LETTER
Mycoplasma genitalium has been implicated as a cause of acute and chronic nonchlamydial nongonococcal urethritis (NCNGU) and pelvic inflammatory disease (PID) (1). In New Zealand, as with many other countries, the preferred treatment for NCNGU is either azithromycin or doxycycline (2). The presence of macrolide resistance in M. genitalium isolates from New Zealand has been reported previously from a small sample size, but there has been no further testing or any continued surveillance reported since then (3).
The Microbiology Department in LabPLUS receives specimens mostly from the clinics of the Auckland Regional Sexual Health Service (ARSHS) for M. genitalium testing, which is performed in-house using TaqMan real-time PCR on an ABI 7500 system (Applied Biosystems Life Technologies, NY, USA). This targets the M. genitalium MgPa adhesion gene, as previously described (4). A PlexPCR M. genitalium ResistancePlus kit (SpeeDx Pvt Ltd., Sydney, Australia), a commercial multiplexed real-time PCR assay that detects the presence of M. genitalium and mutations in the 23S rRNA gene (5), was used for this retrospective study. (Ethics approval was received from the Auckland District Health Board [Ethics: 16/CEN/188]). In the study design, M. genitalium-positive DNA specimens stored at −70°C from 2009 until 2015 were retrieved and retested by the use of a PlexPCR kit. M. genitalium-positive DNA specimens showing mutations in the 23S rRNA gene by the PlexPCR assay had the 23S rRNA gene sequenced on ABI 3130xl sequencer (Applied Biosystems Life Technologies, NY, USA) to confirm the point mutations in the 23S rRNA gene, using a previously published method (6).
Over the period from the start of the M. genitalium testing by LabPLUS in 2009 until 2015, a total of 779 clinical specimens were tested, 149 (19%) of which were positive for M. genitalium. Of the 149 stored M. genitalium-positive samples, 116 (79%) were able to be amplified and further sequenced to confirm the mutation in the 23S rRNA gene detected by PlexPCR. Among the M. genitalium-positive samples recovered for testing by PlexDX PCR, there was agreement in the result with the in-house MgPa real-time assay result. PlexPCR detected mutations in the 23S rRNA gene in 86 of the 116 specimens, all of which were confirmed by in-house sequencing of the 23S rRNA gene. Mutation was detected at both position A2058 (32.5%) and position A2059 (67.5%). The predominant mutants were A2058G and A2059G, while less than 4% of each of A2058C, A2058T, and A2059C were detected.
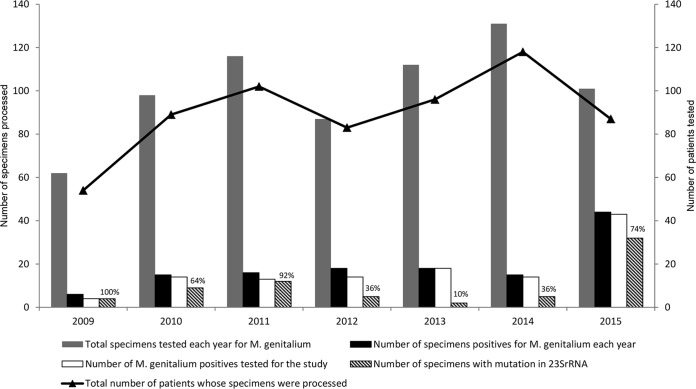
This laboratory-based study shed light on the prevalence of macrolide resistance in the patients presenting with persistent urethritis to the clinics of the ARSHS. Of the 116 specimens tested, 79 were patient specimens sent for testing only once, 17 were patient specimens sent twice within a 1- to 6-month interval to the laboratory for testing, and 3 were specimens from 1 patient who had specimens sent 3 times within a 1- to 3-month interval. Most of the repeat specimens were due to either failed treatment or reinfection. Taking the repeat specimens into consideration, a total of 97 patients were tested in this study. Overall, 70 patients were positive for macrolide resistance among the 97 M. genitalium-positive patients tested, representing 72% of all the patients tested retrospectively. The yearly distribution of the number of specimens processed for Mycoplasma genitalium detection, the number of specimens positive for Mycoplasma genitalium, the number of M. genitalium-positive specimens used for the study, and the number of Mycoplasma genitalium strains with a mutation in the 23S rRNA gene conferring macrolide resistance tested retrospectively from 2009 until 2015 are shown in Fig. 1. The results from this study show high macrolide resistance in Mycoplasma genitalium strains causing infection in the cohort of patients tested in Auckland, New Zealand. The study data support continued testing for macrolide resistance to monitor whether treatment failure is due to antimicrobial resistance.
FIG 1.
The figure shows the yearly distribution of M. genitalium and macrolide resistance detected in the study from 2009 to 2015. The legends are explained below the graph. The percentages indicated on the bars are for macrolide resistance. The black line in the secondary y axis plots the total number of patients tested for M. genitalum, taking into account repeat specimens from the same patient.
Funding was provided by an Auckland District Health Board Charitable Trust grant (Research project A+7436).
ACKNOWLEDGMENT
SpeeDx supplied the PlexPCR M. genitalium ResistancePlus kit for evaluation but played no role in the design or the performance of this study.
REFERENCES
- 1.Taylor-Robinson D, Jensen JS. 2011. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 24:498–514. doi: 10.1128/CMR.00006-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.New Zealand Sexual Health Society. 2015. The New Zealand Sexual Health Society guidelines for urethritis management. http://www.nzshs.org/docman/guidelines/management-of-sexual-health-conditions/180-urethritis-guideline/file.
- 3.Yew HS, Anderson T, Coughlan E, Werno A. 2011. Induced macrolide resistance in Mycoplasma genitalium isolates from patients with recurrent nongonococcal urethritis. J Clin Microbiol 49:1695–1696. doi: 10.1128/JCM.02475-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen JS, Björnelius E, Dohn B, Lidbrink P. 2004. Use of TaqMan 5′ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J Clin Microbiol 42:683–692. doi: 10.1128/JCM.42.2.683-692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabrizi SN, Tan LY, Walker S, Twin J, Poljak M, Bradshaw CS, Fairley CK, Bissessor M, Mokany E, Todd AV, Garland SM. 6 June 2016. Multiplex assay for simultaneous detection of Mycoplasma genitalium and macrolide resistance using PlexZyme and PlexPrime technology. PLoS One doi: 10.1371/journal.pone.0156740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen JS. 2012. Protocol for the detection of Mycoplasma genitalium by PCR from clinical specimens and subsequent detection of macrolide resistance-mediating mutations in region V of the 23S RNA gene. Methods Mol Biol 903:129–139. doi: 10.1007/978-1-61779-937-2_8. [DOI] [PubMed] [Google Scholar]