Abstract
Background: Moderate hyperhomocysteinemia is an attractive target for intervention because it is present in 5–7% of the population and can be reversed by diet. This approach presupposes that hyperhomocysteinemia is directly involved in the disease process. Epidemiologic studies have indicated that moderately elevated homocysteine may contribute to thoracic aortic aneurysm (TAA) dilatation and dissection in humans. In vitro, elevated homocysteine disrupts the structure and function of extracellular matrix components, suggesting that moderate hyperhomocysteinemia may contribute to the development and/or progression of TAA.
Objective: We investigated moderately elevated homocysteine in the development and progression of TAA in a mouse model of Marfan syndrome (MFS) and in isogenic wild-type mice. The MFS mouse is a well-described model of a systemic connective tissue disorder characterized by thoracic aortic dilatation, dissection, and rupture. We used this model as a sensitized indicator system to examine the impact of homocysteine on the progression of TAA.
Methods: Murine fibrillin 1 gene (Fbn1)C1039G/+ MFS and C57BL/6J wild-type mice were fed a cobalamin-restricted diet to induce moderate hyperhomocysteinemia from weaning until the age of 32 wk. Homocysteine and methylmalonic acid were measured and aortic root diameter assessed with the use of echocardiography in mice aged 3, 7, 15, and 32 wk.
Results: Cobalamin-restricted mice exhibited significantly higher homocysteine (P < 0.0001) and methylmalonic acid (P < 0.0001) in the blood. For both strains, no significant difference in thoracic aortic diameter was observed in mice on the cobalamin-restricted diet compared with those on the control diet.
Conclusions: Fbn1C1039G/+ mice are a well-characterized model of progressive aortic root dilation. Hyperhomocysteinemia in the physiologic range did not induce abnormal aortic growth in wild-type mice and did not accelerate or otherwise influence aortic root growth and pathologic progression in mice with an underlying predisposition for aortic dilatation.
Keywords: thoracic aortic aneurysm, homocysteine, methylmalonic acid, Marfan syndrome, vitamin B-12, cobalamin, mouse model
Introduction
Thoracic aortic aneurysm (TAA) is defined as a segmental full-thickness dilation of the aorta that measures ≥50% larger than normal for a given sex, age, and body size. Various etiologies have been associated with TAA; the most common is a degenerative process associated with hypertension, age, and tobacco use. Other causes include atherosclerotic disease, genetic syndromes [e.g., Marfan syndrome (MFS)], bicuspid aortic valve, aortitis, and trauma. Overall, TAA occurs with an estimated annual incidence of 5.9–10.4 cases/100,000 patient-years (1, 2). The most severe complications of TAA include dissection, which may cause arterial occlusion and end-organ ischemia, and rupture, which is usually fatal. Approximately 50% of patients with TAA rupture die before reaching the hospital (3). Emergent surgical repair carries an additional mortality risk of 25–50% (3).
Aneurysm size is the most important determinant of TAA rupture risk. In asymptomatic patients the risk of TAA rupture is near zero for aneurysms <5.0 cm, 1.7%/y for aneurysms 5.0–5.9 cm, and 3.6%/y for aneurysms ≥6.0 cm (4). The risk of rupture, dissection, or death from all causes is 6.5%/y for 5.0–5.9-cm aneurysms and 14.1%/y for aneurysms ≥6.0 cm (4). Elective preemptive surgical repair restores survival to rates similar to those in matched control individuals but carries a ≥2.5% risk of death, even at experienced surgical centers (4). Other perioperative complications include myocardial infarction, stroke, paraplegia, paraparesis, and acute renal failure requiring dialysis. Current medical management of TAA relies on an aggressive antihypertensive treatment to slow aneurysmal growth (5). Additional interventions may include lipid-lowering therapy, smoking cessation, and lifestyle modifications (5). However, even with adequate medical management, many individuals with TAA will meet the criteria for surgical intervention. Given the substantial morbidity and mortality associated with TAA repair, it is important that additional therapies be developed. An understanding of risk factors that contribute to disease severity and progression would assist in this process.
In adults, the normal range of circulating homocysteine is ∼5–14 μmol/L and is known to vary by age and sex (6). Hyperhomocysteinemia is defined as exceeding these concentrations and can be further categorized based on homocysteine concentrations into severe (>100 μmol/L), intermediate (>30–100 μmol/L), and moderate (15–30 μmol/L) (7). Severe hyperhomocysteinemia can be caused by severe cobalamin deficiency or inborn errors of metabolism and is rare (<0.02%); intermediate hyperhomocysteinemia caused by renal failure or moderate to severe cobalamin or folate deficiency is also not common (<1%) (8, 9). Moderate hyperhomocysteinemia can be caused by mild folate or cobalamin deficiency or impaired renal function and is much more common (6, 8). Moderately elevated homocysteine concentrations have been associated with acute thoracic aortic dissection (mean ± SD homocysteine: 22 ± 14 μmol/L) and chronic aortic aneurisms (mean ± SD homocysteine: 17 ± 5 μmol/L) (10). Moderately elevated concentrations of homocysteine have also been observed in individuals with severe cardiovascular manifestations of MFS [median homocysteine: 13.5 μmol/L (IQR: 9, 23.1 μmol/L)] compared with those with no cardiovascular manifestations [median homocysteine: 7.5 μmol/L (IQR: 7, 9 μmol/L)] (11). Similarly, moderate hyperhomocysteinemia has been demonstrated in aortic dilation and dissection outside the thoracic cavity, including abdominal aortic aneurysm (12) and spontaneous cervical artery dissection [median homocysteine: 18.2 μmol/L (IQR: 14.3, 30 μmol/L)] (13). Taken together, these findings suggest that elevated homocysteine concentrations may influence the severity and kinetics of arterial dilation, dissection, and rupture across the vasculature. Some have theorized that hyperhomocysteinemia may contribute to this process by causing structural changes in the extracellular matrix (14). Fibrillin-1 is a major component of the 10–12-nm microfibrils and largely consists of 2 types of disulfide-rich motifs: 47 epidermal growth factor (EGF)-like domains and 7 transforming growth factor β-binding protein-like domains (15). Homocysteine has a higher acid dissociation constant for the thiol group than Cys and may alter the structural integrity, stability, and/or function of fibrillin-1 by reducing disulfide bonds in the Cys-rich protein (16). The damaging effect of hyperhomocysteinemia on fibrillin-1 is supported by experiments that have demonstrated that homocysteine treatment of a fibrillin peptide fragment containing 3 calcium-binding epidermal growth factor domains (domains 32–34) increased the risk for proteolysis (15).
Although current evidence suggests that moderate hyperhomocysteinemia may contribute to TAA pathology, a clear relation has not been established. The hyperhomocysteinemia associated with TAA may simply be a marker for another biological change or environmental exposure. To explore the role of moderate hyperhomocysteinemia in TAA further, we used a nutritional intervention to examine the effects of elevated homocysteine on thoracic aorta diameters in C57BL/6J and MFS mice. In a recent study (17), C57BL/6J mice aged 15 wk maintained on a cobalamin-restricted diet showed significant increases in mean homocysteine concentrations (8.7 or 10.5 μmol/L in mice on different cobalamin-restricted diets compared with 4.2 μmol/L in controls). This moderate hyperhomocysteinemia resulted from a decrease in the activity of methionine synthase, a cobalamin-dependent cytoplasmic enzyme that methylates homocysteine to Met.
MFS is a systemic connective tissue disorder characterized by ocular, skeletal, and cardiovascular system manifestations, including TAA. It is caused by the disruption of the fibrillin-1 domain structure and function with missense mutations that either create or substitute a commonly reported Cys residue (15). Because moderate hyperhomocysteinemia may also modify fibrillin-1, MFS was a particularly informative and sensitive model for our study. In addition, advances in the treatment of MFS currently guide therapy for all causes of TAA. Identifying a risk factor that contributes to TAA in MFS would assist in the development of new therapies for all underlying etiologies.
Methods
Mice.
We studied 2 independent groups of mice. To establish a model of diet-induced hyperhomocysteinemia, wild-type C57BL/6J mice and their dams were maintained either on a control diet with vitamin B-12 or on a standard unpurified diet until being switched to a cobalamin-restricted diet at weaning. Distributions by sex and diet are described in Supplemental Table 1. Aortic roots were not measured in these mice because they were used to determine the long-term biochemical impact of these diets. The mice were maintained at the NIH, and the National Human Genome Research Institute Animal Care and Use Committee approved all experiments that involved dietary manipulations.
Independent parallel experiments were performed with the use of a previously described mouse line harboring the fibrillin-1 gene mutation C1039G (heterozygous Fbn1C1039G/+ MFS mice) (18). All MFS mice were back-crossed (>10 generations) onto the C57BL/6J background, allowing valid comparisons between litters and with wild-type C57BL/6J mice. A total of 43 mice were used in this study. Approximately 50% of the mice were maintained on a cobalamin-restricted diet to raise plasma total homocysteine from weaning (age 3 wk) until the age of 32 wk (a total of 29 wk on the diet). The remaining mice were maintained on a control diet after weaning. Distribution by genotype, sex, and diet are described in Supplemental Table 2. Homocysteine and methylmalonic acid (MMA) measurements were obtained when the mice were aged 15 wk. Aortic root diameter was assessed in vivo in mice aged 3, 7, 15, and 32 wk with the use of echocardiography. Wild-type littermates were used as controls. All experiments were approved by the Johns Hopkins University School of Medicine Animal Care and Use Committee.
Defined diets.
Two soy protein-based mouse diets were custom-blended by Harlan Teklad Laboratories (Supplemental Tables 3 and 4). The cobalamin-restricted diet was derived completely from plant sources and lacked any vitamin B-12. The control diet was supplemented with 50 μg vitamin B-12/kg unpurified diet. Distinguishing food-safe dyes were added to each formulation to ensure that the correct diet was used. Both formulations included the antibiotic succinylsulfathiazole (1% wt:vol) to limit the intestinal production of vitamin B-12 from gut flora. All unpurified diets were sterilized via γ irradiation. In all experiments, food and water were available ad libitum. Weight gain and growth during the course of the experiments were not measured, but no overtly striking differences in size were observed between the different treatment groups.
Blood chemistry.
Blood for serum homocysteine and MMA was collected in mice aged 15 wk via the retro-orbital sinus cavity. A tetracaine ophthalmic anesthetic was used as an adjunct analgesia for retro-orbital sinus bleeding in isoflurane-anesthetized mice. All mice were deeply anesthetized with the use of isoflurane (4–5% isoflurane in 100% O2) until the respiration rate was greatly reduced. The plane of anesthesia was confirmed by the absence of a deep pain response (i.e., withdrawal or twitching) to the pinching of the foot or toe with blunt forceps. Volumes of 0.1–0.2-mL peripheral blood were collected from the retro-orbital sinus cavity into heparinized microcapillary tubes (Sarstedt). Blood was expelled into microcentrifuge tubes, placed on ice, and centrifuged (6800 × g; 10 min; 4°C). The upper plasma layer was immediately transferred to a new microcentrifuge tube, diluted 1:5 in deionized water, and frozen at −80°C. MMA and homocysteine were assayed in diluted plasma samples via HPLC.
Echocardiography.
Echocardiographic assessment was performed in mice aged 3, 7, 15, and 32 wk. Nair hair removal cream was used on all mice the day before echocardiograms. All echocardiograms were performed on mice that were awake and unsedated with the use of the Vevo 660 V1.3.6 imaging system and an RMV603 40-MHz transducer (VisualSonics). The aorta was imaged in the parasternal long-axis view. Three separate measurements of the maximal internal dimension at the sinus of Valsalva were made from distinctly captured images and averaged. All studies were interpreted by a single echocardiographer who was blinded to the genotype and diet.
Statistical analysis.
Statistical analysis was performed with the use of a 2-tailed Mann-Whitney U test. P < 0.05 was considered significant. Statistical analyses were performed with GraphPad Prism version 5.0 for MacOS X.
Results
Cobalamin restriction raised homocysteine and MMA in wild-type C57BL/6J mice.
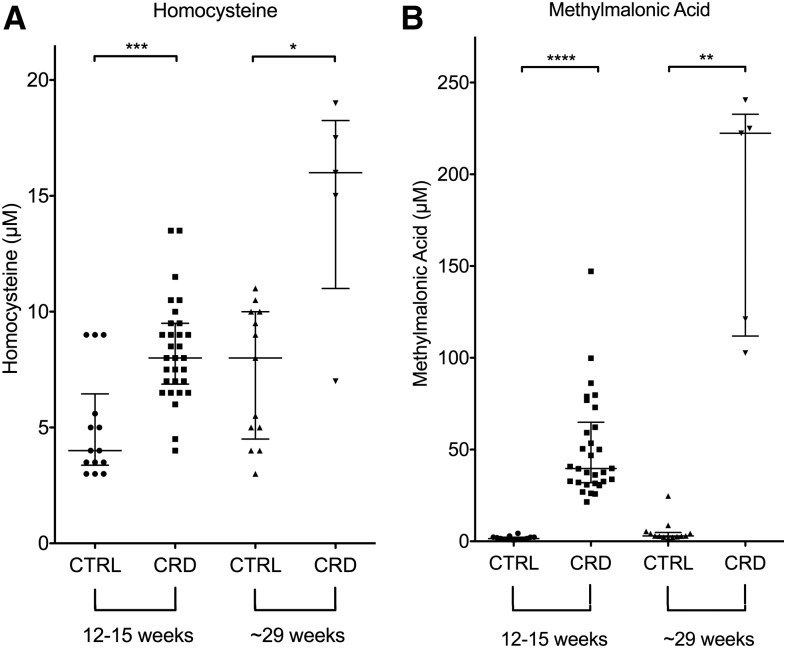
C57BL/6J mice maintained on a cobalamin-restricted diet postweaning exhibited higher homocysteine concentrations at ∼15 and ∼29 wk of age than similarly aged mice on the control diet (Figure 1A). The mice aged 29 wk developed moderate hyperhomocysteinemia compared with similarly aged mice on the control diet (Mann-Whitney U = 7; P = 0.0136) (Figure 1A). The mean homocysteine measurement for the C57BL/6J mice on the cobalamin-restricted diet was 14.9 μmol/L, a 2-fold increase in mean circulating homocysteine induced by dietary cobalamin restriction. Concentrations of circulating homocysteine increased with age and exposure to the cobalamin-restricted diet.
FIGURE 1.
Metabolic measurements of mice aged 12 and 24 wk on the CRD or CTRL diet for 9 and 21 wk, respectively. The squares, circles, and up and down triangles indicate the values obtained from an individual mouse. (A) Homocysteine. (B) Methylmalonic acid. Homocysteine and methylmalonic acid concentrations were significantly elevated in wild-type C57BL/6J mice on the CRD. Homocysteine and methylmalonic acid concentrations in mice aged 29 wk were significantly higher than similarly aged mice on the CTRL diet. Data are presented as medians ± IQRs. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001. CRD, cobalamin-restricted diet; CTRL, control.
To confirm that the dietary manipulations increased homocysteine concentrations by reducing in vivo concentrations of cobalamin, we also measured plasma MMA in all mice (Figure 1B). This metabolite was elevated when the activity of the cobalamin-requiring enzyme, methylmalonyl CoA mutase, was reduced. MMA concentrations in mice aged 29 wk were significantly higher (Mann-Whitney U = 0; P = 0.0016) in those maintained on the cobalamin-restricted diet than those maintained on the control diet (medians: 222.4 and 2.9 μmol/L, respectively). Similar to the homocysteine response, MMA concentrations were notably higher in mice on the cobalamin-restricted diet, and this effect increased with age.
Cobalamin restriction raised homocysteine and MMA in Fbn1C1039G/+ MFS mice.
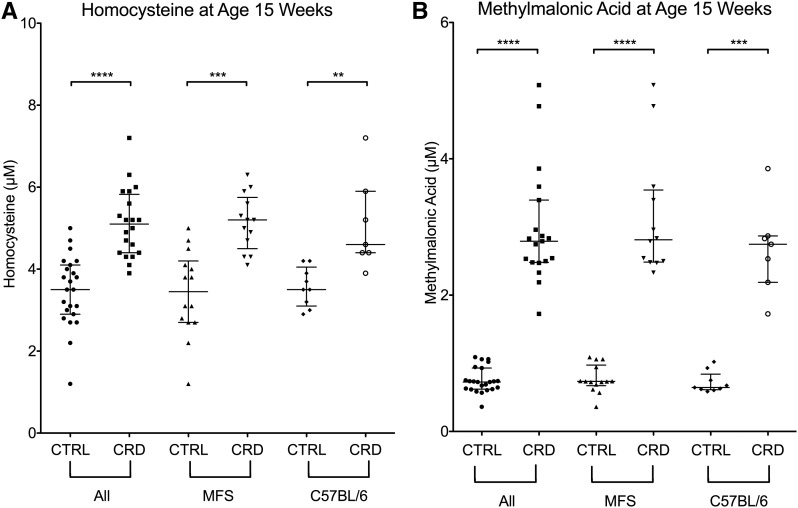
In an independent cohort of C57BL/6J and Fbn1C1039G/+ MFS mice, the same dietary effect on homocysteine and MMA was observed (Figure 2). When these strains were analyzed together, mice aged 15 wk on the cobalamin-restricted diet had a median homocysteine measurement of 5.1 μmol/L compared with 3.5 μmol/L for mice on the control diet. This represented a significant difference between the 2 groups (Mann-Whitney U = 30; P < 0.0001) (Figure 2A). When examined separately, the Fbn1C1039G/+ MFS mice on the cobalamin-restricted diet also had significantly higher homocysteine measurements than Fbn1C1039G/+ MFS mice on the control diet (median: 5.2 and 3.45 μmol/L, respectively) (Mann-Whitney U = 12.5; P = 0.0002). A similar dietary effect on homocysteine was observed in C57BL/6J mice (Figure 2A); an increase of homocysteine concentrations was observed in mice on the cobalamin-reduced diet regardless of genotype. No significant difference was observed in homocysteine measurements of MFS mice on the cobalamin-restricted diet compared with C57BL/6J mice on the cobalamin-restricted diet. Similarly, there was no significant difference in the homocysteine measurements of MFS mice on the control diet compared with C57BL/6 mice on the control diet.
FIGURE 2.
Metabolite measurements of mice aged 15 wk on the CRD or CTRL diet for 12 wk. The squares, circles, triangles, and diamonds indicate the values obtained from an individual mouse. (A) Homocysteine. (B) Methylmalonic acid. Homocysteine and methylmalonic acid concentrations were significantly elevated in mice on the CRD. No significant difference was observed in Fbn1C1039G/+ MFS mice compared with CTRL wild-type C57BL/6J mice in either the CRD or CTRL diet groups. For clarity, an outlying methylmalonic acid measurement (23.17 μmol/L) was not included in the graphical representation of methylmalonic acid values. This datum is included in our statistical analysis. Data are presented as medians ± IQRs. ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001. CRD, cobalamin-restricted diet; CTRL, control; Fbn1, murine fibrillin 1 gene; MFS, Marfan syndrome.
When C57BL/6J and Fbn1C1039G/+ MFS mice were analyzed together, MMA measurements were significantly higher in mice on the cobalamin-restricted diet (median: 2.81 μmol/L) than those on the control diet (median: 0.73 μmol/L) (Mann-Whitney U = 0; P < 0.0001) (Figure 2B). When Fbn1C1039G/+ MFS mice were evaluated separately, significantly different MMA values were also observed in the cobalamin-restricted mice (median: 2.83 μmol/L) compared with those on the control diet (median: 0.73 μmol/L) (Mann Whitney U = 0; P < 0.0001). No difference was noted for MMA measurements between MFS mice and C57BL/6J mice on the cobalamin-restricted diet or between MFS and C57BL/6J mice on the control diet.
Moderately elevated homocysteine did not correlate with thoracic aortic diameter.
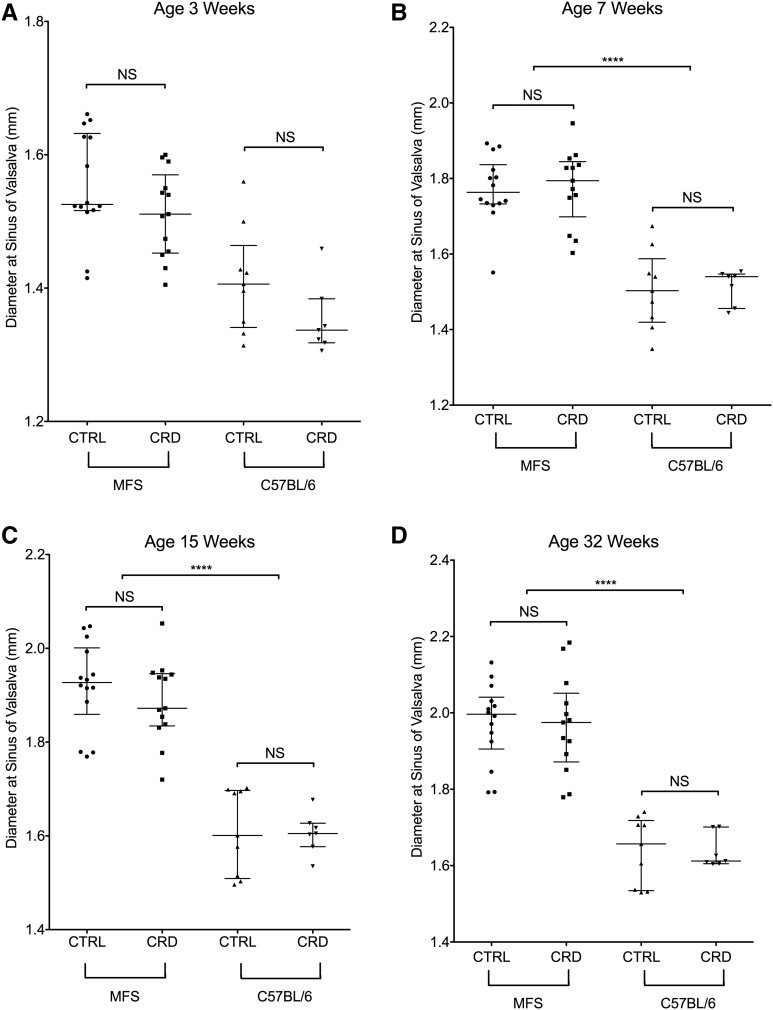
Longitudinal echocardiography was performed on mice aged 3, 7, 15, and 32 wk. Fbn1C1039G/+ MFS mice with or without the cobalamin-restricted diet (higher homocysteine) had enlarged aortic roots (maximal dimension at the sinus of Valsalva) compared with wild-type C57BL/6J mice (aortic root size at 32 wk: 1.97 ± 0.11 compared with 1.64 ± 0.07 mm; P < 0.0001), but no significant difference was observed between Fbn1C1039G/+ MFS mice on the cobalamin-restricted diet (higher homocysteine) compared with MFS mice on the control diet (aortic root size at 32 wk: 1.97 ± 0.10 compared with 1.97 ± 0.13 mm; P = 0.9) (Figure 3). Likewise, no significant difference was noted in the aortic root diameter of wild-type C57BL/6 mice on the cobalamin-restricted diet compared with wild-type C57BL/6J mice on the control diet (aortic root size at 32 wk: 1.64 ± 0.09 compared with 1.64 ± 0.04 mm; P = 0.9).
FIGURE 3.
Echocardiographic measurements. The squares, circles, and up and down triangles indicate the values obtained from an individual mouse. (A) Absolute aortic root diameter measured by echocardiography of wild-type (n = 16) and Fbn1C1039G/+ MFS mice (n = 27) aged 3 wk (baseline measurement before feeding with the diets). (B) Absolute aortic root diameter measurements of mice aged 7 wk on the diet for 4 wk. (C) Absolute aortic root diameter measurements of mice aged 15 wk on the diet for 12 wk. (D) Absolute aortic root diameter measurements of mice aged 32 wk on the diet for 29 wk. There was no significant difference in the aortic root diameter of the Fbn1C1039G/+ MFS mice and CTRL wild-type C57BL/6J mice between the CTRL diet cohort (n = 14) and CRD cohort (n = 13). Data are presented as medians ± IQRs. ∗∗∗∗P < 0.0001. CRD, cobalamin-restricted diet; CTRL, control; Fbn1, murine fibrillin 1 gene; MFS, Marfan syndrome.
Discussion
Epidemiologic studies and some basic laboratory investigations have suggested that hyperhomocysteinemia is positively correlated with thoracic aortic dilation, dissection, and rupture. Moderate hyperhomocysteinemia produced by genetic changes and diet is present in 5–7% of the population and in many cases may be reversed with nutritional interventions (6, 19). Therefore, it presents an attractive target for medical therapy and merits further investigation.
We used a nutritional intervention to raise homocysteine 1.5–2-fold above the normal physiologic concentrations in mice and to examine its role in TAA. We demonstrated an elevated mean homocysteine in the moderate range (∼15 μmol/L) in mice aged 29 wk when they were placed on the cobalamin-restricted diet at weaning (Figure 1A). To confirm a similar trend in both genotypes, a single measurement of homocysteine and MMA was obtained in all mice aged 15 wk (Figure 2). This nutritional intervention allowed us to measure the effect of moderately elevated homocysteine in normal mice and in mice genetically engineered to be highly susceptible to TAA. Mice heterozygous for a Cys substitution in an epidermal growth factor-like domain of fibrillin-1 (Fbn1C1039G/+) are a well-characterized model of MFS with progressive aortic root dilation starting as early as 2 wk of age. By 7 wk of age, the aortic root of Fbn1C1039G/+ mice is significantly larger than the aortic root of wild-type mice, making this an appealing model of TAA (20). In addition, in vitro studies have demonstrated that elevated homocysteine may alter the structure and function of fibrillin-1, making MFS an even more attractive system in which to study the effect of homocysteine on TAA.
We found no significant differences in the thoracic aortic diameter between the mice fed the control and cobalamin-restricted diets in either the MFS or C57BL/6J cohorts. The lack of any demonstrated effect of homocysteine on the thoracic aortic diameter of MFS mice argues against a substantial direct role for the metabolite in TAA pathology in the context of cobalamin deficiency. A possible limitation of our work was the use of an animal model to recapitulate aspects of human disease. Mice on standard lab diets have steady-state homocysteine concentrations that are lower than the normal range for humans [3–5 μmol/L in wild-type C57BL/6J mice (21–27) and 6–10 and 8–12 μmol/L in men and women, respectively (6)]. The diet manipulations used in this study produced homocysteine concentrations that were comparable to elevated concentrations observed in humans, i.e., ∼15 μmol/L at the experimental endpoint. If the absolute versus relative concentrations were critical, we may not have observed a change in aortic parameters because the upper range of homocysteine concentrations observed in humans is higher than that observed in mice aged 32 wk. It is worth noting that our experimental model was designed to mimic the physiologic concentrations commonly observed in humans rather than manipulating the system to produce supraphysiologic concentrations of homocysteine. Our observations do not rule out the possibility that greatly elevated concentrations of homocysteine would influence the progression of TAA. In the same vein, we note that although monitoring for subtle prelesion changes was beyond the scope of this study, a histology of the mice maintained on the cobalamin-restricted diet may have revealed the cellular effects observed in TAA, such as the loss of smooth muscle cell organization, loss of elastic fibers, or evidence of inflammation (28).
The most severe complications of TAA, including rupture and death, are associated with an increased aneurysmal diameter. Medical management is often insufficient in preventing progressive thoracic aortic dilatation, and surgical intervention carries a considerable risk of morbidity and mortality. Additional therapeutic strategies for TAA are needed. Animal studies that investigate promising interventions are part of this process. Equally important is the publication of negative results. Studies such as ours that use well-developed hypotheses and simple experimental design avoid duplicated scientific effort and assist in directing research toward better interventions.
Acknowledgments
We thank Faith Pangilinan for critical advice and suggestions. The authors’ responsibilities were as follows—JR, BK, HCD, and LCB: designed the research; JR, BK, D Bernard, and D Bedja: conducted the research; JR and BK: analyzed the data and wrote the paper; HCD and LCB: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Footnotes
Abbreviations used: Fbn1, murine fibrillin 1 gene; MFS, Marfan syndrome; MMA, methylmalonic acid; TAA, thoracic aortic aneurysm.
References
- 1.Bickerstaff LK, Pairolero PC, Hollier LH, Melton LJ, Van Peenen HJ, Cherry KJ, Joyce JW, Lie JT. Thoracic aortic aneurysms: a population-based study. Surgery 1982;92:1103–8. [PubMed] [Google Scholar]
- 2.Clouse WD, Hallett JW Jr., Schaff HV, Gayari MM, Ilstrup DM, Melton LJ III. Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 1998;280:1926–9. [DOI] [PubMed] [Google Scholar]
- 3.Abdolrazaghi M, Navidbakhsh M, Hassani K. Abdominal and thoracic aortic aneurysm modeling. Am J Appl Sci 2008;5:1052–8. [Google Scholar]
- 4.Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, Elefteriades JA. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002;73:17–27. [DOI] [PubMed] [Google Scholar]
- 5.Booher AM, Eagle KA. Diagnosis and management issues in thoracic aortic aneurysm. Am Heart J 2011;162:38–46.e1. [DOI] [PubMed] [Google Scholar]
- 6.McCully KS. Homocysteine and vascular disease. Nat Med 1996;2:386–9. [DOI] [PubMed] [Google Scholar]
- 7.Kang SS, Wong PW, Malinow MR. Hyperhomocyst(e)inemia as a risk factor for occlusive vascular disease. Annu Rev Nutr 1992;12:279–98. [DOI] [PubMed] [Google Scholar]
- 8.Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, Johnston C, Engbaek F, Schneede J, McPartlin C, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem 2004;50:3–32. [DOI] [PubMed] [Google Scholar]
- 9.Guttormsen AB, Ueland PM, Nesthus I, Nygard O, Schneede J, Vollset SE, Refsum H. Determinants and vitamin responsiveness of intermediate hyperhomocysteinemia (> or = 40 micromol/liter). The Hordaland Homocysteine Study. J Clin Invest 1996;98:2174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sbarouni E, Georgiadou P, Analitis A, Chaidaroglou A, Marathias A, Degiannis D, Voudris V. High homocysteine and low folate concentrations in acute aortic dissection. Int J Cardiol 2013;168:463–6. [DOI] [PubMed] [Google Scholar]
- 11.Giusti B, Porciani MC, Brunelli T, Evangelisti L, Fedi S, Gensini GF, Abbate R, Sani G, Yacoub M, Pepe G. Phenotypic variability of cardiovascular manifestations in Marfan syndrome. Possible role of hyperhomocysteinemia and C677T MTHFR gene polymorphism. Eur Heart J 2003;24:2038–45. [DOI] [PubMed] [Google Scholar]
- 12.Takagi H, Umemoto T, Group A. A meta-analysis of circulating homocysteine levels in subjects with versus without abdominal aortic aneurysm. Int Angiol 2015;34:229–37. [PubMed] [Google Scholar]
- 13.Pezzini A, Del Zotto E, Padovani A. Hyperhomocysteinemia: a potential risk factor for cervical artery dissection following chiropractic manipulation of the cervical spine. J Neurol 2002;249:1401–3. [DOI] [PubMed] [Google Scholar]
- 14.Takagi H, Umemoto T. Homocysteinemia is a risk factor for aortic dissection. Med Hypotheses 2005;64:1007–10. [DOI] [PubMed] [Google Scholar]
- 15.Whiteman P, Hutchinson S, Handford PA. Fibrillin-1 misfolding and disease. Antioxid Redox Signal 2006;8:338–46. [DOI] [PubMed] [Google Scholar]
- 16.Hubmacher D, Sabatier L, Annis DS, Mosher DF, Reinhardt DP. Homocysteine modifies structural and functional properties of fibronectin and interferes with the fibronectin-fibrillin-1 interaction. Biochemistry 2011;50:5322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh S, Sinha JK, Putcha UK, Raghunath M. Severe but not moderate vitamin B12 deficiency impairs lipid profile, induces adiposity, and leads to adverse gestational outcome in female C57BL/6 mice. Front Nutr 2016;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest 2004;114:172–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietrzik K, Bronstrup A. Causes and consequences of hyperhomocyst(e)inemia. Int J Vitam Nutr Res 1997;67:389–95. [PubMed] [Google Scholar]
- 20.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 2006;312:117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baumbach GL, Sigmund CD, Bottiglieri T, Lentz SR. Structure of cerebral arterioles in cystathionine beta-synthase-deficient mice. Circ Res 2002;91:931–7. [DOI] [PubMed] [Google Scholar]
- 22.da Costa KA, Gaffney CE, Fischer LM, Zeisel SH. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am J Clin Nutr 2005;81:440–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dayal S, Arning E, Bottiglieri T, Boger RH, Sigmund CD, Faraci FM, Lentz SR. Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke 2004;35:1957–62. [DOI] [PubMed] [Google Scholar]
- 24.Dayal S, Bottiglieri T, Arning E, Maeda N, Malinow MR, Sigmund CD, Heistad DD, Faraci FM, Lentz SR. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine beta-synthase-deficient mice. Circ Res 2001;88:1203–9. [DOI] [PubMed] [Google Scholar]
- 25.Ernest S, Hosack A, O’Brien WE, Rosenblatt DS, Nadeau JH. Homocysteine levels in A/J and C57BL/6J mice: genetic, diet, gender, and parental effects. Physiol Genomics 2005;21:404–10. [DOI] [PubMed] [Google Scholar]
- 26.Lentz SR, Erger RA, Dayal S, Maeda N, Malinow MR, Heistad DD, Faraci FM. Folate dependence of hyperhomocysteinemia and vascular dysfunction in cystathionine beta-synthase-deficient mice. Am J Physiol Heart Circ Physiol 2000;279:H970–5. [DOI] [PubMed] [Google Scholar]
- 27.Tan H, Jiang X, Yang F, Li Z, Liao D, Trial J, Magera MJ, Durante W, Yang X, Wang H. Hyperhomocysteinemia inhibits post-injury reendothelialization in mice. Cardiovasc Res 2006;69:253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Hamamsy I, Yacoub MH. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat Rev Cardiol 2009;6:771–86. [DOI] [PubMed] [Google Scholar]