ABSTRACT
Urethral swabs are the samples of choice for point-of-care Gram stain testing to diagnose Neisseria gonorrhoeae infection and nongonococcal urethritis (NGU) in men. As an alternative to urethral swabs, meatal swabs have been recommended for the collection of urethral discharge to diagnose N. gonorrhoeae and Chlamydia trachomatis infection in certain populations by nucleic acid amplification testing (NAAT), as they involve a less invasive collection method. However, as meatal swabs could be sampling a reduced surface area and result in fewer collected epithelial cells compared to urethral swabs, the adequacy of meatal swab specimens to collect sufficient cellular material for Gram stain testing remains unknown. We enrolled 66 men who underwent either urethral or meatal swabbing and compared the cellular content and Gram stain failure rate. We measured the difference in swab cellular content using the Cepheid Xpert CT/NG sample adequacy control crossing threshold (SACCT) and determined the failure rate of Gram stain smears (GSS) due to insufficient cellular material. In the absence of discharge, meatal smears were associated with a significant reduction in cellular content (P = 0.0118), which corresponded with a GSS failure rate significantly higher than that for urethral swabs (45% versus 3%, respectively; P < 0.0001). When discharge was present, there was no difference among results from urethral and meatal swabs. Therefore, if GSS testing is being considered for point-of-care diagnosis of N. gonorrhoeae infection or NGU in men, meatal swabs should be avoided in the absence of a visible discharge.
KEYWORDS: Chlamydia trachomatis, Gram stain, meatal swabs, Neisseria gonorrhoeae, urethral swabs
INTRODUCTION
Chlamydia trachomatis and Neisseria gonorrhoeae infections continue to rise, with 1.4 million and >350,000 cases reported, respectively, in the United States in 2015 (1); nongonococcal urethritis (NGU) remains the most common form of urethritis in men (2). For screening at-risk individuals, the Centers for Disease Control and Prevention (CDC) recommends highly sensitive nucleic acid amplification testing (NAAT) of urine or a urethral swab of urethral secretions (3). Meatal swabs have been suggested as a less invasive alternative to urethral swabs for specimen collection for NAAT and are amenable to self-obtained patient collection because they elicit less discomfort (4). Although not yet FDA approved or CDC recommended for NAAT in men, meatal swabs are recommended for NAAT in prepubertal boys with discharge, given concerns about urethral trauma from swabs (3, 5). In men, self-obtained meatal swabs appear to be equivalent to clinician-collected urethral swabs for diagnosing C. trachomatis/N. gonorrhoeae infection by NAAT, although there have been mixed reports of the sensitivity of this sample type (6, 7). Currently, no rapid (<30-min) point-of-care NAAT is available to diagnose N. gonorrhoeae infection or NGU, and Gram stain smear (GSS) testing of urethral secretions remains the test of choice in settings where rapid diagnosis is needed and microscopy can be performed. The 2015 Sexually Transmitted Diseases Treatment Guidelines specify that GSS testing of male urethral secretions is appropriate for diagnosing N. gonorrhoeae infection or NGU, although no preferred swab type is indicated for specimen collection (5). Meatal swabs are recommended as an alternative for collecting urethral secretions in specific populations, though they have yet to be approved for NAAT in men. In contrast to NAAT, which amplifies nucleic acids from lysed cells, GSS testing requires collection of intact cells. As meatal swabs may sample a smaller surface area and a higher proportion of cornified squamous epithelium from the meatus than urethral swabs, it is possible that the number of intact cells collected using a meatal swab may be insufficient to reliably diagnose N. gonorrhoeae infection or NGU by GSS testing. To address this concern, we enrolled both symptomatic and asymptomatic men and systematically assigned them to undergo either meatal or urethral swabbing, and we measured the swabs' cellular content using the Cepheid Xpert CT/NG sample adequacy control crossing threshold (SACCT) (8) and determined the failure rate of GSS testing for each swab type. The SACCT is an internal control of the Xpert CT/NG assay and denotes the cycle number at which human hydroxymethylbilane synthase, a single-copy housekeeping gene, is first detected by real-time PCR amplification. Included in each assay to ensure specimen sample adequacy (9), the SACCT is inversely proportional to the amount of cellular material present in the specimen. The primary outcome of our study was to determine, compared with urethral swabs, if meatal swabs were associated with a lower cellular content and a higher GSS failure rate. A secondary objective was to determine how meatal swabs were influenced by the presence or absence of discharge.
RESULTS
Sixty-six men were included in this study (Table 1). Participants were 20 to 69 years of age (mean, 29 years), and 56 (89%) were black. Twenty-seven men (41%) had visible discharge on genital examination. Swab collection (meatal versus urethral) was alternated such that 33 men provided a urethral swab and 33 men had a meatal swab taken. No difference in age, race, symptoms, prior STI history, or number of sex partners in the past 30 days was identified between the groups. Nine (14%) men had Gram-negative intracellular diplococci (GNID) present on GSS, and 32 men (48%) were diagnosed with NGU. The Xpert CT/NG assay, performed on both swabs and voided urine specimens, diagnosed 20 men with C. trachomatis and/or N. gonorrhoeae infection; 8 men were positive for C. trachomatis alone, 4 men for both C. trachomatis and N. gonorrhoeae, and 8 men for N. gonorrhoeae alone.
TABLE 1.
Characteristics of study participants
| Characteristica | Patients (n = 66) | Urethral swab (n = 33) | Meatal swab (n = 33) | P valueb |
|---|---|---|---|---|
| Age (mean [range]) | 29 (20–69) | 29 (20–60) | 29 (20–69) | 0.9720 |
| Race (n [%]) | 0.2920 | |||
| AA | 56 (89) | 28 (85) | 28 (93)c | |
| Caucasian | 7 (11) | 5 (15) | 2 (7)c | |
| Other | 0 | 0 | 0c | |
| Prior STI (n [%]) | 43 (65) | 25 (76) | 18 (55) | 0.0724 |
| Partners in past 30 days (mean [range]) | 1.6 (0–7) | 1.8 (0–7) | 1.5 (0–4) | 0.4472 |
| Discharge present (n [%]) | 27 (41) | 14 (42) | 13 (39) | 0.8040 |
| Gram stain (n [%]) | ||||
| QNS | 16 (24) | 1 (3) | 15 (45) | <0.0001 |
| GNID positive | 9 (14) | 3 (9) | 6 (18) | 0.2891 |
| GNID negative | ||||
| PMNs | ||||
| <2 | 9 (14) | 7 (21) | 2 (6) | 0.0749 |
| 2–5 | 12 (18) | 11 (33) | 1 (3) | 0.0011 |
| ≥5 | 20 (30) | 11 (33) | 9 (27) | 0.5989 |
| Xpert swab results (n [%]) | ||||
| Negative | 46 (70) | 25 (76) | 21 (64) | 0.2912 |
| C. trachomatis positive | 8 (12) | 2 (6) | 6 (18) | 0.1556 |
| C. trachomatis positive, N. gonorrhoeae positive | 4 (6) | 2 (6) | 2 (6) | >0.999 |
| N. gonorrhoeae positive | 8 (12) | 4 (12) | 4 (12) | >0.999 |
| SACCT (mean [range]) | 24.8 (21–33) | 23.9 (21–27) | 25.6 (21–33) | 0.0026 |
| Urine SACCT (mean [range]) | 26.1 (19–32) | 26.3 (19–32) | 25.9 (19–32) | 0.6147 |
AA, African American; STI, sexually transmitted infection; GNID, Gram-negative intracellular diplococci; PMN, polymorphonuclear neutrophil; QNS, quantity of cells not sufficient.
Significance evaluated using Fisher's exact test or t test, as appropriate.
Three missing.
To assess whether meatal swabs were associated with a decrease in cellular content, we compared the SACCT of meatal swabs to that of urethral swabs. Meatal swabs were associated with significantly higher SACCT values, indicating that they contained less cellular material than did the urethral swabs (mean, 25.6 versus 23.9; P = 0.0026) (Table 1). This difference in cellular content did not affect the performance of the NAAT since the swab sample NAAT results were 100% concordant with the urine NAAT results (data not shown).
We then assessed the failure rate of GSS prepared using urethral and meatal swabs, with failure defined as an absence of cellular material on microscopy (i.e., quantity of cells not sufficient [QNS]). The GSS QNS rate for meatal swabs was significantly higher than that for urethral swabs (45% versus 3%, respectively; P < 0.0001). Despite no difference in the frequency of signs or symptoms of urethritis between the two groups, meatal swabs were associated with significantly lower numbers of GSS with polymorphonuclear leukocytes (PMNs) between 2 and 5 (3% versus 33%; P = 0.0011) (Table 1) than urethral swabs, which is reflected in the high GSS failure rate.
To determine if the increased QNS rate in meatal GSS was associated with less cellular content, we stratified the SACCT results by QNS status. As shown in Fig. 1A, Gram stains identified as QNS were associated with a higher meatal swab SACCT (mean, 27.4 versus 24.1, respectively; P = 0.0002), indicating that the meatal swabs used to prepare the GSS contained less cellular material than urethral swabs.
FIG 1.
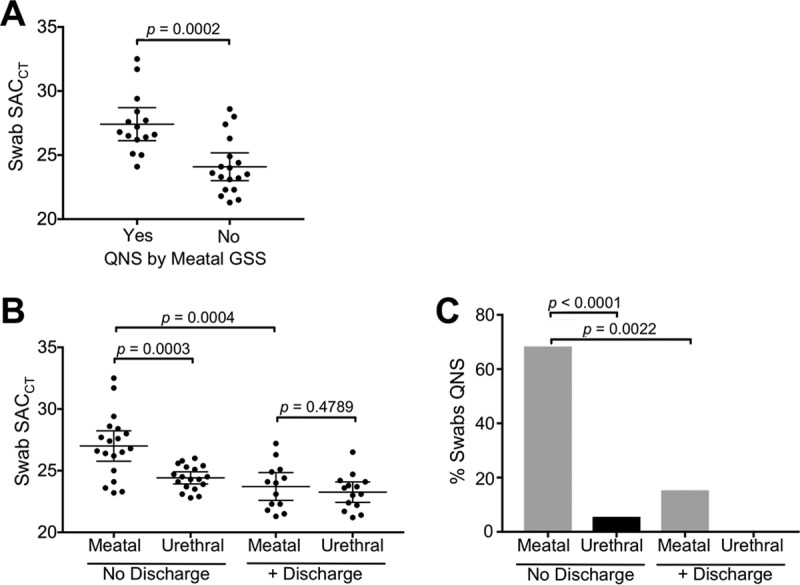
Comparison of cellular content and percentage QNS in meatal versus urethral swabs in the presence or absence of urethral discharge. (A) Meatal swabs that resulted as QNS by GSS are associated with a significantly higher SACCT than those with satisfactory GSS, indicating that they contain less cellular material. (B) In the absence of discharge, meatal swabs have a significantly higher SACCT than urethral swabs from men without discharge and meatal swabs from men with discharge. In the presence of discharge, there was no difference in the SACCTs from meatal and urethral swabs. (C) In the absence of discharge, meatal swabs were significantly more likely to result as QNS by GSS than were urethral swabs from men without discharge and meatal swabs from men with discharge. In the presence of discharge, there was no difference between the swab types. Horizontal lines and whiskers denote the mean and 95% confidence interval, respectively. SACCT values were obtained from the Xpert CT/NG assay.
Given our finding that meatal swabs are associated with significantly less cellular material and a higher GSS failure rate than are urethral swabs, we were interested in establishing how meatal swabs performed when sampling discharge. As shown in Fig. 1B, in the absence of discharge, meatal swabs collected significantly less cellular material than urethral swabs (mean SACCT, 24.4 versus 27.0, respectively; P = 0.0003). If discharge was present, the meatal swabs collected significantly more cellular material than meatal swabs from men without discharge (mean, SACCT 27.0 versus 23.7, respectively; P = 0.0004). Further, in the setting of visible discharge, no difference in the cellular content collected from the meatal and urethral swabs was identified (mean, SACCT 23.7 versus 23.3, respectively; P = 0.4789). In an evaluation of the GSS failure rate of swabs in the presence or absence of discharge, the highest QNS rates were seen in meatal swabs from men without discharge compared to meatal swabs from men with discharge (68% versus 15%, respectively; P = 0.0022) (Fig. 1C) and compared to urethral swabs in men without discharge (68% versus 6%, respectively; P < 0.0001) (Fig. 1C). In the presence of discharge, meatal swabs were associated with a slight nonsignificant increase in the QNS rate compared to that of the urethral swabs (15% versus 0%, respectively; P = 0.1373) (Fig. 1C).
We then compared the diagnoses (using both Xpert and GSS results) of all QNS results to assess the number of N. gonorrhoeae or NGU diagnoses that could have been missed by GSS failure. Of the 16 QNS GSS results, none were N. gonorrhoeae positive, but three NGU diagnoses (one C. trachomatis infection diagnosed by NAAT and two non-C. trachomatis NGU [defined by discharge on exam]) were included, which highlights the fact that GSS failures from meatal swabs could delay the time to effective treatment (i.e., missed opportunity to evaluate PMNs in point-of-care testing) in the absence of discharge (data not shown).
DISCUSSION
Evaluation by GSS of urethral secretions from a urethral or meatal swab remains the test of choice for rapid diagnosis of N. gonorrhoeae or NGU in men with urethritis symptoms, though no preferred swab type is recommended by the 2015 Sexually Transmitted Diseases Treatment Guidelines for collecting urethral secretions (5). In this study, we compared meatal and urethral swabs from men and used the Cepheid Xpert CT/NG SACCT to measure the difference in the amounts of swab cellular material and determine the failure rate of GSS testing for each swab type. Not surprisingly, we found that meatal swabs collected significantly less cellular material than urethral swabs in the absence of urethral discharge. Moreover, meatal swabs were associated with a high GSS failure rate, eliciting a 12-fold increase in QNS GSS rates over those for urethral swabs in men without discharge. In the presence of urethral discharge, there was no difference in the cellular content or GSS failure rates between the swab types, indicating that the collection quality of meatal swabs is highly susceptible to the sampling surface area of the urethral meatus and/or that meatal swabs may be collecting increased numbers of nonintact cells, which are limitations that may be overcome by urethral sampling. Additionally, the limitations of meatal swabs may only significantly affect Gram stain testing, which is dependent on the collection of intact cells to evaluate for the presence of GNID and PMNs, whereas NAAT is much more sensitive and less likely to be significantly influenced by changes in the cellular content of the swabs. In fact, NAAT comparing urine and either swab type demonstrated a 100% concordance rate for the diagnosis of C. trachomatis and/or N. gonorrhoeae infection in our study, despite the meatal swabs containing less cellular material.
Although the NAAT performance of self-collected meatal swabs appears comparable to that of clinician-collected urethral swabs (6) and superior to urine for diagnosis of C. trachomatis and N. gonorrhoeae infection and trichomonas (7), no study has compared the performance of meatal swabs to urethral swabs for Gram stain point-of-care testing to evaluate urethritis (5). To our knowledge, our study is the first to demonstrate that, in the absence of urethral discharge, meatal swabs are inferior to urethral swabs for collecting cellular material, which increases the failure rate of GSS testing. In addition, although there was no difference in the signs or symptoms between the swab groups, meatal swabs were associated with significantly fewer numbers of GSS with a PMN count of 2 to 5 (Table 1), suggesting that underdiagnosis of NGU cases may also result when meatal swabs are used for GSS testing.
Our findings have important implications for the use of meatal swabs for collecting urethral secretions, a practice that may be increasing given that meatal swabs have several advantages over urethral swabs for NAAT. Meatal swabs are likely better tolerated than urethral swabs, as demonstrated in a comparison study of men who received both a urethral and meatal swab, which found that 76% of men preferred the meatal swab (4). Another study attempted to quantify, using a visual analogue pain scale, the discomfort associated with urethral swab collection and found that collection with a urethral swab using the standard technique (inserting a swab 2 cm into the distal urethra) elicited a median pain score of 52 mm (on a 100-mm pain scale), which correlated with moderate (>30 mm) to severe (>54 mm) pain (10). Furthermore, multiple studies have identified discomfort from urethral swabbing as a major factor that causes men to delay seeking health care (11–13), and participants in adolescent focus groups expressed strong negative emotions when asked about the urethral swab testing process (12, 13). In addition, the ease of obtaining a meatal swab facilitates self-collection for NAAT, allowing patients to self-collect specimens at home or in a clinical setting where regular interval screening of asymptomatic high-risk men is performed. Given that 77% of men preferred self-collection with a meatal swab to provider collection (14) and more than 90% of men report self-collection with a penile-meatal swab as “easy” or “very easy” (6, 14), it is likely that NAAT of self-collected meatal swabs may play an important future role in screening asymptomatic men in busy high-flow clinical settings or nonclinical settings.
Our study has several limitations. We could not evaluate the performance of meatal swabs versus urethral swabs for the diagnosis of NGU or N. gonorrhoeae infection by Gram staining, as our study was not powered to evaluate that outcome. Also, the majority of men in our study were African American, which is reflective of demographic characteristics of clients attending the sexually transmitted disease (STD) clinic, and our results may not be generalizable to other non-STD clinic populations. Additionally, the Gram stains were prepared by multiple clinicians, all highly proficient at preparing and reading Gram stain smears. Although no difference in either swab cellular content or the rate of QNS GSS results was associated with any individual clinician (data not shown), we cannot rule out the possibility that differences in provider collection techniques may have influenced our results.
In conclusion, in the absence of visible urethral discharge, the use of meatal swabs for point-of-care diagnosis of N. gonorrhoeae infection or NGU by GSS testing was associated with a significant decrease in swab cellular content and an increase in GSS failure rates compared to the use of urethral swabs. Therefore, in the absence of discharge, meatal swabs should be avoided when considering point-of-care testing for N. gonorrhoeae infection or NGU in men.
MATERIALS AND METHODS
We recruited men ≥19 years old from the Jefferson County Department of Health (JCDH) STD clinic in Birmingham, Alabama, excluding only men who had voided within the past 60 min and those who had received antibiotics with C. trachomatis/N. gonorrhoeae activity within the past 30 days. After informed consent was obtained, a directed physical exam was performed for the presence or absence of discharge, and men were alternately assigned to undergo either a urethral or meatal swab for specimen collection using a dacron swab. To collect a urethral swab, a highly experienced clinician inserted a swab 2 cm into the urethra and rotated during insertion and extraction. For meatal swab specimen collection, the swab was positioned perpendicular to the urethral meatus and rolled back and forth several times across the meatus for 5 s. Following specimen collection, swabs were immediately rolled onto the surface of a glass microscope slide in preparation for Gram staining of urethral secretions and then placed in an Xpert CT/NG (Cepheid, Sunnyvale, CA) specimen transport container and stored at 4°C. Following meatal or urethral swab collection, an initial void urine specimen was collected for C. trachomatis and N. gonorrhoeae testing.
Gram staining of the smear was performed using a standard laboratory procedure, and all smears were read by a highly experienced clinician (J.R.S.) who was blinded to the physical exam findings and Xpert CT/NG assay results. After identifying an area of high cellularity using low magnification, the presence or absence of Gram-negative intracellular diplococci (GNID) and the average number of leukocytes from five contiguous high-power fields were determined and recorded using high magnification with oil immersion (×1,000). GSS were labeled quantity cells not sufficient (QNS) if no cells were identified by scanning with either low- or high-power magnification. NGU was diagnosed if no GNID were seen and either (i) a discharge was present on exam or (ii) ≥2 PMNs per high-power field were seen by GSS testing.
Xpert CT/NG testing was performed on both urethral and meatal swab specimens and postswab urine specimens within 7 days of collection as described in the package insert. For quantification of specimen cellularity, the Xpert CT/NG sample adequacy control crossing threshold (SACCT) was used. We also tested the performance of each swab type to prepare a GSS by determining the failure rate of GSS testing, defined as lacking a sufficient number of cells to evaluate the specimen. This study was approved by the institutional review board of the University of Alabama at Birmingham and the research review committee at JCDH. Bivariate comparisons were evaluated using an unpaired t test, while Fisher's exact or a chi-square test was used to test differences between groups. Significance was reported as a P value of <0.05 using Prism v7.0b software (GraphPad Software, Inc., San Diego, CA).
ACKNOWLEDGMENTS
We are grateful to Paula Dixon and Austin Culver for their assistance with specimen processing and testing.
J.R.S. has received honoraria, consulting fees, or research support from Cepheid, BD Diagnostics, LabCorp., and Hologic. B.V.D.P. has received honoraria, consulting fees, or research support from Abbott Molecular Diagnostics, Atlas Genetics, BD Diagnostics, Beckman Coulter, Great Basin, Scientific, Cepheid, Hologic, Rheonix, and Roche Diagnostics. E.W.H. has received honoraria, research support, or consulting fees from Cepheid, BD Diagnostics, Gen-Probe Hologic, Roche Diagnostics, and Cempra Pharmaceuticals. S.J.J. and K.J.A. report no conflicts of interest.
Reagents and test kits for this study were supplied by Cepheid (Sunnyvale, CA).
This work was funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health Sexually Transmitted Infection Cooperative Research Center (grant U19AI113212, to E.W.H., principal investigator).
REFERENCES
- 1.Centers for Disease Control and Prevention. 2015. Sexually transmitted disease surveillance 2014. Atlanta: U.S. Department of Health and Human Services. [Google Scholar]
- 2.Moi H, Blee K, Horner PJ. 2015. Management of non-gonococcal urethritis. BMC Infect Dis 15:294. doi: 10.1186/s12879-015-1043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and prevention. 2014. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 63(RR-02):1–19. [PMC free article] [PubMed] [Google Scholar]
- 4.Lamba H, Davies J, Murphy S, Shafi M. 2001. Detection of chlamydia on meatal swabs. Sex Transm Infect 77:224. doi: 10.1136/sti.77.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Workowski KA, Bolan GA; Centers for Disease Control and Prevention. 2015. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 6.Dize L, Barnes P Jr, Barnes M, Hsieh Y-H, Marsiglia V, Duncan D, Hardick J, Gaydos CA. 2016. Performance of self-collected penile-meatal swabs compared to clinician-collected urethral swabs for the detection of Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Mycoplasma genitalium by nucleic acid amplification assays. Diagn Microbiol Infect Dis 86:131–135. doi: 10.1016/j.diagmicrobio.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dize L, Agreda P, Quinn N, Barnes MR, Hsieh YH, Gaydos CA. 2013. Comparison of self-obtained penile-meatal swabs to urine for the detection of C. trachomatis, N. gonorrhoeae and T. vaginalis. Sex Transm Infect 89:305–307. doi: 10.1136/sextrans-2012-050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan SJ, Van Der Pol B, Hook EW III. 2017. Utilization of the Cepheid Xpert CT/NG sample adequacy control to determine the influence of the urethral swab on cellular content in post-swab versus pre-swab urine. Sex Transm Dis 44:67–68. doi: 10.1097/OLQ.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bristow C, Adachi K, Nielsen-Saines K, Ank B, Morgado MG, Watts H, Veloso VG, Pilotto JH, Joao EC, Klausner JD. 2014. Characteristics of the sample adequacy control (SAC) in the Cepheid Xpert CT/NG assay in female urine specimens. J Microbiol Exp 1:1–5. doi: 10.15406/jmen.2014.01.00026. [DOI] [Google Scholar]
- 10.Apoola A, Herrero-Diaz M, FitzHugh E, Rajakumar R, Fakis A, Oakden J. 2011. A randomised controlled trial to assess pain with urethral swabs. Sex Transm Infect 87:110–113. doi: 10.1136/sti.2010.042861. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong B. 2003. The young men's clinic: addressing men's reproductive health and responsibilities. Perspect Sex Reprod Health 35:220–225. doi: 10.1363/3522003. [DOI] [PubMed] [Google Scholar]
- 12.Blake DR, Kearney MH, Oakes JM, Druker SK, Bibace R. 2003. Improving participation in chlamydia screening programs: perspectives of high-risk youth. Arch Pediatr Adolesc Med 157:523–529. doi: 10.1001/archpedi.157.6.523. [DOI] [PubMed] [Google Scholar]
- 13.Tilson EC, Sanchez V, Ford CL, Smurzynski M, Leone PA, Fox KK, Irwin K, Miller WC. 2004. Barriers to asymptomatic screening and other STD services for adolescents and young adults: focus group discussions. BMC Public Health 4:21. doi: 10.1186/1471-2458-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chai SJ, Aumakhan B, Barnes M, Jett-Goheen M, Quinn N, Agreda P, Whittle P, Hogan T, Jenkins WD, Rietmeijer CA, Gaydos CA. 2010. Internet-based screening for sexually transmitted infections to reach nonclinic populations in the community: risk factors for infection in men. Sex Transm Dis 37:756–763. doi: 10.1097/OLQ.0b013e3181e3d771. [DOI] [PMC free article] [PubMed] [Google Scholar]


