Abstract
Background
Weight regain after successful weight loss interventions is common.
Objective
To establish the efficacy of a weight loss maintenance program compared with usual care in obese adults.
Design
2-group, parallel, randomized trial stratified by initial weight loss (<10 kg vs. ≥10 kg), conducted from 20 August 2012 to 18 December 2015. Outcome assessors were blinded to treatment assignment. (ClinicalTrials.gov: NCT01357551)
Setting
3 primary care clinics at the Veterans Affairs Medical Center in Durham and Raleigh, North Carolina.
Patients
Obese outpatients (body mass index ≥30 kg/m2) who lost 4 kg or more of body weight during a 16-week, group-based weight loss program.
Intervention
The maintenance intervention, delivered primarily by telephone, addressed satisfaction with outcomes, relapse-prevention planning, self-monitoring, and social support. Usual care involved no contact except for study measurements.
Measurements
Primary outcome was mean weight regain at week 56. Secondary outcomes included self-reported caloric intake, walking, and moderate physical activity.
Results
Of 504 patients in the initial program, 222 lost at least 4 kg of body weight and were randomly assigned to maintenance (n = 110) or usual care (n = 112). Retention was 85%. Most patients were middle-aged white men. Mean weight loss during initiation was 7.2 kg (SD, 3.1); mean weight at randomization was 103.6 kg (SD, 20.4). Estimated mean weight regain was statistically significantly lower in the intervention (0.75 kg) than the usual care (2.36 kg) group (estimated mean difference, 1.60 kg [95% CI, 0.07 to 3.13 kg]; P = 0.040). No statistically significant differences in secondary outcomes were seen at 56 weeks. No adverse events directly attributable to the intervention were observed.
Limitations
Results may not generalize to other settings or populations. Dietary intake and physical activity were self-reported. Duration was limited to 56 weeks.
Conclusion
An intervention focused on maintenance-specific strategies and delivered in a resource-conserving way modestly slowed the rate of weight regain in obese adults.
Interventions targeting dietary change, physical activity, and behavioral strategies yield clinically significant weight loss of at least 5% (1, 2). After this weight loss, most people tend to regain 1 to 2 kg per year, with faster rates of regain in the earlier years (3). To reduce weight regain, various strategies have been evaluated, including sequential dieting, meal replacements, medications, and behavioral approaches (4). Behavioral approaches help people learn and habitualize behaviors for navigating situations that might lead to lapses and relapse (5).
One behavioral approach is teaching patients maintenance-specific skills thought to differ from initiation-specific skills (6–8). Some trials evaluating maintenance skills did not have a specific weight loss requirement, so maintenance-specific skills could not be fully tested (9–13). One notable exception is the WLM (Weight Loss Maintenance) trial, in which participants who lost at least 4 kg were randomly assigned to an Internet, a personal contact, or a self-directed maintenance intervention (14). Although the personal contact group had modestly less regain at 30 months than the other 2 groups, the intervention was resource intensive, involving monthly telephone calls and in-person visits every 4 months. Efficacious maintenance interventions are needed to help participants adopt maintenance-specific skills while being delivered in a resource-conserving way.
We evaluated the efficacy of a maintenance intervention compared with usual care among patients who lost at least 4 kg in a weight loss program. Our innovative maintenance intervention focused on cognitive and behavioral processes involved in weight loss maintenance and was designed to conserve resources by being delivered primarily by telephone with decreasing frequency of contact.
Methods
Design Overview
The design was a 2-group, parallel, randomized, controlled trial. Patients who lost at least 4 kg during a 16-week weight loss program were randomly assigned 1:1 to the maintenance or usual care group. The primary hypothesis was the group difference in mean weight regain at week 56; all follow-up weight measurements were assessed by blinded personnel. The first patient gave informed consent on 20 August 2012; the last date of follow-up was 18 December 2015. The study protocol was approved by the institutional review boards of Durham Veterans Affairs (VA) Medical Center and Duke University Medical Center before data collection. Although body composition was specified in the protocol, these specific data from the bioelectric impedance scales were not entered into our database.
Setting
Participants were recruited from 3 primary care clinics at the VA Medical Center in Durham and Raleigh, North Carolina. Group sessions were held at the Duke Center for Living and comprised patients from the 3 VA clinics.
Eligibility Screening and Participants
The study was conducted in 6 cohorts, each recruited over 6 to 8 weeks. Eligibility for the weight loss initiation program was determined in a 3-step process involving an electronic medical record data pull, a screening telephone call, and an in-person screening visit. Details of screening procedures were reported and are available in the protocol (Supplement 1, available at Annals.org) (15). In brief, patients identified via the data pull were mailed a recruitment letter. Patients called study staff in response to the recruitment letters or flyers posted at the VA clinics, or study staff called patients referred by providers through a consult in the electronic medical record. Patients who passed the telephone screen were scheduled for an in-person visit to determine their final eligibility, and eligible patients provided written informed consent. To be included in the study, patients had to be aged 18 to 75 years, have a body mass index (BMI) of 30 kg/m2 or greater, have a primary care provider, agree to attend visits, and have access to a telephone and reliable transportation. Exclusion criteria included liver disease; type 1 diabetes; most recent hemoglobin A1c in past 6 months 12% or greater; average systolic blood pressure over the past year and most recent blood pressure 160 mm Hg or greater; history of weight loss surgery; dementia, severe psychiatric illness, or substance abuse; weight loss of 10 lb or more in the previous 3 months; current enrollment in a lifestyle program; current weight loss medication; pregnancy or plans to become pregnant in the next 6 months; breastfeeding; lack of birth control if premenopausal; organ transplant recipient; heart issues in the past 3 months; cancer not in remission; pacemaker or defibrillator (because of the use of a bio-electronic impedance scale); emotional problems that would impede intervention adherence or interacting in a group environment; and inability to stand for measurements. Eligible patients were offered up to 6 group meeting times and chose one that they would attend every 2 weeks for 16 weeks (8 sessions total).
The weight loss program was an abbreviated version of a previous protocol that focused on calorie and fat restriction (16). It included education and strategies for behavior initiation as outlined in our theoretical model, including goal setting and self-monitoring of dietary intake and physical activity. Weight loss of 4 kg or more during this program was the final eligibility criterion for randomization; thus, recording of weight within 1 week of the first scheduled group visit (week −16) and at the end of the weight loss initiation intervention (week 0) was required for patients to be considered for random assignment. A uniform 4-kg criterion was used because it is clinically significant for the average participant (17) and was used in a previous weight loss maintenance trial (14).
Randomization and Interventions
Patients were randomly assigned 1:1 to maintenance intervention or usual care within initial weight loss strata (<10 kg vs. ≥10 kg) in block sizes of 4. The randomization scheme was generated via a uniform random-number generator and loaded into a tracking database. It could be accessed only by the study statistician or information technology personnel. Either the study’s registered dietitian or the project coordinator randomly assigned the participants by using the tracking database, and participants were given their assignment by telephone within 1 week after their week-0 visit. Intervention participants were asked to return at week 2 for the first maintenance group session, whereas usual care participants were scheduled for the follow-up visit at week 14. The first participant was randomly assigned on 31 January 2013.
The maintenance intervention involved transitions from initiation to maintenance skills and from group visits to individual telephone calls, as well as decreased frequency of contact (18). The intervention period was 42 weeks, followed by 14 weeks of no contact. Group visits occurred at weeks 2, 6, and 10. Individual telephone calls were made at weeks 4, 8, 12, 16, 20, 24, 32, and 40. Group sessions addressed maintenance caloric intake, weight self-monitoring, increasing and maintaining physical activity, obtaining social support from friends and family, and relapse prevention. All calls had a uniform structure that focused on 4 maintenance constructs outlined in our theoretical model and were deemed acceptable in a pilot test: satisfaction with outcomes, relapse-prevention planning, self-monitoring, and social support (19). To make salient satisfaction with outcomes, participants reviewed “before” and “after” photographs and were asked to discuss outcomes of weight loss that continued to motivate them. They then identified high-risk situations in which relapse might occur and developed a plan to navigate those situations. Next, they specified a frequency of self-weighing and used a 1.36-kg (3-lb) regain relapse threshold. If relapse occurred, the registered dietitian guided the patient to reinitiate and self-monitor his or her diet or physical activity. Finally, participants identified a primary support person and supportive behaviors and were encouraged to share their plans with their support person.
The group sessions and telephone calls were delivered by 1 of 2 registered dieticians. Training included a review of theoretical principles and calls with mock participants, with feedback from the investigators. A co-investigator attended each group session and used a fidelity checklist to ensure that all protocoled elements were addressed. All maintenance telephone calls were recorded. Each week, the principal investigator, at least 1 co-investigator, and the registered dietitians met for 1 hour to review randomly selected intervention calls, and the investigators completed fidelity checklists and provided feedback.
Usual care was chosen as the comparator to mimic the typical patient experience of no further intervention after participating in a weight loss program. The VA’s MOVE! clinical weight loss program (20) was not used as a comparator because it focuses on weight loss. MOVE! and referral to a nutritionist were available as part of usual care. Participants in the intervention group were asked not to enroll in MOVE! or other lifestyle programs or to consult a nutritionist during the intervention, whereas usual care participants were told they could do both. In a post hoc chart review, we determined that 2 participants randomly assigned to usual care and 3 to the intervention group attended a MOVE! orientation visit but had no further involvement in the program during the 56-week maintenance phase.
Outcomes and Follow-up
All participants were scheduled for assessment appointments at weeks 0, 14, 26, 42, and 56 (weight loss initiation occurred at week −16 to week 0). All measurements were conducted by a research associate blinded to group assignment. Participants received $20 for the first 4 visits and $40 for the assessment at week 56.
Weight was assessed at weeks 0, 14, 26, 42, and 56, with week 0 as the initial weight and mean weight regain at week 56 as the primary end point. Weight was measured on a calibrated digital scale, with patients wearing light clothing and no shoes. Height, recorded to enable calculation of BMI, was assessed with a stadiometer. Weight and height were double-entered into an electronic case report form. Waist circumference was measured at weeks 0, 26, and 56 by using a nonelastic tape measure placed on the skin in a horizontal plane around the abdomen at the level of the umbilicus.
Dietary intake was evaluated with the Block Brief 2000 food frequency questionnaire (FFQ) with a 3-month recall period at weeks 0, 26, and 56. Participants completed the FFQ either in person or at home and returned it by mail. We analyzed estimated total daily energy intake (in kilocalories) because this corresponded to the intervention. Daily physical activity was assessed by using the short version of the International Physical Activity Questionnaire (IPAQ). We focused on weekly metabolic minutes of walking and moderate physical activity because they were emphasized in our intervention.
Veterans Affairs expenditures for admissions and outpatient visits for the 392-day period after the week-0 weight date were determined by inpatient and outpatient VA claims files maintained in the Corporate Data Warehouse. Expenditures were inflation-adjusted to 2016 dollars.
During the in-person screening visit, self-reported race, ethnicity, sex, education, current tobacco use, and previous weight loss attempt were assessed. Age was calculated from self-reported birth date. At weeks 0, 26, and 56, additional constructs were assessed that relate to our theoretical model (Supplement 1) but are not reported here.
Adverse events were reported by participants to a team member during intervention contacts or outcome assessments rather than being assessed systematically. Details and dates of protocol amendments and quality control procedures are described in Supplement 1.
Statistical Analysis
The sample size estimate was based on the hypothesis that patients randomly assigned to receive the maintenance intervention would have less mean weight regain at week 56 than those assigned to usual care (21, 22). From a previous study (23), the SD at week 0 was estimated as 24.6 kg, the correlation between weeks 0 and 56 as 0.95, and the dropout rate at week 56 as 10%. To detect a difference of 3.5 kg with 90% power and a type I error rate of 5%, we determined that 230 randomly assigned patients (115 in each group) were needed. This effect size represents approximately a 3% difference between groups, which is the lower threshold for clinically significant weight reduction (17).
For the primary analysis of weight, we used a general linear model with an unstructured covariance to account for the correlation of patients’ repeated measurements over time (by using PROC MIXED in SAS, version 9.4 [SAS Institute]). The outcome variable in this model was the study-measured weight (in kilograms) at weeks 0, 14, 26, 42, and 56. Model parameters included a common intercept (which constrains the baseline means to be equal); initial weight loss stratum (centered); indicator variables for weeks 14, 26, 42, and 56; and indicators for the maintenance intervention interacted with each follow-up time point indicator variable (24). Contrast statements were used to estimate the difference between the groups in weight regain from week 0 and each follow-up. The primary hypothesis was tested by examining the estimated difference in mean weight regain at week 56, 95% CI, and P value. In a post hoc sensitivity analysis, the assumption of equal mean weights at baseline was dropped.
Between-group differences in mean energy intake (kilocalories) at weeks 26 and 56 also were estimated and tested via a general linear model (PROC MIXED) with unstructured covariance. Incidence rate ratios (IRRs) and 95% CIs comparing the weekly number of metabolic minutes of walking and moderate physical activity between groups at weeks 26 and 56 were estimated by using generalized linear mixed models under a negative binomial distribution with a log link (PROC GLIMMIX) to account for overdispersion (25); correlation of patients’ repeated measures over time was accounted for with a random intercept. Model parameters for the mean energy and physical activity models included a common intercept, initial weight loss stratum (centered), indicator variables for weeks 26 and 56, and interaction of the maintenance intervention with the indicator variables for weeks 26 and 56 (see Supplement 2 [available at Annals.org] for the SAS code).
All available data, including information from participants who subsequently discontinued the study, were used for analyses. Primary and secondary outcome analyses were conducted with full likelihood methods, which are valid under a missing-data framework in which the missing values may depend on intervention group, initial weight loss stratum, and any observed outcome values (26). We also conducted a sensitivity analysis in which additional predictors of missing weight values at week 56 were explored and included in a multiple imputation model (predictors are listed in the Supplement 2). Ten multiple imputations for week-56 weights were generated via the MCMC option in PROC MI and analyzed with a general linear model including only parameters for a common intercept, initial weight loss stratum (centered), week-56 indicator variable, and interaction of the maintenance intervention with the week-56 indicator variable. The model estimates were combined by using PROC MIANALYZE (SAS, version 9.4). Following the recommendations of MacLean and colleagues (3), we conducted post hoc analyses with the multiply imputed weights at week 56 to derive the percentage of patients whose weights at week 56 remained at least 5% lower than those at week −16, and the change in weight from weeks 0 to 56 as a percentage of weight at week 0 and weight lost during the initiation phase. The values presented for these post hoc analyses were averaged across the 10 imputed data sets.
Between-group differences in mean and median VA total expenditures for the 56-week maintenance phase were examined via t tests and Wilcoxon rank-sum tests, respectively. Labor and capital costs for delivering the intervention also were calculated. Capital costs were estimated from market rates, and labor costs were calculated as the product of the time reported on the interventionists’ activity logs and their hourly wages, obtained from information in the General Schedule salary table for Durham, North Carolina. Because a VA payer perspective was taken, only VA costs were calculated.
Role of the Funding Source
The study was funded by the Health Services Research and Development service of the Department of Veterans Affairs, which did not have a role in study design, execution, statistical analysis, manuscript preparation or interpretation, or the decision to submit this paper for publication.
Results
Participants and Intervention Adherence
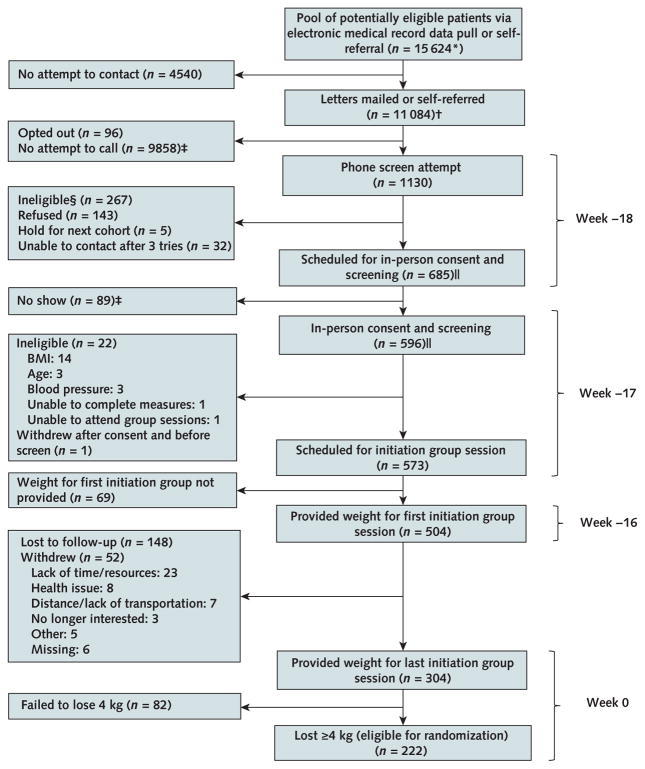
As shown in the Appendix Figure, 1130 participants called in response to recruitment letters, flyers, or recommendations from their providers. Of those, 685 were scheduled for in-person screening, after which 573 were eligible and scheduled for a weight loss group meeting. Of the eligible patients, 69 did not initiate (that is, provide a weight at week −16). Of the 504 participants who initiated, 82 participated in the program but lost insufficient weight, 200 did not return for outcome assessments, and 222 participated in the program and lost enough weight to be randomly assigned to a group (110 to maintenance intervention and 112 to usual care).
Appendix Figure.
MAINTAIN study phase 1 patient flow (weight loss initiation).
BMI = body mass index; MAINTAIN = Maintenance After Initiation of Nutrition Training.
* Exclusion criteria determined by data pull included most recent serum creatinine value >2.0 mg/dL in men or >1.7 mg/dL in women; liver disease; type 1 diabetes; most recent hemoglobin A1c value in the past 6 mo ≥12%; average systolic blood pressure over the past year ≥160 mm Hg and most recent blood pressure reading ≥160 mm Hg; and history of weight loss surgery, dementia, severe psychiatric illness, or substance abuse.
† 10 807 patients were mailed letters; 38 were mailed letters as well as being self-referred; 239 were self-referred, with no letter sent.
‡ Obtained by subtraction.
§ Potential reasons for ineligibility assessed by telephone included BMI ≥29 kg/m2 based on self-reported weight and height (reduced threshold to allow for error in reporting); self-reported age <18 or >75 y; weight loss ≥10 lb in the previous 3 mo; current enrollment in a lifestyle program; history of weight loss surgery; current use of weight loss medication or appetite suppressant; pregnancy or plan to become pregnant in upcoming 6 mo, breastfeeding, or lack of birth control if premenopausal (female); organ transplant recipient; type 1 diabetes; heart disease with new treatment in past 3 mo; liver disease; cancer not in remission; pacemaker or defibrillator (because of the use of a bioelectronic impedance scale); major depression or emotional problems that would prevent the participant from following a diet closely or interacting with others in a group environment; illicit drug use or alcohol problems in the past year; inability to stand for study measurements; lack of desire to lose weight; lack of agreement to attend study visits; and no access to telephone or reliable transportation.
|| Two of the 267 patients ineligible at phone screen (1 because of BMI and 1 because of age) are included in both the “Scheduled for in-person consent and screening” and “In-person consent and screening” boxes. One was ineligible at phone screen because of BMI <30 kg/m2 but then was erroneously rescreened in person and excluded at that point for the same reason. The other patient was listed as excluded because of age >75 y at both phone and in-person screen. Both exclusions were erroneous because the patient was aged 75 y at both time points; however, the patient was not included in the study after the in-person screen.
Randomly assigned participants lost an average of 7.2 kg during initiation; at randomization, their mean weight was 103.6 kg and mean BMI was 34.0 kg/m2 (Table). Most patients were middle-aged (mean, 62 years), white (58%), male (84%), high school graduates (98%), and retired (56%) and had attempted weight loss previously (82%). Self-reported dietary intake and physical activity at week 0 are reported in the Table.
Table.
Characteristics of Participants, Overall and by Treatment Group and Time Point*
| Characteristic | Overall (n = 222) | Usual Care (n = 112) | Maintenance (n = 110) |
|---|---|---|---|
| Measured at week −17 (in-person screening) | |||
| Demographic | |||
|
| |||
| Mean age (SD), y | 61.8 (8.3) | 62.0 (8.3) | 61.5 (8.3) |
|
| |||
| Race, n (%) | |||
| White | 129 (58.1) | 62 (55.4) | 67 (60.9) |
|
| |||
| Black | 83 (37.4) | 45 (40.2) | 38 (34.5) |
|
| |||
| Multiracial/other | 6 (2.7) | 3 (2.7) | 3 (2.7) |
|
| |||
| Female, n (%) | 34 (15.3) | 17 (15.2) | 17 (15.5) |
|
| |||
| High school graduate, n (%) | 217 (97.7) | 111 (99.1) | 106 (96.4) |
|
| |||
| Current tobacco user, n (%) | 14 (6.3) | 9 (8.0) | 5 (4.5) |
|
| |||
| Employment status, n (%)† | |||
|
| |||
| Working full or part time | 61 (27.5) | 28 (25.0) | 33 (30.0) |
|
| |||
| Retired | 124 (55.9) | 65 (58.0) | 59 (53.6) |
|
| |||
| Other/disabled | 35 (15.8) | 18 (16.1) | 17 (15.5) |
|
| |||
| Attempted weight loss previously, n (%) | 183 (82.4) | 89 (79.5) | 94 (85.5) |
| Measured at week −16 (first initiation group meeting) | |||
| Anthropometric | |||
|
| |||
| Mean weight (SD), kg | 110.8 (20.6) | 112.2 (21.7) | 109.3 (19.5) |
|
| |||
| Mean BMI (SD), kg/m2 | 36.3 (6.1) | 36.9 (6.5) | 35.7 (5.5) |
|
| |||
| BMI ≥35 kg/m2, n (%) | 104 (46.8) | 56 (50.0) | 48 (43.6) |
| Measured at week 0 (randomization) | |||
| Anthropometric | |||
|
| |||
| Mean weight loss during initiation phase (SD), kg‡ | 7.2 (3.1) | 7.2 (3.4) | 7.2 (2.8) |
|
| |||
| Mean weight (SD), kg | 103.6 (20.4) | 105.0 (21.0) | 102.1 (19.8) |
|
| |||
| Mean waist circumference (SD), inches | 44.0 (5.7) | 44.5 (6.0) | 43.5 (5.4) |
|
| |||
| Mean BMI (SD), kg/m2 | 34.0 (6.1) | 34.6 (6.4) | 33.3 (5.7) |
|
| |||
| BMI ≥35 kg/m2, n (%) | 76 (34.2) | 44 (39.3) | 32 (29.1) |
|
| |||
| Initial weight loss strata, n (%) | |||
| 4 to <10 kg | 194 (87.4) | 98 (87.5) | 96 (87.3) |
|
| |||
| ≥10 kg | 28 (12.6) | 14 (12.5) | 14 (12.7) |
|
| |||
| Mean diet (SD) | |||
| Energy intake, kcal | 1178.2 (570.5) | 1159.3 (513.3) | 1197.9 (626.6) |
|
| |||
| Total calories from fat, % | 37.1 (8.7) | 36.5 (8.5) | 37.8 (9.0) |
|
| |||
| Total calories from carbohydrates, % | 46.5 (10.3) | 47.1 (10.0) | 45.8 (10.6) |
|
| |||
| Total calories from protein, % | 17.5 (3.7) | 17.5 (4.0) | 17.4 (3.4) |
|
| |||
| Mean physical activity (SD), metabolic min/wk | |||
| Walking§ | 748 (934) | 697 (894) | 801 (975) |
|
| |||
| Moderate-intensity physical activity§ | 788 (1095) | 779 (1205) | 798 (977) |
|
| |||
| Vigorous-intensity physical activity§ | 1225 (2061) | 1211 (2286) | 1240 (1814) |
|
| |||
| Total physical activity|| | 2788 (3109) | 2701 (3449) | 2877 (2729) |
BMI = body mass index.
Missing values were included in the calculation of the percentages and are specified here by randomization group: race (usual care, 2; maintenance, 2); sex (usual care, 1; maintenance, 0); education level (usual care, 0; maintenance, 1); tobacco use (usual care, 1; maintenance, 0); employment status (usual care, 1; maintenance, 1); attempted weight loss previously (usual care, 1; maintenance, 0); week 0 waist circumference (usual care, 2; maintenance, 1); week 0 energy intake and percentage of total calories from fat, carbohydrates, and protein (usual care, 12; maintenance, 14); walking (usual care, 0; maintenance, 2); moderate-intensity physical activity (usual care, 1; maintenance, 0); and total physical activity (usual care, 1; maintenance, 2).
Patients could select >1 status; the category reported in the table was assigned preferentially on the basis of the order of employment statuses listed in the table.
Week −16 minus week 0.
Metabolic minutes per week were calculated by multiplying self-reported weekly minutes of walking and moderate- and vigorous-intensity physical activity by 3.3, 4.0, and 8.0, respectively.
Calculated by summing individuals’ metabolic minutes per week for walking and moderate- and vigorous-intensity physical activity. The mean value does not equal the sum of the mean components because of individuals with missing data, noted above.
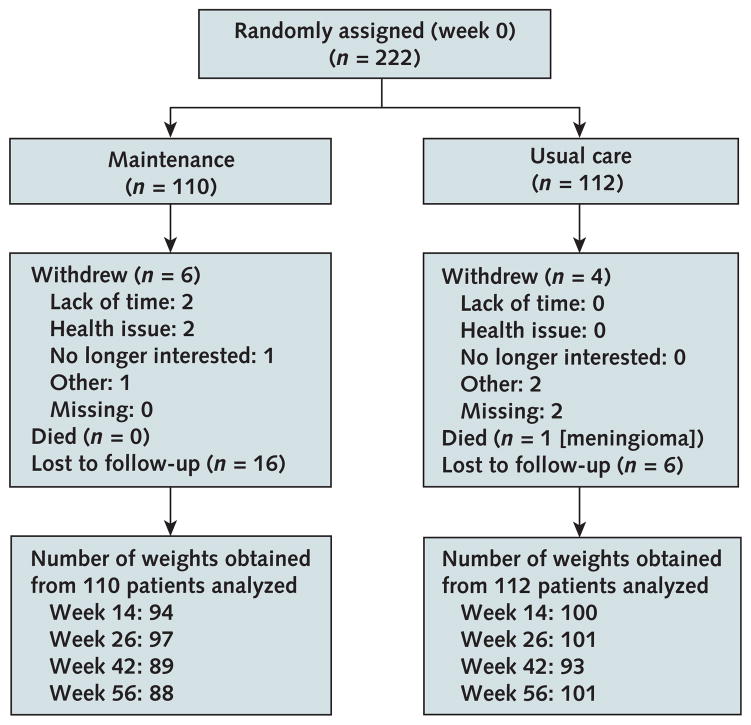
Patients in the intervention group attended 0 to 3 maintenance meetings (mean, 2.07 [SD, 1.06]) and participated in 2 to 8 telephone calls (mean, 7.34 [SD, 1.43]). Retention for week-56 assessments in the usual care and maintenance groups was 90% and 80% for weight (Figure 1), 78% and 76% for FFQ, and 89% and 79% for IPAQ, respectively. The median length of follow-up was 397.0 days (interquartile range, 381.5 to 407.0) in the usual care and 391.5 days (interquartile range, 351.0 to 404.0) in the intervention group. No crossover of assignments occurred. One patient in the usual care group died of meningioma. Reported adverse events were knee pain (n = 1), low blood pressure (n = 1), bradycardia (n = 1), and anxiety (n = 1) in the intervention group, and heart failure (n = 2) and myocardial infarction (n = 1) among patients not randomly assigned.
Figure 1.
MAINTAIN study phase 2 patient flow (weight loss maintenance).
MAINTAIN = Maintenance After Initiation of Nutrition Training. Detailed inclusion and exclusion criteria for phase 2 (randomized clinical trial) of the MAINTAIN study are described in the Methods section and the Appendix Figure (available at Annals.org). In brief, patients included in the study were aged 18 to 75 years with a body mass index ≥30 kg/m2. Patients whose weight could or should not be measured with a bioelectric impedance scale and women who were pregnant or breastfeeding were excluded. In addition, patients with a history of any of the following were excluded: kidney or liver disease, type 1 diabetes, elevated systolic blood pressure, surgical or recent behavioral or pharmaceutical weight loss attempts, mental health or substance abuse issues, recently treated heart disease, cancer not in remission, or receipt of an organ transplant.
Primary Outcome
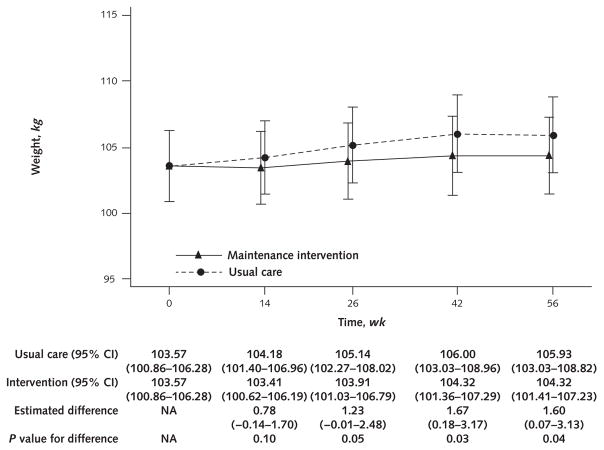
Estimated mean weight loss at each time point is shown in Figure 2. Estimated weight regain was statistically significantly lower in the intervention (0.75 kg) than the usual care (2.36 kg) group (estimated mean difference, 1.60 kg [95% CI, 0.07 to 3.13 kg]; P = 0.040) at the primary end point of 56 weeks and at week 42 (estimated mean difference, 1.67 kg [CI, 0.18 to 3.17 kg]; P = 0.029) (Figure 2). Dropping the assumption of equal baseline weights did not meaningfully change the results (estimated mean weight regain, 1.59 kg [CI, 0.07 to 3.11 kg]; P = 0.040). Results of the analysis on the multiply imputed weight data were similar to the model only on observed data (estimated mean difference at 56 weeks, 1.65 kg [CI, 0.10 to 3.19 kg]; P = 0.036). Using individuals’ multiply imputed weight values at week 56, we conducted several post hoc descriptive analyses. Estimated weight regain from weeks 0 to 56 as a percentage of week 0 weight was 0.8% and 2.4% for the maintenance and usual care groups, respectively. Estimated weight regain from weeks 0 to 56 as a percentage of weight loss during initiation was 14.6% and 41.9% for the maintenance and usual care groups, respectively. Finally, weights at week 56 were at least 5% lower (about 5.5 kg) than those at baseline before the weight loss initiation phase (week −16) for 57.8% of maintenance group participants, compared with 34.3% of those receiving usual care.
Figure 2.
Model-estimated weights, differences in weights, and associated 95% CIs, by treatment group and week.
Bars are 95% CIs. Weight estimates for each time point were calculated by using a general linear model with an unstructured covariance to account for the correlation of patients’ repeated measurements over time. Model parameters included a common intercept (which constrained the baseline means to be equal); initial weight loss stratum (centered); indicator variables for weeks 14, 26, 42, and 56; and indicators for the maintenance intervention interacted with each follow-up time point indicator variable. Contrast statements were used to estimate the difference between the groups in weight regain from week 0 to each of the 4 follow-up time points. The model-estimated weight correlation between time points ranged from 0.99 (weeks 0 and 14) to 0.97 (weeks 0 and 56). NA = not applicable (because the model constrained the baseline means to be equal).
Secondary Outcomes
A statistically significant difference in self-reported energy intake (kilocalories) was noted between the maintenance (estimated mean, 1176.06 kcal) and usual care (estimated mean, 1399.50 kcal) groups at week 26 (estimated mean difference, −223.44 kcal [CI, −395.92 to −50.96 kcal]; P = 0.011). However, the between-group difference was no longer statistically significant at 56 weeks (estimated mean, 1226.84 kcal for maintenance and 1352.34 kcal for usual care; estimated mean difference, −125.49 kcal [CI, −280.71 to 29.72 kcal]; P = 0.112). No differences were seen in estimated rates of walking or moderate physical activity between the maintenance and usual care groups at week 26 (walking IRR, 1.38 [CI, 0.85 to 2.23; P = 0.19]; moderate physical activity IRR, 1.01 [CI, 0.54 to 1.90; P = 0.96]) or week 56 (walking IRR, 1.03 [CI, 0.63 to 1.68; P = 0.91]; moderate physical activity IRR, 1.07 [CI, 0.57 to 2.03; P = 0.83]). No between-group differences were noted in estimated waist circumference at week 26 (estimated mean difference, −0.36 in [CI, −1.03 to 0.30 in]; P = 0.28) or week 56 (estimated mean difference, −0.55 in [CI, −1.32 to 0.21]; P = 0.153).
Intervention Cost and Health Care Expenditures
Intervention costs were $88.58 and $276.19 per participant for the initiation and maintenance interventions, respectively. No differences were seen in mean and median VA total expenditures by group during the 56-week maintenance phase (mean, $11 932 [SD, $20 714] in the maintenance group and $14 876 [SD, $32 795] in the usual care group [P = 0.42]; median, $4292 [interquartile range, $13 429] in the maintenance group and $5584 [interquartile range, $11 438] in the usual care group [P = 0.37]).
Discussion
Despite the efficacy of behavioral weight loss initiation programs, maintenance has remained the holy grail of weight loss research. Our study provides evidence that focusing on maintenance-specific skills may help people maintain much of their initial weight loss 56 weeks later. Participants maintained their weight even though the intervention decreased in frequency, shifted from in-person to telephone delivery, and involved no intervention contact in the final 14 weeks.
We compare our findings to those of other maintenance trials identified in an English-language PubMed search in November 2016 (6, 10–14, 27–29). The trial most similar in design was the WLM trial, in which patients who lost at least 4 kg in a 6-month weight loss phase were randomly assigned to an Internet, a personal contact, or a self-directed maintenance intervention (14). At 12 months (the time point that aligned most closely with our primary end point of 56 weeks), the treatment difference between the personal contacts and self-directed interventions was 1.6 kg. Our treatment difference was identical despite notable distinctions in our study, including a shorter initial weight loss phase, a larger proportion of male participants, and a lower rate of patient eligibility after the weight loss initiation phase (44% in our trial vs. 61% in WLM). Our treatment difference is similar in magnitude to that of trials that had different designs (such as those involving participants who had lost weight before enrollment and thus varied in recency of weight loss and level of dietary education and skills [6, 13]), tested maintenance interventions of different content, often involved in-person delivery, and involved more frequent contact (6, 13, 27, 28).
One would expect to see the differences in weight regain reflected in secondary measures of caloric intake and physical activity. The lack of statistically significant differences in our secondary outcomes at week 56 may be the result of measurement error due to the limited reliability and validity of self-report measures of dietary intake and physical activity. In addition, participants’ behavioral plans for maintaining weight loss varied in whether they focused on maintaining a constant dietary pattern or incorporating physical activity, which may have masked treatment differences in the 2 behaviors.
Another limitation to our study was the relatively short duration of intervention and follow-up determined by our funding mechanism. In addition, generalizability is limited because initiation of the weight loss program was lower among people who were younger, lacked a support person, and had less encouragement for making dietary changes (15). Furthermore, compared with participants who lost enough weight in the initial weight loss phase, dropout in the initial phase was more likely among younger participants and tobacco users, and weight loss less than 4 kg was greater among nonwhite participants and was associated with greater controlled (extrinsic) motivation for physical activity. Generalizability might be enhanced by developing strategies to promote initiation and retention among these subgroups.
Strengths of our study include the use of a strong efficacy design (3), a high retention rate, the use of a conceptual model to inform the intervention, and regular fidelity assessments. In addition, our intervention was designed with implementation in mind. Its script is standardized so that it can be programmed into a custom software package and store participant responses for future reference. Although the protocol focuses on maintenance, it allows participants to refocus on weight loss initiation processes if a weight regain threshold is surpassed. The intervention also was designed to be low cost so that more resources could be devoted to initial weight loss relative to maintenance. Studies have underscored the superiority of in-person, group-based initiation intervention delivered at least twice per month compared with less intense intervention delivered by other methods, as reflected in the current obesity guideline (17) and coverage of weight loss treatment by the Centers for Medicare & Medicaid Services. Our maintenance intervention was delivered primarily by telephone, with decreasing frequency of contact.
In conclusion, a low-cost intervention focusing on maintenance constructs modestly reduced weight regain. Future research may extend the duration of intervention or follow-up to evaluate the durability of treatment effects. Electronic medical record data might be used to evaluate longer-term effects in the absence of further intervention. Future research should evaluate whether other behavior maintenance strategies would improve weight maintenance further. Additional efforts also might determine optimal implementation strategies for incorporating efficacious weight maintenance interventions into clinical programs, such as identifying an appropriate referral process, addressing barriers to initiation and retention in a comprehensive weight management program, and identifying optimal staff training and fidelity monitoring processes. By incorporating a weight maintenance intervention into clinical or commercial weight loss programs, the effect of efficacious weight loss programs may be increased.
Acknowledgments
The authors thank Jahdai Dawes, Marsha Turner, and Terry Ervin for assisting with recruitment and conducting measurements. They also thank Lesa Powell and Aviel Alkon for programming and database development and Lynn Van Scoyoc for creating data sets and variables necessary for health utilization analyses.
Grant Support: This study was funded by a grant awarded to Drs. Voils and Yancy by the Department of Veterans Affairs Health Services Research and Development (VA HSR&D) Service (IIR 11-040). Efforts on this study or manuscript also were made possible by a VA Research Career Scientist award to Dr. Voils (RCS 14-443), a career development award to Dr. McVay (K23 HL127334), and a VA Research Career Scientist award to Dr. Maciejewski (RCS 10-391).
Primary Funding Source: Veterans Affairs Health Services Research and Development Service.
Footnotes
Disclaimer: The views represented in this article represent those of the authors and not those of the U.S. Department of Veterans Affairs or U.S. government.
Reproducible Research Statement: Study protocol: See Supplement 1 (available at Annals.org). Statistical code: Available from the Supplement 2 (available at Annals.org) or from Dr. Olsen (maren.olsen@va.gov). Data set: Available through a data use agreement from Dr. Olsen (maren.olsen@va.gov).
Author Contributions: Conception and design: C.I. Voils, M.K. Olsen, J.M. Gierisch, L. Gaillard, W.S. Yancy.
Analysis and interpretation of the data: C.I. Voils, M.K. Olsen, J.M. Gierisch, M.A. McVay, J.M. Grubber, M.L. Maciejewski, W.S. Yancy.
Drafting of the article: C.I. Voils, M.K. Olsen, J.M. Grubber.
Critical revision for important intellectual content: C.I. Voils, M.K. Olsen, J.M. Gierisch, M.A. McVay, J.M. Grubber, W.S. Yancy.
Final approval of the article: C.I. Voils, M.K. Olsen, J.M. Gierisch, M.A. McVay, J.M. Grubber, L. Gaillard, J. Bolton, M.L. Maciejewski, E. Strawbridge, W.S. Yancy.
Provision of study materials or patients: C.I. Voils, J.M. Gierisch, L. Gaillard, J. Bolton, E. Strawbridge.
Statistical expertise: M.K. Olsen, J.M. Grubber, M.L. Maciejewski.
Obtaining of funding: C.I. Voils, M.K. Olsen, J.M. Gierisch, W.S. Yancy.
Administrative, technical, or logistic support: C.I. Voils, J.M. Grubber, L. Gaillard, J. Bolton, M.L. Maciejewski, E. Strawbridge, W.S. Yancy.
Collection and assembly of data: J.M. Grubber, L. Gaillard, J. Bolton, E. Strawbridge.
Disclosures: Dr. Voils, Ms. Bolton, and Ms. Strawbridge report grants from VA HSR&D during the conduct of the study. Dr. Maciejewski reports grants from VA HSR&D during the conduct of the study, and nonfinancial support from Amgen and grants from the Agency for Healthcare Research and Quality and National Committee for Quality Assurance outside the submitted work. Dr. Yancy reports grants from the Department of Veterans Affairs during the conduct of the study, and personal fees from University of Pennsylvania/Weight Watchers International and grants from the National Institutes of Health outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M16-2160.
References
- 1.Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: a randomized trial. Ann Intern Med. 2010;153:147–57. doi: 10.7326/0003-4819-153-3-201008030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005;353:2111–20. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 3.MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, et al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity (Silver Spring) 2015;23:7–15. doi: 10.1002/oby.20967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dombrowski SU, Knittle K, Avenell A, Araújo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. BMJ. 2014;348:g2646. doi: 10.1136/bmj.g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothman AJ, Sheeran P, Wood W. Reflective and automatic processes in the initiation and maintenance of dietary change. Ann Behav Med. 2009;38(Suppl 1):S4–17. doi: 10.1007/s12160-009-9118-3. [DOI] [PubMed] [Google Scholar]
- 6.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 7.Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem-solving therapy in the long-term management of obesity. J Consult Clin Psychol. 2001;69:722–6. [PubMed] [Google Scholar]
- 8.Rothman AJ. Toward a theory-based analysis of behavioral maintenance. Health Psychol. 2000;19:64–9. doi: 10.1037/0278-6133.19.suppl1.64. [DOI] [PubMed] [Google Scholar]
- 9.Pekkarinen T, Kaukua J, Mustajoki P. Long-term weight maintenance after a 17-week weight loss intervention with or without a one-year maintenance program: a randomized controlled trial. J Obes. 2015;2015:651460. doi: 10.1155/2015/651460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeffery RW, Levy RL, Langer SL, Welsh EM, Flood AP, Jaeb MA, et al. A comparison of maintenance-tailored therapy (MTT) and standard behavior therapy (SBT) for the treatment of obesity. Prev Med. 2009;49:384–9. doi: 10.1016/j.ypmed.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perri MG, McAllister DA, Gange JJ, Jordan RC, McAdoo G, Nezu AM. Effects of four maintenance programs on the long-term management of obesity. J Consult Clin Psychol. 1988;56:529–34. doi: 10.1037//0022-006x.56.4.529. [DOI] [PubMed] [Google Scholar]
- 12.Perri MG, Limacher MC, Durning PE, Janicke DM, Lutes LD, Bobroff LB, et al. Extended-care programs for weight management in rural communities: the treatment of obesity in underserved rural settings (TOURS) randomized trial. Arch Intern Med. 2008;168:2347–54. doi: 10.1001/archinte.168.21.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West DS, Gorin AA, Subak LL, Foster G, Bragg C, Hecht J, et al. Program to Reduce Incontinence by Diet and Exercise (PRIDE) Research Group. A motivation-focused weight loss maintenance program is an effective alternative to a skill-based approach. Int J Obes (Lond) 2011;35:259–69. doi: 10.1038/ijo.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, et al. Weight Loss Maintenance Collaborative Research Group. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–48. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 15.Voils CI, Grubber JM, McVay MA, Olsen MK, Bolton J, Gierisch JM, et al. Recruitment and retention for a weight loss maintenance trial involving weight loss prior to randomization. Obes Sci Pract. 2016;2:355–65. doi: 10.1002/osp4.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yancy WS, Jr, Mayer SB, Coffman CJ, Smith VA, Kolotkin RL, Geiselman PJ, et al. Effect of allowing choice of diet on weight loss: a randomized trial. Ann Intern Med. 2015;162:805–14. doi: 10.7326/M14-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Voils CI, Gierisch JM, Olsen MK, Maciejewski ML, Grubber J, McVay MA, et al. Study design and protocol for a theory-based behavioral intervention focusing on maintenance of weight loss: the Maintenance After Initiation of Nutrition TrAINing (MAINTAIN) study. ContempClinTrials. 2014;39:95–105. doi: 10.1016/j.cct.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Voils CI, Gierisch JM, Yancy WS, Jr, Sandelowski M, Smith R, Bolton J, et al. Differentiating behavior initiation and maintenance: theoretical framework and proof of concept. Health Educ Behav. 2014;41:325–36. doi: 10.1177/1090198113515242. [DOI] [PubMed] [Google Scholar]
- 20.Kahwati LC, Lance TX, Jones KR, Kinsinger LS. RE-AIM evaluation of the Veterans Health Administration’s MOVE! Weight Management Program. Transl Behav Med. 2011;1:551–60. doi: 10.1007/s13142-011-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60:1234–8. doi: 10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Keppel G. Design and Analysis: A Researcher’s Handbook. 3. Englewood Cliffs, NJ: Prentice-Hall; 1991. [Google Scholar]
- 23.Yancy WS, Jr, Westman EC, McDuffie JR, Grambow SC, Jeffreys AS, Bolton J, et al. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch Intern Med. 2010;170:136–45. doi: 10.1001/archinternmed.2009.492. [DOI] [PubMed] [Google Scholar]
- 24.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- 25.Diggle PJ, Heagerty P, Liang K, Zeger SL. Analysis of Longitudinal Data. New York: Oxford Univ Pr; 2002. [Google Scholar]
- 26.Olsen MK, Stechuchak KM, Edinger JD, Ulmer CS, Woolson RF. Move over LOCF: principled methods for handling missing data in sleep disorder trials. Sleep Med. 2012;13:123–32. doi: 10.1016/j.sleep.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Kiernan M, Brown SD, Schoffman DE, Lee K, King AC, Taylor CB, et al. Promoting healthy weight with “stability skills first”: a randomized trial. J Consult Clin Psychol. 2013;81:336–46. doi: 10.1037/a0030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherwood NE, Crain AL, Martinson BC, Anderson CP, Hayes MG, Anderson JD, et al. Enhancing long-term weight loss maintenance: 2 year results from the Keep It Off randomized controlled trial. Prev Med. 2013;56:171–7. doi: 10.1016/j.ypmed.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perri MG, McAdoo WG, McAllister DA, Lauer JB, Jordan RC, Yancey DZ, et al. Effects of peer support and therapist contact on long-term weight loss. J Consult Clin Psychol. 1987;55:615–7. doi: 10.1037/0022-006X.55.4.615. [DOI] [PubMed] [Google Scholar]