Abstract
BACKGROUND
Intravenous iron is used widely in hemodialysis, yet there are limited data on the effectiveness of contemporary dosing strategies or formulation type.
METHODS
We conducted a retrospective cohort study using data from the clinical database of a large dialysis provider (years 2004–2008) merged with administrative data from the United States Renal Data System to compare the effects of intravenous iron use on anemia management. Dosing comparisons were (1) bolus (consecutive doses ≥100 mg exceeding 600 mg during one month) versus maintenance (all other iron doses during the month); and (2) high (> 200 mg over 1 month) versus low dose (≤200 mg over 1 month). Formulation comparison was administration of ferric gluconate versus iron sucrose over one month. Outcomes were hemoglobin, epoetin dose, transferrin saturation, and serum ferritin during 6 weeks of follow-up.
RESULTS
We identified 117,050 patients for the dosing comparison, and 66,207 patients for the formulation comparison. Bolus dosing was associated with higher average adjusted hemoglobin (+0.23 g/dl, 95% C.I. 0.21–0.26), transferrin saturation (+3.31% 95% C.I. 2.99–3.63), serum ferritin (+151 mcg/L, 95% C.I. 134.9–168.7), and lower average epoetin dose (-464 units 95% C.I. -583 to -343) compared to maintenance. Similar trends were observed with high dose iron versus low dose. Iron sucrose was associated with higher adjusted average hemoglobin (+0.16 g/dl, 95% C.I. 0.12–0.19) versus ferric gluconate.
CONCLUSIONS
Strategies favoring large doses of intravenous iron or iron sucrose lead to improved measures of anemia management. These potential benefits should be weighed against risks, which currently, remain incompletely characterized.
INTRODUCTION
Intravenous (IV) iron is now an integral component of anemia management among patients with end-stage renal disease.1 Originally considered an adjuvant to erythropoeisis stimulating agents (ESA), its use has steadily risen over the past decade.2 In the United States, contemporary practice patterns for IV iron vary by both dose and formulation. For example, some dialysis clinics administer large repletion or bolus doses of iron over consecutive dialysis sessions on an intermittent, as-needed basis.3 Others provide low-dose administrations of iron every 1 to 2 weeks to maintain iron stores4, or a combination of maintenance and bolus dosing.3 Formulation patterns for intravenous iron primarily consist of iron sucrose and ferric gluconate despite the availability of five different agents. Both formulations are iron-carbohydrate complexes, but possess varying pharmacokinetic and pharmacodynamic properties that may differentially affect anemia management.5–7
Despite the growing use of iron, there are sparse contemporary data about the benefits of IV iron use in the US dialysis population. Previous studies of dosing patterns have not directly compared dosing strategies that are now used in practice. 8,9 Furthermore, they have limited sample size and follow-up, potentially reducing generalizability to patients currently receiving dialysis. Studies comparing ferric gluconate and iron sucrose have similar limitations.10,11
To address this gap in the literature, we conducted a large-scale observational study using data from one of the largest national dialysis providers linked with the United States Renal Data System (USRDS). Our goal was to examine the comparative effectiveness of dosing strategies and formulation types on clinical parameters of anemia management – hemoglobin level, epoetin alfa (EPO) dose, transferrin saturation (TSAT), and serum ferritin -- in a cohort that is representative of contemporary patients receiving hemodialysis.
METHODS
Data Sources
The data used for this study came from the clinical research database of a large dialysis provider and the United States Renal Data System (USRDS). The dialysis provider owns and manages over 1,500 outpatient dialysis facilities located throughout the U.S. in urban, rural, and suburban areas. Their clinical database captures detailed clinical, laboratory, and treatment data on patients receiving care at all of their dialysis units. All data are collected using standardized electronic health record systems. For this study, we used the clinical data to obtain detailed information on iron formulation and dosing, ESA use and dosing, and clinical laboratory values (e.g., hemoglobin, transferrin saturation, serum ferritin). We also used data from the USRDS, a national data system that collects, analyzes, and distributes information about the treatment of ESRD in the U.S. Funded by the National Institutes of Diabetes Digestive and Kidney disease, the USRDS collaborates with several entities, including the Centers for Medicare and Medicaid Services (CMS), to create a detailed data system on ESRD patients. Our USRDS data originated from CMS and included data from the Medical Evidence Report Form, the Medicare Enrollment database, the ESRD Death Notification Form, and the standard analytic files, which contain final action claims data.12
We examined 5 years of data (2004 – 2008) from the clinical database to identify the cohort. These data were merged with data from the USRDS to obtain information on demographic characteristics, health care use (e.g., hospitalizations, outpatient care), and additional clinical characteristics (e.g., comorbidities).
Study Design
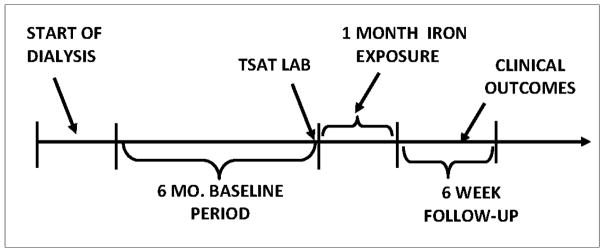
We utilized a retrospective cohort design in which we established a 6-month baseline period (to identify potential confounders and effect modifiers), a one-month iron exposure period, and a 6 week follow-up period. Figure 1 diagrams our specific implementation of the cohort design. The index date of the exposure period was anchored on a TSAT lab, as this information is used to guide iron administration.
Figure 1.
Study Design
Cohort Identification
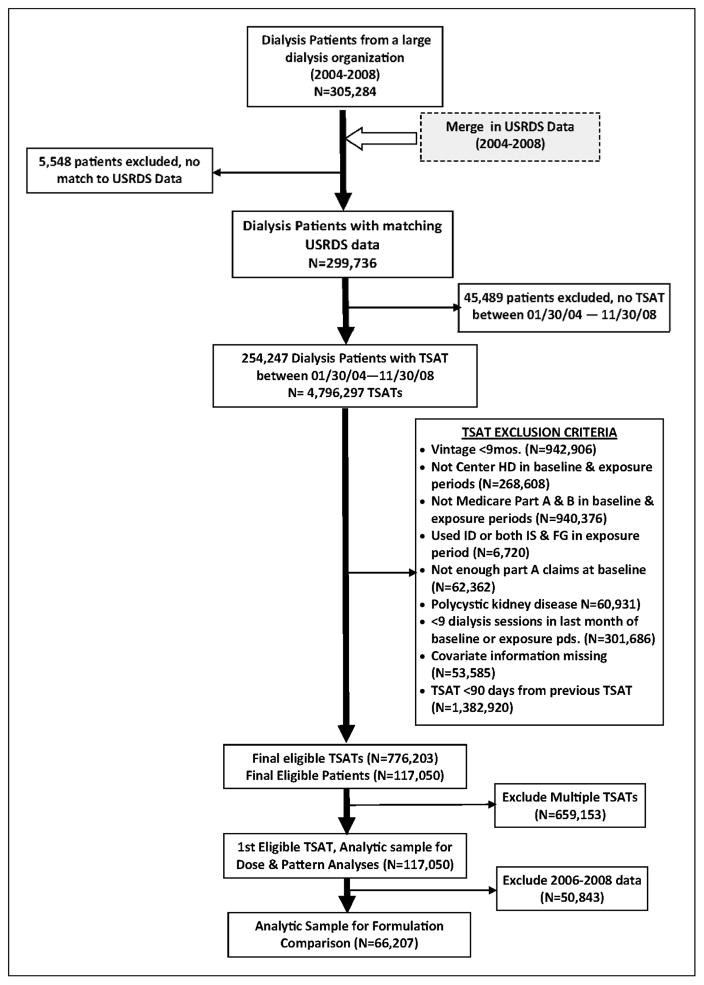
Figure 2 outlines the creation of our sample. We considered a mix of incident and prevalent patients. After merging the clinical and USRDS data, we identified individuals who had one or more TSAT labs between January 30, 2004 and November 30, 2008. The January 30th date was chosen to allow for a month of dialysis, which is typically followed by a TSAT lab. The November 30th date was chosen to allow for the 1-month exposure period and at least one day of follow-up (December 31, 2008).
Figure 2.
Creation of Study Cohorts
Data on TSAT labs were excluded if the patient: 1) had a dialysis vintage less than 9 months (which accounted for the 6-month baseline period and an additional 3 months from the start of dialysis to allow for stability in the CMS claims processing)12; 2) was not in center hemodialysis for the baseline and exposure period; 3) did not have Medicare Part A and Part B coverage; 4) received iron dextran or both ferric gluconate and iron sucrose in the exposure period; 5) had an insufficient amount of Part A claims at baseline (i.e., <120 days of Part A claims), suggesting incomplete data; 6) had polycystic kidney disease; and 7) had fewer than 9 dialysis sessions in the last month of baseline or during the exposure period. The last exclusion criterion was set to eliminate potential bias since individuals who did not receive the usual number of dialysis treatments in the month may have been too sick to do so. We also excluded TSAT records with missing covariate information. The exposure assessment period for each patient was anchored on the first qualifying TSAT lab.
Study Variables
Exposures
There were two primary exposures of interest: (1) dosing pattern, and (2) formulation type.
For the dosing pattern comparison, the exposures were high dose versus low dose iron, and bolus versus maintenance dosing. Based on the distribution of the IV iron dosing, we defined high dose as >200 mg of IV iron in the one month exposure period. Low dose was defined as ≤200 mg of IV iron. We also created a no iron category for individuals who received no iron during the one month exposure period.
A month was classified as a "bolus month" if it contained administrations of iron on consecutive dialysis sessions of at least 100 mg. We also classified a month as a bolus month if it contained two or more administrations of iron >100mg that had the potential to exceed 600mg within 30 days based on spacing between the doses in the sequence. For example, two consecutive iron doses of 200 mg each, within 10 days, would qualify as a bolus dose according to our definition. Months that had no bolus dosing patterns were classified as “maintenance months”. We also included a no iron category.
For the formulation comparison, the two exposures were iron sucrose and sodium ferric gluconate, measured using native codes from the clinical database. Individuals who received no iron were also included in the analyses.
Outcomes
We examined four clinical measures of anemia management during the follow-up period at a varying frequency: (1) weekly hemoglobin, (2) monthly TSAT, (3) monthly ferritin, and (4) weekly average EPO dose. All outcome variables were obtained from the clinical database.
Covariates
We included several covariates in our cohort analyses. The table in the Appendix presents specific definitions for the covariates, which included demographic and medical characteristics such as laboratory and anemia management variables, and co-morbidity measures based on the Elixhauser classification.13 With an extensive list of co-morbidities, we decided, a-priori, which ones to include in a parsimonious model, and others to add during sensitivity analyses.
Statistical Analysis
To assess the relation between the exposures (i.e., iron formulation and dosing practices) and the outcomes, (i.e., measures of anemia management) linear models estimated by Generalized Estimating Equations (GEE) were used. Covariates were grouped and entered consecutively into the model in the following manner using blocks of variables (Table 2, Appendix). Individuals were censored by death (for the hospitalization outcomes), loss to follow-up, receipt of a transplant, or administratively by the end of available data.
RESULTS
After merging clinical data with the USRDS, we identified a total of 117,050 patients who met our study entry requirements for the dosing comparison, and 66,207 patients for the formulation comparison. The patient characteristics of the primary cohort stratified by dosing pattern and formulation type are presented in Table 1. When comparing the high to low dose, approximately 29% of the sample received high dose iron, 34% received low dose iron, and 36% percent received no iron. In the bolus and maintenance comparisons, most individuals received maintenance dosing (47.4 percent), with 16.3 percent receiving bolus dosing. The groups had generally similar covariate distributions, both in the high and low dose comparison and in the bolus and maintenance comparison, with some notable exceptions. For example, the mean hospital days in the past month were slightly higher for the bolus versus maintenance group. The frequency of historical infections was also greater in the high and bolus dose patients than in the low and maintenance dose patients, respectively. The majority of cohort participants were from the year 2004, the first year of the observation period, and included both prevalent and incident patients; in the subsequent years, only incident patients were included.
Table 1.
Patient Characteristics at Baseline, Stratified by Dosing Pattern and Formulation Type
| Dosing Patterns (N=117,050) | Formulation Type (N=66,207) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Characteristics | High (29.3%) | Low (34.4%) | Bolus (16.3%) | Maintenance (47.4%) | Non-user (36.4%) | FG (12.3%) | IS (48.6%) | Non-user (39.1%) |
| Demographic | ||||||||
|
| ||||||||
| Mean (SD) Age, y | 60.7 (15.3) | 61.5 (15.2) | 60.3 (15.4) | 61.5 (15.2) | 61.6 (15.2) | 60.7 (15.2) | 61.1 (15.2) | 61.5 (15.1) |
| Female (%) | 44.9 | 44.6 | 45.3 | 44.6 | 46.3 | 45.2 | 45.5 | 47.0 |
| Race (%) | ||||||||
| White | 54.8 | 54.2 | 53.7 | 54.8 | 52.0 | 48.4 | 52.4 | 49.1 |
| Black | 40.3 | 39.8 | 41.6 | 39.5 | 41.1 | 48.0 | 41.5 | 43.7 |
| Other | 4.8 | 6.0 | 4.7 | 5.7 | 6.9 | 3.6 | 6.1 | 7.2 |
| Medicaid (%) | 48.8 | 47.4 | 50.1 | 47.4 | 49.2 | 49.3 | 49.4 | 51.3 |
| Region (%) | ||||||||
| Midwest | 19.1 | 17.0 | 18.2 | 17.9 | 16.9 | 15.4 | 17.3 | 16.5 |
| Northeast | 13.0 | 13.1 | 12.0 | 13.5 | 12.3 | 16.8 | 12.0 | 12.1 |
| South | 50.5 | 46.6 | 54.1 | 46.4 | 48.7 | 57.0 | 47.0 | 48.1 |
| West | 17.4 | 23.3 | 15.7 | 22.3 | 22.0 | 10.2 | 23.1 | 22.8 |
| Year (%) | ||||||||
| 2004 | 39.0 | 40.5 | 38.1 | 40.4 | 48.7 | 76.6 | 72.8 | 80.1 |
| 2005 | 15.2 | 15.8 | 15.2 | 15.6 | 13.2 | 23.4 | 27.2 | 19.9 |
| 2006 | 15.5 | 15.6 | 16.2 | 15.3 | 13.1 | |||
| 2007 | 15.6 | 14.6 | 15.9 | 14.8 | 13.3 | |||
| 2008 | 14.8 | 13.5 | 14.7 | 13.9 | 11.7 | |||
|
| ||||||||
| Clinical | ||||||||
|
| ||||||||
| ESRD Reason (%) | ||||||||
| Diabetes | 47.6 | 46.3 | 47.0 | 46.9 | 44.5 | 43.8 | 45.9 | 43.4 |
| Glomerulonephritis | 11.1 | 11.4 | 11.1 | 11.3 | 12.3 | 12.7 | 12.1 | 13.0 |
| Hypertension | 29.9 | 30.8 | 30.2 | 30.5 | 30.5 | 31.2 | 30.8 | 31.0 |
| Other | 11.4 | 11.5 | 11.7 | 11.3 | 12.7 | 12.4 | 11.2 | 12.6 |
| Mean (SD) Vintage, y | 2.3 (3.7) | 2.4 (3.8) | 2.3 (3.7) | 2.4 (3.8) | 3.1 (4.2) | 3.1 (4.0) | 2.9 (4.0) | 3.8 (4.3) |
| Mean (SD) BMI | 27.3 (7.1) | 27.1 (6.8) | 27.0 (7.1) | 27.3 (6.9) | 26.4 (6.5) | 27.0 (7.1) | 26.9 (6.8) | 26.2 (6.5) |
|
| ||||||||
| Anemia Management | ||||||||
|
| ||||||||
| Catheter (%) | 31.7 | 28.8 | 33.4 | 29.1 | 28.0 | 33.2 | 27.6 | 26.9 |
| Mean (SD) Albumin, g/dL | 3.8 (0.4) | 3.9 (0.4) | 3.7 (0.4) | 3.8 (0.4) | 3.8 (0.4) | 3.8 (0.4) | 3.8 (0.4) | 3.9 (0.4) |
| Mean (SD) Index TSAT, % | 24.4 (10.4) | 30.3 (11.5) | 22.1 (10.4) | 29.5 (11.1) | 34.6 (15.9) | 27.1 (11.1) | 27.8 (11.2) | 34.2 (15.6) |
| Mean (SD) Ferritin, mcg/L | 440 (293) | 502 (371) | 431 (355) | 488 (332) | 761 (568) | 524 (537) | 466 (288) | 771 (546) |
| Mean (SD) Hemoglobin, g/dL | 12.1 (1.4) | 12.2 (1.3) | 11.9 (1.5) | 12.3 (1.3) | 12.1 (1.4) | 12.1 (1.3) | 12.2 (1.4) | 12.1 (1.4) |
| Mean (SD) Iron, mg | 352 (346) | 212 (231) | 385 (425) | 239 (227) | 105 (248) | 271 (278) | 271 (309) | 100 (246) |
| Mean (SD) EPO during baseline, units | 113,097 (101,774) | 79,475 (82,277) | 128,086 (109,392) | 83,556 (84,098) | 74,895 (83,963) | 99302 (99993) | 96206 (93734) | 76064 (83604) |
| Mean (SD) EPO during exposure, units | 108,306 (103,128) | 78,162 (81,880) | 124,901 (111,247) | 80,738 (83,609) | 80,506 (87,550) | 95278 (100068) | 94683 (95125) | 81457 (88414) |
| Blood transfusion (%) | 9.6 | 6.1 | 11.7 | 6.3 | 8.1 | 8.0 | 7.1 | 7.6 |
|
| ||||||||
| Comorbidities | ||||||||
|
| ||||||||
| Mean (SD) Hosp. Days, last mo. | 0.8 (2.2) | 0.6 (1.8) | 1.0 (2.4) | 0.6 (1.8) | 0.6 (2.0) | 0.7 (1.9) | 0.7 (2.0) | 0.6 (1.9) |
| Infection, last month (%) | 16.0 | 12.5 | 18.2 | 12.6 | 12.2 | 13.0 | 12.8 | 11.2 |
| Comorbidities during baseline | ||||||||
| Pneumonia (%) | 12.9 | 9.9 | 14.3 | 10.2 | 10.9 | 11.1 | 10.8 | 10.5 |
| Sepsis (%) | 16.1 | 11.8 | 18.3 | 12.2 | 12.4 | 12.0 | 13.4 | 11.4 |
| Vascular access (%) | 18.3 | 14.3 | 20.3 | 14.7 | 13.4 | 18.1 | 15.6 | 12.9 |
| Diabetes (%) | 59.4 | 55.2 | 60.0 | 56.1 | 53.2 | 51.3 | 55.0 | 50.4 |
| Ischemic stroke (%) | 12.8 | 11.0 | 14.0 | 11.1 | 11.1 | 10.7 | 10.9 | 10.1 |
| Myocardial Infarction (%) | 4.8 | 3.6 | 5.6 | 3.6 | 3.6 | 4.2 | 3.9 | 3.5 |
| COPD, Asthma (%) | 21.7 | 18.0 | 23.2 | 18.5 | 17.2 | 19.0 | 17.9 | 16.0 |
| Cancer (%) | 9.9 | 9.0 | 10.6 | 9.0 | 9.6 | 9.1 | 8.9 | 9.1 |
| GI bleeding (%) | 6.9 | 4.3 | 8.0 | 4.6 | 4.6 | 5.8 | 5.3 | 4.4 |
| Other heart problems (%) | 68.4 | 61.0 | 70.9 | 62.2 | 60.5 | 61.7 | 61.6 | 57.8 |
| Hypertensive disease (%) | 83.1 | 77.6 | 85.4 | 78.3 | 75.7 | 76.9 | 77.1 | 72.4 |
| Rheumatic heart disease (%) | 4.4 | 3.4 | 4.9 | 3.5 | 3.6 | 4.0 | 3.6 | 3.3 |
| Psychiatric disorder (%) | 5.3 | 4.0 | 6.0 | 4.2 | 4.3 | 4.7 | 4.3 | 3.9 |
| Auto immune disorder (%) | 4.2 | 3.6 | 4.5 | 3.6 | 3.7 | 3.5 | 3.7 | 3.4 |
| Pulmonary circulatory disease (%) | 4.9 | 3.6 | 5.4 | 3.8 | 3.5 | 3.9 | 3.5 | 2.9 |
| Peptic Ulcer disease (%) | 4.0 | 2.8 | 4.5 | 3.0 | 3.0 | 3.8 | 3.6 | 3.1 |
| Liver disease (%) | 4.2 | 3.5 | 4.7 | 3.5 | 3.8 | 4.4 | 3.5 | 3.5 |
| Other neurological disorders (%) | 9.8 | 8.1 | 10.9 | 8.2 | 8.9 | 8.3 | 8.4 | 8.2 |
| Substance use disorder (%) | 10.2 | 7.5 | 11.8 | 7.7 | 7.1 | 9.4 | 7.9 | 6.5 |
| Anemia due to blood loss (%) | 4.1 | 2.6 | 4.7 | 2.8 | 2.9 | 2.6 | 2.7 | 2.2 |
For the formulation cohort, a total of 32,156 patients comprised the iron sucrose group while 8,149 patients made up the ferric gluconate group. The two groups had comparable distributions of race, gender, vintage, hospitalization rates, and baseline laboratory parameters. The baseline EPO and iron exposures were also similar between the two groups, as were the distributions of comorbidities. The ferric gluconate group, however, had a higher prevalence of catheter use, 33.2% versus 27.6%.
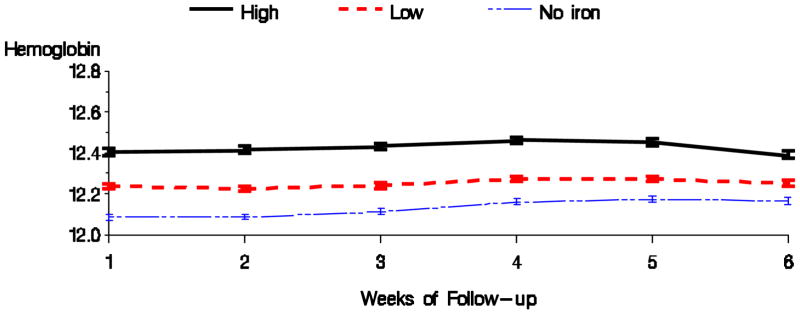
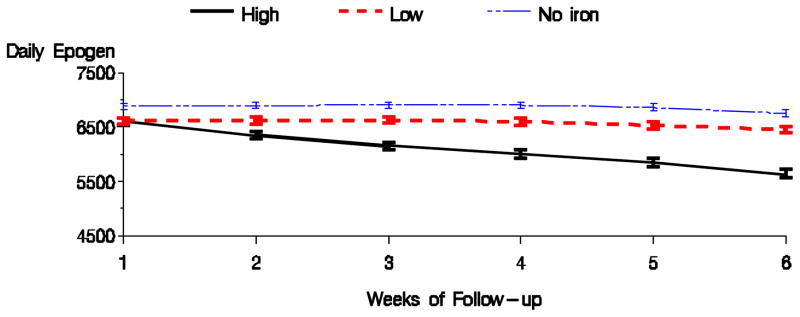
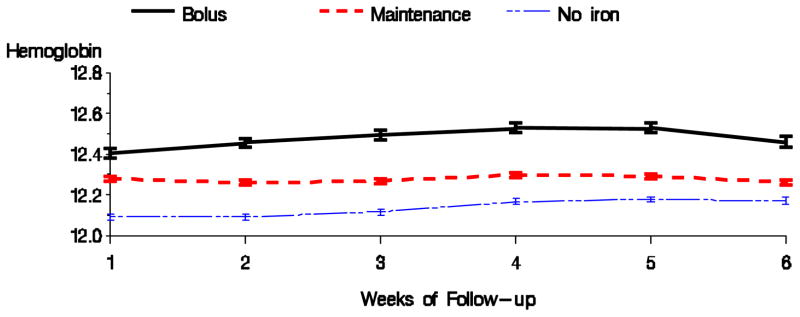
Tables 2 shows the results for the high versus low dose comparison with respect to anemia management parameters for the 1 month exposure and 6 week follow-up period. High dose of intravenous iron, compared with low iron dose, was associated with higher hemoglobin, TSAT, and ferritin, and with a lower EPO dose. Figures 3 & 4 display the average hemoglobin and EPO dose respectively for the high, low, and no iron dosing groups during the follow-up period. Bolus dosing of IV iron was associated with higher hemoglobin, TSAT, and ferritin, and lower EPO dose in weeks 4 and 6, compared to maintenance (Table 3). Figures 5 & 6 display the average hemoglobin and EPO dose respectively for the bolus, maintenance, and no iron groups.
Table 2.
Differences* in Anemia Management Parameters for High vs Low Dose Users (Mean and 95% CI)
| Parameter | Hemoglobin (g/dL) | Epogen (units) | TSAT (%) | Ferritin (ng/ml) |
|---|---|---|---|---|
| Week 02 | 0.19 (0.17, 0.21) | −283 (−371, −195) | -- | -- |
| Week 04 | 0.19 (0.17, 0.21) | −606 (−701, −511) | 3.28 (2.99, 3.56) | 148.4 (133.3, 163.6) |
| Week 06 | 0.15 (0.12, 0.17) | −819 (−919, −719) | -- | -- |
Fully adjusted model: covariates included : EPO dose during exposure period, age, sex, race, baseline hemoglobin, ferritin, index TSAT; pneumonia, vascular access infection, sepsis in baseline; any infection in last month; most recent vascular access is catheter, vintage, albumin, baseline EPO dose, baseline iron dose; history of diabetes, stroke, myocardial infarction, chronic obstructive pulmonary disease, cancer, gasterointestinal bleeding in baseline; hospital days in past month; body mass index
Figure 3.
Average hemoglobin by dosing pattern of IV iron (Fully adjusted model).
Figure 4.
Average EPO dose by dosing pattern of IV iron (Fully adjusted model).
Table 3.
Differences* in Anemia Management Parameters for Bolus and Maintenance Dose Users (Mean and 95% CI)
| Parameter | Hemoglobin | Epogen | TSAT | Ferritin |
|---|---|---|---|---|
| Week 02 | 0.2 (0.17, 0.22) | 46 (−67, 158) | -- | -- |
| Week 04 | 0.23 (0.21, 0.26) | −463 (−583, −343) | 3.31 (2.99, 3.63) | 151.8 (134.9, 168.7) |
| Week 06 | 0.2 (0.17, 0.23) | −883 (−1010, −757) | -- | -- |
Fully adjusted model: covariates included : EPO dose during exposure period, age, sex, race, baseline hemoglobin, ferritin, index TSAT; pneumonia, vascular access infection, sepsis in baseline; any infection in last month; most recent vascular access is catheter, vintage, albumin, baseline EPO dose, baseline iron dose; history of diabetes, stroke, myocardial infarction, chronic obstructive pulmonary disease, cancer, gasterointestinal bleeding in baseline; hospital days in past month; body mass index
Figure 5.
Average hemoglobin by dosing pattern of IV iron (Fully adjusted model).
Figure 6.
Average EPO dose by dosing pattern of IV iron (Fully adjusted model).
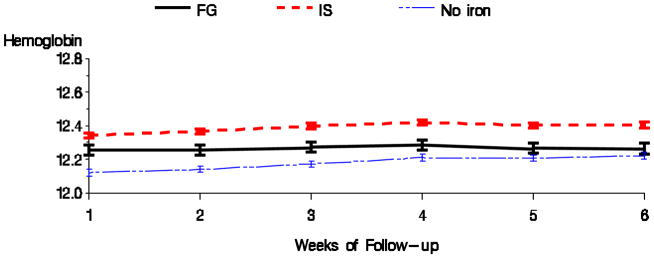
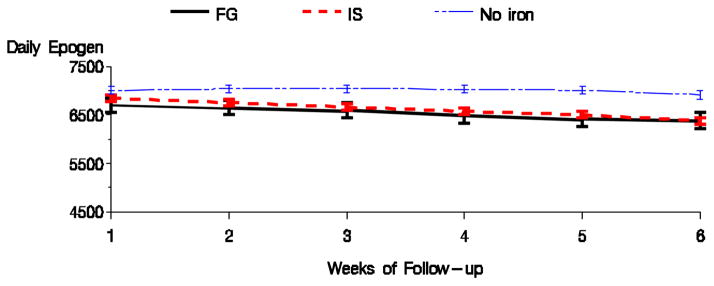
Table 4 displays the differences in the anemia management parameters for the formulation comparison. Ferric gluconate was associated with lower average hemoglobin than iron sucrose during the follow-up period. This difference was significant in all models, including the sensitivity analysis (results not shown). There were no significant differences in EPO dose, TSAT, or serum ferritin between the formulations in the fully adjusted model. Figures 7 & 8 display the mean hemoglobin and EPO dose during the follow-up period for the two formulation groups.
Table 4.
Differences* in Anemia Management Parameters for Ferric Gluconate and Iron Sucrose Users (Mean and 95% CI)
| Parameter | Hemoglobin | Epogen | TSAT | Ferritin |
|---|---|---|---|---|
| Week 02 | −0.13 (−0.17, −0.1) | −80 (−237, 77) | -- | -- |
| Week 04 | −0.16 (−0.19, −0.12) | −59 (−232, 114) | −0.17 (−0.68, 0.34) | 12.4 (−22.8, 47.7) |
| Week 06 | −0.16 (−0.2, −0.12) | 38 (−145, 222) | -- | -- |
Fully adjusted model: covariates included : EPO dose during exposure period, age, sex, race, baseline hemoglobin, ferritin, index TSAT; pneumonia, vascular access infection, sepsis in baseline; any infection in last month; most recent vascular access is catheter, vintage, albumin, baseline EPO dose, baseline iron dose; history of diabetes, stroke, myocardial infarction, chronic obstructive pulmonary disease, cancer, gasterointestinal bleeding in baseline; hospital days in past month; body mass index
Figure 7.
Average hemoglobin by formulation of IV iron (Fully adjusted model).
Figure 8.
Average EPO dose by formulation of IV iron (Fully adjusted model).
The addition of the 5th covariate block for the purpose of sensitivity analysis, did not alter our findings for either dosing comparison (high versus low, or bolus versus maintenance) or for the formulations comparison (ferric gluconate versus iron sucrose), results not shown.
DISCUSSION
Among patients with ESRD anemia is a common condition14 that is associated with increased morbidity, mortality, and risk of hospitalization.15 Erythropoesis stimulating agents and intravenous iron have been used in combination to manage anemia. While recent clinical trial data have provided more precise information on the efficacy and safety of ESAs 16–18 contemporary data for intravenous iron is sparse.
We therefore examined the comparative effectiveness of dose and formulation of intravenous iron on anemia management, using schedules and formulations reflective of current practice in the United States. Our data show that large doses of intravenous iron, whether bolus or high dose, are associated with higher hemoglobin, TSAT, and ferritin, and with lower ESA dose. We also found that iron sucrose is associated with higher level of hemoglobin compared to ferric gluconate. The findings for the dose and formulation comparisons did not change meaningfully with the addition of several sets of pre-specified covariates and potential confounders. To our knowledge, this is the largest study to compare intravenous iron either by dosing pattern or by formulation.
Previous studies of bolus dosing8.9 have demonstrated a similar beneficial effect on anemia parameters, albeit with markedly smaller numbers of patients. The patients included in our study cohort, furthermore, had numerous co-morbidities that are representative of the conditions patients who receive IV iron may have, including recent hospitalizations, infections, and catheters for dialysis access. Such patients are often excluded in clinical trials of intravenous iron, but may be especially sensitive to the effects of intravenous iron administration. This analysis furthermore suggested a dose response effect. Iron is essential in the production of the heterocyclic porphyrin ring of hemoglobin, and therefore, a relatively large iron dose should result in a higher hemoglobin value than a relatively small dose, as seen in our data. Bolus dosing was associated with a greater absolute increase in hemoglobin high dose IV iron (though not compared directly).
With respect to the formulation comparisons, two previous clinical trials comparing these iron sucrose and ferric gluconate did not find a statistically significant difference between the agents with respect to attained hemoglobin, TSAT, or ferritin values. 11,12 Interestingly, one of the studies reported that the attained hemoglobin value at 6 months in the iron sucrose group was 0.3 g/dl higher than in the ferric gluconate group, a difference that may not have reached statistical significance because of insufficient power. 11 Indeed, intrinsic differences in properties of the two agents could help explain our observations.5–7 Ferric gluconate is taken up quickly by the reticuloendothelial system (RES) and eliminated rapidly from the plasma, while iron sucrose is taken up more slowly and thus has a longer plasma half-life. Furthermore, iron sucrose has a lower binding coefficient than transferrin and there is a significant amount of direct transfer of iron from iron sucrose to transferrin in the plasma. 19,20
While the findings of the study ostensibly re-affirm current trends in clinical practice -- a trend for larger doses of iron, and predominance of iron sucrose-- they also indirectly raise questions about safety. The mean adjusted difference in achieved hemoglobin was only 0.2 mg/dl between groups of patients receiving either high or bolus dosing, and those receiving low or maintenance dosing. This benefit comes at a potential cost, a 2 to 3 fold greater intravenous iron exposure. Experimental studies have demonstrated risk associated with intravenous iron21–24, though clinical studies have yet to clearly show an increased risk of infection25 or mortality.26,27 Recent clinical observations suggest that large doses of intravenous iron in the patients with chronic kidney disease results in radiographic evidence of excess tissue iron not detectable by serum laboratories. 28–30 The long-term effects of excess tissue iron from intravenous products are not known.
The findings of this study should be interpreted in the context of the following limitations. First, our study was non-experimental and, therefore, could have been confounded by unobserved differences between users of the dosing strategies and formulations. We saw an increased prevalence of medical comorbidities among patients receiving either high or bolus dosing of iron. However, our robust sample size allowed us to control for a very large number of these covariables, and to perform sensitivity analyses. The point estimates did not significantly change the point estimates with either of these methods, suggesting that unobserved confounding may be minimal. Also, we saw little difference between the patients exposed to iron sucrose and those exposed to ferric gluconate. A second limitation is that our study design required survival until 9 months after the start of dialysis, which may have excluded patients with a high prevalence of serious co-morbidities. While excluding such patients may ultimately limit the generalizability of our results, it would allow for a more valid interpretation of the effect of exposure, intravenous iron, on the outcome, measures of anemia management. A third limitation to the current analysis is that relatively fewer individuals were available for the formulation comparison than for the dosing comparisons ( 66,207 versus 117,050). This is likely the result of a secular trend during the observation period when large dialysis organizations chose iron sucrose because of favorable pricing structures, intuitive dosing (multiples of 50 mg rather than 62.5 mg), and ease of administration (vials rather than glass ampules).
Nonetheless, the study findings may help inform decision making about iron management in an era of new incentives but minimal data from clinical trials. Dialysis providers and ESRD networks now face an environment of pay-for-performance, including expanded capitated reimbursement (the ESRD Prospective Payment System, which includes costs for ESAs and iron supplementation, but not blood transfusions) 31, a dynamic Quality Incentive Program that is mandated by law to include quality metrics on anemia32, and black box warnings about high hemoglobin levels33. In such a context, the study data favor a strategy of low or maintenance IV iron, as individuals given these doses would be, on average, less likely to have large or rapid increases in hemoglobin than if given bolus or high dose intravenous iron. The data also demonstrate that low and maintenance intravenous iron are associated with a higher epogen dose. Indeed, the data highlight the very real competing incentives that providers now face, minimizing operational costs by finding the “right mix” of ingredients for the treatment of anemia (ESAs, iron supplementation, blood transfusion) while maximizing patient safety. Maximizing the latter should be the undisputed and universal goal. In the absence of data on clinical safety of large doses of intravenous iron, furthermore, the prudent choice would be for moderation.34,35
In conclusion, our results suggest that large doses of intravenous iron lead to increased hemoglobin, improved iron status, and reduced epoetin dose. We also find that iron sucrose may be more effective than ferric gluconate with respect to hemoglobin outcomes. On-going research into the safety of intravenous iron dosing strategies and formulation choices will be crucial to help weigh the benefits of greater iron use, reported here, against its risks, which remain poorly quantified. Ultimately, a clinical trial comparing dose and formulation may be necessary.36
Acknowledgments
Funding Source: Agency For Healthcare Research and Quality, Contract No. HHSA290200500401
This project was funded under Contract No. HHSA290200500401 from the Agency for Healthcare Research and Quality (AHRQ), US Department of Health and Human Services (DHHS) as part of the Developing Evidence to Inform Decisions about Effectiveness (DECIDE) program. The authors thank DaVita Clinical Research for providing data for this study. DaVita Clinical Research had no role in the design or implementation of this study, nor on the decision to publish.
Appendix
Table 1.
Definition of Covariates
| COVARIATE | DEFINITION | SOURCE |
|---|---|---|
|
| ||
| Demographic | ||
|
| ||
| Age | Categorized as: 16–45; 46–60; 61–75; >75 yrs. | USRDS |
|
| ||
| Sex | Male or female | USRDS |
|
| ||
| Race | White, Black, Other | USRDS |
|
| ||
| Medicaid Eligibility | Indicator for dual eligibility during any part of the baseline | USRDS |
|
| ||
| Year of treatment | 2004, 2005, 2006, 2007, 2008 | Clinical Database |
|
| ||
| Census Region | Based on location of last dialysis center in baseline period: Northeast, South, Midwest, West | USRDS |
|
| ||
| Clinical | ||
|
| ||
| Vintage | Categorized as 0; 1–3; 4 or more yrs. | USRDS |
|
| ||
| ESRD Reason | Diabetes, Glomerulonephritis, hypertension, other | USRDS |
|
| ||
| BMI | Categorized as underweight, normal, overweight, obese | Clinical Database & USRDS |
|
| ||
| Anemia Management | ||
|
| ||
| Access | Most recent vascular access (catheter vs fistula/graft) prior to TSAT index date | Clinical Database |
|
| ||
| EPO dose (baseline) | Total EPO dose, (quintiles) | Clinical Database |
|
| ||
| EPO dose (exposure) | Total EPO dose, (tertiles plus a no-use category) | Clinical Database |
|
| ||
| Index TSAT | Last TSAT at baseline (quintiles) | Clinical Database |
|
| ||
| Iron dose | Total dose at last month of baseline, categorized as none, low (1–200 mg), or high (>200mg) | Clinical Database |
|
| ||
| Hemoglobin | Most proximal Hb lab prior to index TSAT date (<10,10–11,>11–12,>12–13,>13) | Clinical Database |
|
| ||
| Ferritin | Most proximal serum ferritin prior to index TSAT date (quintiles) | Clinical Database |
|
| ||
| Albumin | At baseline (<3.3, 3.3–3.9, >3.9) | Clinical Database |
|
| ||
| Comorbidities | ||
|
| ||
| Hospital days in last month of baseline | Categorized as 0, 1–3, <=4 | USRDS, Medicare Part A Claims |
|
| ||
| Infection in last month | Any hospital admission in the last month with one of the following ICD-9-CM diagnostic codes as the principal diagnostic code: 001–139, 254.1, 320–326, 331.81, 372– 372.39, 373.0–373.2, 382–382.4, 383.0, 386.33, 386.35, 388.60, 390–393, 421–421.1, 422.0, 422.91–422.93, 460–466, 472–474.0, 475–476.1, 478.21–478.24, 478.29, 480–490, 491.1, 494, 510–511, 513.0, 518.6, 519.01, 522.5, 522.7, 527.3, 528.3, 540–542, 566–567.9, 569.5, 572–572.1, 573.1– 573.3, 575–575.12, 590–590.9, 595–595.4, 597–597.89, 598, 599.0, 601–601.9, 604–604.9, 607.1, 607.2, 608.0, 608.4, 611.0, 614–616.1, 616.3–616.4, 616.8, 670, 680–686.9, 706.0, 711–711.9, 730–730.3, 730.8–730.9, 790.7–790.8, 996.60– 996.69, 997.62, 998.5, and 999.3. | USRDS, Medicare Part A Claims |
| Any claims with the following HCPCS codes for antibiotic use in last month of baseline: J3370, J0690, J0713, J0692, J0696, J1580, J3260, J0278, J1840, J1956. | USRDS, Medicare Part A & B Claims | |
| Any indication of IV antibiotic use in the last month of baseline. | Clinical Database | |
|
| ||
| Pneumonia | Any ICD-9-CM diagnostic code of 481.xx – 486.xx in baseline period | USRDS, Medicare Part A & B Claims |
|
| ||
| Vascular Access Infection | Any ICD-9-CM diagnostic code of 996.62 in baseline period | |
|
| ||
| Sepsis | Any ICD diagnostic code 038.xx, 995.90, 995.91, 995.92 in baseline period | |
|
| ||
| Diabetes | Any ICD-9-CM diagnostic code of 250.xx in baseline period | |
|
| ||
| Ischemic Stroke | Any ICD-9-CM diagnostic code of 434.01, 434.11, 434.91, 435, 436, 437, 438, V12.54 in baseline period | |
|
| ||
| MI | Any ICD-9-CM diagnostic code of 410.xx in baseline period | |
|
| ||
| COPD | Any ICD-9-CM diagnostic code of 490.xx-496.xx, 505.xx, 506.4 in baseline period | |
|
| ||
| Cancer | Any ICD-9-CM diagnostic code of 173.3, 173.9, 174.0–175.9, 179–195, 196–199, 232.9, 233.0, 233.1, 300.29, 338.3, 789.51, 795.82, 799.4, V67.2, 200, 201, 202.0–202.3, 202.50- 203.01,203.8, 238.6, 273.3 in baseline period | |
|
| ||
| GI bleeding | Any ICD-9-CM diagnostic code of 578.xx in baseline period | |
|
| ||
| Additional Comorbidities for Sensitivity Analyses | ||
|
| ||
| Pulmonary circulation disease | Any ICD-9-CM diagnostic code of 415.xx-417.xx in baseline period | USRDS, Medicare Part A & B Claims |
|
| ||
| Peptic Ulcer Disease | Any ICD-9-CM diagnostic code of 530.2, 531.xx-534.xx, V12.71 in baseline period | |
|
| ||
| Liver disease | Any ICD-9-CM diagnostic code of 070.32, 070.33, 070.54, 456.0, 456.1, 456.20, 456.21, 571.0, 571.2, 571.3, 571.4, 571.5, 571.6, 571.8, 571.9, 572.3, 572.8, V42.7 in baseline period | |
|
| ||
| Other neurological problem | Any ICD-9-CM diagnostic code 331.9, 332.0, 333.4, 333.5, 334–335, 340, 341, 345.0, 345.1, 345.4, 345.5, 345.8, 345.9, 348.1, 348.3, 780.3, 784.3 in baseline period | |
|
| ||
| Substance abuse | Any ICD-9-CM diagnostic code 303.xx-305.xx in baseline period | |
|
| ||
| Ischemic Heart disease, other heart disease, peripheral vascular disease, history of CABG, Stent, PTCA | Any ICD-9-CM diagnostic code of 411.xx-414.xx, 420.xx- 429.xx, 785.o, V45.0, v53.3, 402.11, 402.91, 404.11, 404.12, 404.91, 404.93, 093.2, 746.3–746.6, v42.2, v43.3, v43.4441.xx- 443.xx, 447.1, 557.1, 557.9, 444.xx-445.xx; Procedure codes of 00.66, 92982, 92985, 36.06, 36.07, 92980, 33510–33519 in baseline period | |
|
| ||
| Hypertension | Any ICD-9-CM diagnostic code of 401.xx-405.xx, except 402.11, 402.91, 404.11, 404.13, 404.91, 404.93 in baseline period | |
|
| ||
| Rheumatic heart disease | Any ICD-9-CM diagnostic code of 393.xx -398.xx in baseline period | |
|
| ||
| Psychiatric problems | Any ICD-9-CM diagnostic code 295.xx-298.xx in baseline period | |
|
| ||
| Autoimmune disorders | Any ICD-9-CM diagnostic code of 564.1, 696.0, 696.1, 695.4, 710.0, 701.0, 710, 714, 720, 725 in baseline period | |
|
| ||
| Blood loss anemia | Any ICD-9-CM diagnostic code of 280.0 in baseline period | |
|
| ||
| Transfusion | Indicator for receipt of one or more transfusions during the baseline period, based on HCPCS codes P9010, P9011, P9016, P9021, P9022, P9038, P9039, P9040, 36430 and ICD- 9 codes 99.03, 99.04 | |
Table 2.
Groupings of variables (Block) used in models
| Block | Variables |
|---|---|
| 1 | Epogen (EPO) dose during exposure period |
| 2 | Block 1 & age, sex, race |
| 3 | Block 2 & baseline hemoglobin, ferritin, and index TSAT |
| 4 (Fully adjusted model) | Block 3 & pneumonia, vascular access infection, or sepsis in past 6 months; any infection in past month; most recent vascular access is catheter; vintage; baseline albumin; baseline EPO dose; baseline iron dose; history of diabetes, stroke, myocardial infarction, chronic obstructive pulmonary disease, cancer, or gasterointestinal bleeding in past 6 months; number of hospital days in past month, and body mass index |
| 5 (Sensitivity analysis) | Block 4 & reason for ESRD (Diabetes,glomerulonephritis, hypertension, other); history of the following in the past 6 months: heart problems (combining ischemic heart disease, other heart, peripheral vascular disease, coronary artery bypass grafting/coronary artery stenting/percutaneous coronary angioplasty), hypertension, rheumatic heart disease, psychiatric problems, transfusion, autoimmune disorder, blood loss anemia, pulmonary circulation disease, ulcer, liver disease, other neurological problem, substance use.; year; geographic region; Medicaid eligibility |
Footnotes
All authors had access to the data and a role in writing the manuscript
Conflict of Interest: Dr. Brookhart—investigator initiated support from Amgen, and advisory boards for Amgen, Pfizer, and Rockwell Medical. Dr. Winkelmayer—scientific advisory board for Amgen and Fibrinogen
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coyne DW, Kaponian T, Suki W, et al. Ferric gluconate is highly efficacious in anemic Hemodialysis patients with high serum ferritin and low transferring saturation: Results of the Dialysis Patients Response to IV Iron with Elevate Ferritin (DRIVE) Study. J Am Soc Nephrol. 2007;18:975–984. doi: 10.1681/ASN.2006091034. [DOI] [PubMed] [Google Scholar]
- 2.Freburger JK, Ng LJ, Bradbury BD, et al. Changing patterns of anemia management in US Hemodialysis patients. Am J Med. 2012;125:906–914. doi: 10.1016/j.amjmed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Thakuria M, Ofsthun NJ, Mullon C, Diaz-Buxo JA. Anemia management in patients receiving chronic hemodialysis. Semin Dial. 2011;24:597–602. doi: 10.1111/j.1525-139X.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 4.Yong K, Kairaitis L. Effects of proactive iron and erythropoiesis-stimulating agent protocol implementation on achieving clinical guideline targets for anaemia in a satellite haemodialysis patient cohort. Nephrology (Carlton) 2010;15:288–293. doi: 10.1111/j.1440-1797.2009.01184.x. [DOI] [PubMed] [Google Scholar]
- 5.American Regent Laboratories I. Venofer (iron sucrose) package insert. Shirley, NY: 2003. [Google Scholar]
- 6.Seligman PA, Dahl NV, Strobos J, et al. Single-dose pharmacokinetics of sodium ferric gluconate complex in iron-deficient subjects. Pharmacotherapy. 2004;24:574–583. doi: 10.1592/phco.24.6.574.34750. [DOI] [PubMed] [Google Scholar]
- 7.Warady BA, Seligman PA, Dahl NV. Single-dosage pharmacokinetics of sodium ferric gluconate complex in iron-deficient pediatric hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:1140–1146. doi: 10.2215/CJN.00830207. [DOI] [PubMed] [Google Scholar]
- 8.Nissenson AR, Lindsay RM, Swan S, et al. Sodium ferric gluconate complex in sucrose is safe and effective in hemodialysis patients: north american clinical trial. Am J Kidney Dis. 1999;33:471–482. doi: 10.1016/s0272-6386(99)70184-8. [DOI] [PubMed] [Google Scholar]
- 9.Charytan C, Levin N, Al-Saloum M, et al. Efficacy and safety of iron sucrose for iron deficiency in patients with dialysis-associated anemia: north american clinical trial. Am J Kidney Dis. 2001;37:300–307. doi: 10.1053/ajkd.2001.21293. [DOI] [PubMed] [Google Scholar]
- 10.Kosch M, Bahner U, Bettger H, et al. A randomized, controlled, parallel-group trial on efficacy and safety of iron sucrose (venofer) vs iron gluconate (ferrlicit) in haemodialysis patients treated with rHuEpo. Nephrol Dial Transplant. 2001;16:1239–1244. doi: 10.1093/ndt/16.6.1239. [DOI] [PubMed] [Google Scholar]
- 11.Sheashaa H, El-Husseini A, Sabry A, et al. Parenteral iron therapy in treatment of anemia in end-stage renal disease patients: a comparative study between iron saccharate and gluconate. Nephron Clin Pract. 2005;99:c97–c101. doi: 10.1159/000083766. [DOI] [PubMed] [Google Scholar]
- 12.Researcher's Guide to the USRDS Database: 2010 ADR Edition. Bethesda, MD: National Institutes of Health, NIDDK; 2011. [Google Scholar]
- 13.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 14.USRDS. 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda MD: US Renal Data System, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2009. [Google Scholar]
- 15.Collins AJ, Li S, St Peter W, et al. Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39% J Am Soc Nephrol. 2001;12:2465–2473. doi: 10.1681/ASN.V12112465. [DOI] [PubMed] [Google Scholar]
- 16.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 17.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 19.Besarab A, Coyne DW. Iron supplementation to treat anemia in patients with chronic kidney disease. Nat Rev Nephrol. 2010;6:699–710. doi: 10.1038/nrneph.2010.139. [DOI] [PubMed] [Google Scholar]
- 20.Danielson BG, Salmonson T, Derendorf H, Geisser P. Pharmacokinetics of iron(III)-hydroxide sucrose complex after a single intravenous dose in healthy volunteers. Arzneimittel-Forschung. 1996;46:615–621. [PubMed] [Google Scholar]
- 21.Parkkinen J, von Bonsdorff L, Peltonen S, et al. Catalytically active iron and bacterial growth in serum of haemodialysis patients after i.v. iron-saccharate administration. Nephrol Dial Transplant. 2000;15:1827–1834. doi: 10.1093/ndt/15.11.1827. [DOI] [PubMed] [Google Scholar]
- 22.Lim CS, Vaziri ND. The effects of iron dextran on the oxidative stress in cardiovascular tissues of rats with chronic renal failure. Kidney Int. 2004;65:1802–1809. doi: 10.1111/j.1523-1755.2004.00580.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuo KL, Hung SC, Wei YH, Tarng DC. Intravenous iron exacerbates oxidative DNA damage in peripheral blood lymphocytes in chronic hemodialysis patients. J Am Soc Nephrol. 2008;19:1817–1826. doi: 10.1681/ASN.2007101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonnweber T, Therul, Seifert M, et al. Impact of iron treatment on immune effector function and cellular iron status of circulating monocytes in dialysis patients. Nephrol Dial Transplant. 2011;26:977–987. doi: 10.1093/ndt/gfq483. [DOI] [PubMed] [Google Scholar]
- 25.Hoen B, Paul-Dauphin A, Hestin D, Kessler M. EPIBACDIAL: a multicenter propective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol. 1998;9:869–876. doi: 10.1681/ASN.V95869. [DOI] [PubMed] [Google Scholar]
- 26.Feldman HI, Joffe M, Robinson B, et al. Administration of parenteral iron and mortality among hemodialysis patients. J Am Soc. 2004;15:1623–1632. doi: 10.1097/01.asn.0000128009.69594.be. [DOI] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Regidor DL, McAllister CJ, et al. Time-dependent associtations between iron and mortality in hemodialysis patients. J Am Soc Nephrol. 2006;15:3070–3080. doi: 10.1681/ASN.2005040423. [DOI] [PubMed] [Google Scholar]
- 28.Cavanese C, Bergamo D, Ciccone G, et al. Validation of serum ferritin values by magnetic susceptometry in predicting iron overload in dialysis patients. Kidney Int. 2004;65:1091–1098. doi: 10.1111/j.1523-1755.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 29.Ferrari P, Kulkarni H, Harrison C, et al. Serum iron markers are inadequate for guiding iron repletion in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:77–83. doi: 10.2215/CJN.04190510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rostoker G, Griuncelli M, Loridon C, et al. Hemodialysis-associated hemosiderosis in the era of erythropoesis-stimulating agents. Am J Med. 2012;125:991–999. doi: 10.1016/j.amjmed.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Medicare Program; End-Stage Renal Disease Prospective Payment System and Quality Incentive Program; Ambulance Fee Schedule; Durable Medical Equipment; and Competitive Acquisition of Certain Durable Medical Equipment, Prosthetics, Orthotics and Supplies; Final Rule. [Accessed October 14, 2012];Federal Register. 2011 76(218) Website: http://www.gpo.gov/fdsys/pkg/FR-2011-11-10/pdf/2011-28606.pdf. [PubMed] [Google Scholar]
- 32.Medicare Program; End-Stage Renal Disease Prospective Payment System, Quality Incentive Program, and Bad Debt Reductions for All Medicare Providers; Proposed Rule. [Accessed October 14, 2012];Federal Register. 77(133) Website: http://www.gpo.gov/fdsys/pkg/FR-2012-07-11/pdf/2012-16566.pdf. [PubMed] [Google Scholar]
- 33.FDA Drug Safety Communication. [Accessed October 14];Modified dosing recommendations to improve the safe use of Erythropoiesis-Stimulating Agents (ESAs) in chronic kidney disease. http://www.fda.gov/drugs/drugsafety/ucm259639.htm.
- 34.Spiegel DM, Chertow GM. Lost without directions: lesson from the anemia debate and the drive study. Clin J Am Soc Nephrol. 2009;4:1009–1010. doi: 10.2215/CJN.00270109. [DOI] [PubMed] [Google Scholar]
- 35.Vaziri ND. Epidemic of iron overload in dialysis population caused by intravenous iron products: a plea for moderation. Am J Med. 2012;125:951–952. doi: 10.1016/j.amjmed.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Coyne DW. It’s time to compare anemia management strategies in hemodialysis. Clin J Am Soc Nephrol. 2010;5:740–742. doi: 10.2215/CJN.02490409. [DOI] [PubMed] [Google Scholar]