 76:549–550. doi: 10.1016/j.diagmicrobio.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Steele TW, McDermott SN. 1984. The use of membrane filters applied directly to the surface of agar plates for the isolation of Campylobacter jejuni from feces. Pathology 16:263–265. doi: 10.3109/00313028409068535. [DOI] [PubMed] [Google Scholar]
- 6.Goossens H, Vlaes L, De Boeck M, Pot B, Kersters K, Levy J, De Mol P, Butzler JP, Vandamme P. 1990. Is “Campylobacter upsaliensis” an unrecognized cause of human diarrhea? Lancet 334:584–586. doi: 10.1016/0140-6736(90)90359-D. [DOI] [PubMed] [Google Scholar]
- 7.Vandenberg O, Houf K, Douat N, Vlaes L, Retore P, Butzler JP, Dediste A. 2006. Antimicrobial susceptibility of clinical isolates of non-jejuni/coli campylobacters and arcobacters from Belgium. J Antimicrob Chemother 57:908–913. doi: 10.1093/jac/dkl080. [DOI] [PubMed] [Google Scholar]
- 8.Engberg J, On SLW, Harrington CS, Gerner Smidt P. 2000. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by reevaluation of isolation methods for campylobacters. J Clin Microbiol 38:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen HL, Ejlertsen T, Nielsen H. 2015. Polycarbonate filtration technique is noninferior to mCCDA for isolation of Campylobacter species from stool samples. Diagn Microbiol Infect Dis 83:11–12. doi: 10.1016/j.diagmicrobio.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen HL, Engberg J, Ejlertsen T, Nielsen H. 2013. Clinical manifestations of Campylobacter concisus infection in children. Pediatr Infect Dis J 32:1194–1198. doi: 10.1097/INF.0b013e31829f0aff. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen HL, Kirk KF, Bodilsen J, Ejlertsen T, Nielsen H. 2016. Azithromycin vs placebo for the clinical outcome of Campylobacter concisus diarrhea in adults: a randomized, double blinded, placebo-controlled clinical trial. PLoS One 11:e0166395. doi: 10.1371/journal.pone.0166395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nielsen HL, Engberg J, Ejlertsen T, Bucker R, Nielsen H. 2012. Short-term and medium-term clinical outcomes of Campylobacter concisus infection. Clin Microbiol Infect 18: E459–E465. doi: 10.1111/j.1469-0691.2012.03990.x. [DOI] [PubMed] [Google Scholar]
- 13.Bullman S, Corcoran D, O'Leary J, Lucey B, Byrne D, Sleator RD. 2011. Campylobacter ureolyticus: an emerging gastrointestinal pathogen? FEMS Immunol Med Microbiol 61:228–230. doi: 10.1111/j.1574-695X.2010.00760.x. [DOI] [PubMed] [Google Scholar]


