Abstract
IMPORTANCE
Although nevi with a peripheral rim of globules (peripheral globular nevi [PGN]) observed with dermoscopy are associated with enlarging melanocytic nevi, their actual growth dynamics remain unknown. Because change is a sensitive but nonspecific marker for melanoma, beginning to understand the growth patterns of nevi may improve the ability of physicians to differentiate normal from abnormal growth and reduce unnecessary biopsies.
OBJECTIVE
To study the growth dynamics and morphologic evolution of PGN on dermoscopy.
DESIGN, SETTING, AND PARTICIPANTS
A total of 84 participants with 121 PGN from September 1, 1999, through May 1, 2013, were identified retrospectively. Cohorts were recruited from the Memorial Sloan Kettering Cancer Center; Melanoma Unit of the Hospital Clinic, University of Barcelona; and Study of Nevi in Children. All 3 cohorts underwent longitudinal monitoring with serial dermoscopic imaging of their PGN. Data analysis was performed from May 1, 2014, through April 1, 2015.
MAIN OUTCOMES AND MEASURES
Establishment of the natural growth curve of PGN. The secondary aim was to establish the median time to growth cessation in those PGN for which the size eventually stabilized and/or had begun to decrease during the study period.
RESULTS
The median duration of follow-up was 25.1 (range, 2.0–114.4) months. Most of the nevi (116 [95.9%]) enlarged at some point during sequential monitoring. The rate of increase in the surface area of PGN varied among cohorts and ranged from −0.47 to 2.26mm2/mo (mean rate, 0.25 [95% CI, 0.14–0.36]mm2/mo). The median time to growth cessation in the 26 PGN that stabilized or decreased in size (21.5%) was 58.6 months. All lesions changed in a symmetric manner and 91 (75.2%) displayed a decrease in the density of peripheral globules over time.
CONCLUSIONS AND RELEVANCE
Nevi displaying a peripheral globular pattern enlarged symmetrically with apparent growth cessation occurring during a span of 4 to 5 years. Our results reiterate the important concept that not all growth is associated with malignancy.
Nevi are dynamic yet benign neoplasms with a life cycle that includes inception, growth, senescence, and involution.1 Although growth is a normal part of the nevus life cycle, limited data are available to help physicians distinguish normal from abnormal growth. Data on the change in the surface area of nevi are primarily based on cross-sectional studies, and few studies provide long-term follow-up of nevus growth characteristics.2–4 Longitudinal tracking of nevus growth can be time-consuming and difficult to standardize using conventional clinical photography.2,4 Since the development of dermoscopy, serial digital dermoscopic imaging (SDDI) is used increasingly to track changes in nevi over time.5 Dermoscopy also provides a fixed distance and a fiducial ruler during image capture, which facilitate standardization of repeated measurements.
Peripheral globular nevi (PGN) are a distinct subset of enlarging nevi that display a single peripheral rim of brown globules when examined with dermoscopy and have a homogeneous, globular, reticular, or mixed central pattern. The histologic characteristics of peripheral globules correspond to junctional melanocytic nests, and nevi with this pattern are most commonly seen in younger individuals.6,7 The presence of peripheral globules is thought to suggest that the nevus is in an active growth phase.
Because change is a sensitive marker for melanoma, the observed growth of a nevus can be disconcerting to a physician. Dermoscopists recognize that peripheral globules are indicative of a benign nevus but acknowledge that patient-related factors may influence management decisions.6 Knowledge of the normal growth trajectory of such lesions may also inform the physician’s management decision.8 In this study, we retrospectively examined the change in the surface area of PGN from 3 disparate cohorts to study the normal growth curve of these nevi.
Methods
The study was approved by the institutional review boards of Memorial Sloan Kettering Cancer Center (MSKCC); the Hospital Clinic, University of Barcelona (HCB); and Boston University. We adhered to the principles set forth in the Declaration of Helsinki. Informed consent was waived, and the patient data were deidentified.
Study Sample
Peripheral globular nevi monitored with serial dermoscopic images (≥2)were identified retrospectively from 3 participant cohorts. We followed up participants through their existing medical records. Nevi with a peripheral rim of globules that were deemed to have benign morphologic features as determined by expert dermoscopists (J.M., S.P., and A.A.M.) at the baseline visit were included in the study. Nevi undergoing biopsy at any subsequent evaluation were included in the study, with the date of the biopsy considered the final evaluation.
Memorial Sloan Kettering Cancer Center is a tertiary referral center in New York, and the patient population of this clinic consists of patients at high risk form elanoma. The MSKCC selects patients for SDDI who are undergoing cutaneous surveillance using total-body photography. Lesions that are new or changing are photographed with dermoscopy and subsequently monitored using SDDI. For the MSKCC cohort, PGN were retrospectively selected from consecutive lesions chosen for SDDI by 1 dermatologist (A.A.M.) from June 1, 2005, through May 1, 2013. The HCB cohort also consisted of patients at high risk for melanoma; the study population was described by Salerni et al9 as 45% with a personal history of melanoma and 58% with more than 200 nevi. The HCB cohort was selected from patients undergoing total-body dermoscopic imaging by 3 dermatologists (J.B., J.M., and S.P.) at the Melanoma Unit of the HCB. The 50 PGN were then retrospectively selected from consecutively obtained dermoscopic images of patients from September 1, 1999, through October 11, 2011. The third cohort was drawn from participants in the Study of Nevi in Children (SONIC). SONIC is a prospective, population-based study in Framingham, Massachusetts, that includes children and adolescents followed up from ages 11 through 17 years with imaging performed at baseline (11 years), year 3 (14 years), year 4 (15 years), and year 6 (17 years). Nineteen consecutive PGN first imaged between November 1, 2004, and October 24, 2007, were retrospectively selected for inclusion. The study recruitment and methods have been described previously.10,11
Imaging Process, Surface Area Measurement, Morphologic Observations, and Definitions
All dermoscopic images were acquired using standard contact dermoscopy at a magnification factor of ×10. Each image was stored digitally in a secure database without compression. Dermoscopic images from the MSKCC cohort were obtained with a digital camera (D90 SLR; Nikon) with a 60-mm lens (Macro Nikkor; Nikon) and a dermoscopy attachment (EpiLume; Canfield Imaging Systems, Inc) and stored in 3008 × 2000-pixel resolution. The images from the HCB cohort were obtained with a digital camera (DermLiteFoto; 3GEN LLC) and stored in 640 × 480-pixel resolution. Images from the SONIC cohort were obtained using a digital camera (S2 SLR; Fuji) and 60-mm lens (Macro Nikkor)with a dermoscopy attachment (EpiLume) and stored in 3016 × 2014-pixel resolution. Lesion surface area for the MSKCC and SONIC cohorts was calculated from dermoscopic images for each index nevus using a measurement tool incorporated into the image archiving tool (DermaGraphix or Mirror; Canfield Scientific). Spectral and spatial calibration for standardization between image sets was accomplished by measuring fiducial markers in each of the images. Nevus growth was defined as an increase in surface area in subsequent evaluation points. We determined test-retest reliability for lesion area measurements on a random subset of 20 lesion images at 2 time points, with high intrarater reliability (intraclass correlation coefficient, 0.89).
Lesion surface area for the HCB cohort was calculated from dermoscopic images for each index nevus using a measurement tool incorporated into the image archiving tool(MoleMax 2; Derma Instruments). Using a test set of 10 images from the HCB cohort, size measurements were also calculated using the MSKCC’s archiving tool and were found to be reliable and reproducible (intraclass correlation coefficient, 0.92).
Growth cessation was defined as a lesion for which the surface area initially increased and subsequently did not increase or decreased in successive evaluation points. All lesions had distinct globules present at the periphery at the baseline dermoscopic evaluation, but were further classified into 4 groups based on the dermoscopic pattern present at the center of the lesion (globular, reticular, homogeneous, and combined reticular and globular) by experts in the dermoscopic assessment of pigmented lesions (J.M., S.P., and A.A.M.). Qualitative morphologic observations regarding symmetry of growth and changes in globule size and density (concentration per unit area classified as high, medium, low, very low, or none) were assessed at baseline and compared at successive evaluations by an expert dermoscopist (A.A.M.) for all lesions. Participant age was measured in years and was assessed as the age at the first imaging session. Anatomic location was categorized as trunk vs other.
Statistical Analysis
Data analysis was preformed from May 1, 2014, through April 1, 2015. Descriptive statistics and graphical methods were used to characterize the study participants, the study lesions, and the size (surface area) of the lesions at the evaluation points. Scatterplots with Lowess curves12 were used to depict mean lesion growth characteristics as a function of observation time. Interrater reliability for lesion measurement was assessed for a subset of study lesions using the intraclass correlation coefficient. Because the intervals between lesion evaluation points varied between individuals, we used multilevel growth-curve models to assess the change in lesion growth during the evaluation period. With this approach, we can model the shape of the individual lesion growth trajectories over time and assess how these trajectories differ randomly and by subject-level criteria, such as age or anatomic location and dermoscopic pattern. For these models, the outcome variable was lesion surface area measured in square millimeters. Time was measured in months elapsed since the initial imaging session and was evaluated on a linear and a quadratic scale. Interaction terms were created to assess differences in the rate of lesion growth by study cohort characteristics while controlling for potential differences in age, sex, and lesion anatomic location. All statistical analyses were performed in STATA (version 12.1; StataCorp LP).
Results
Participant and Nevus Characteristics
The study included 84 participants, of whom 65 contributed 1 PGN and 19 contributed multiple PGN. In total, we included 121 PGN from the MSKCC (n = 52), HCB (n = 50), and SONIC (n = 19) cohorts (Table). A statistically significant difference existed in the mean ages of the participants from each cohort, with the mean age of MSKCC participants being the highest (Table). Most of the participants were younger than 50 years, with only 5 PGN contributed from participants older than 50 years (4.1%). The median number of SDDIs obtained per nevus was 3 for the MSKCC and HCB cohorts and 4 for the SONIC cohort. The median time of follow-up for all cohorts was 25.1 (range, 2.0–114.4) months. Nevi from the MSKCC cohort were followed up for a median of 15 months, whereas the HCB and SONIC nevi were followed up for a median of 30 and 72 months, respectively. Most of the nevi were located on the trunk, including 51 (98.1%) in the MSKCC, 39 (78.0%) in the HCB, and 19 (100%) in the SONIC cohorts. Nevi on the trunk were a mean of 2.00 (95% CI, 0.45–3.55) mm2 larger than those located on other anatomic sites (P = .01). After adjusting for participant age at study inception, sex, and central dermoscopic pattern, no differences in baseline nevus size were noted.
Table.
Participant and Lesion Characteristics
| Characteristica | MSKCC Cohort (n = 52) | HCB Cohort (n = 50) | P Valueb,c | SONIC Cohort (n = 19) | P Valueb,d |
|---|---|---|---|---|---|
| Age, mean (SD)e | 32.8 (11.4) | 27.4 (8.5) | .008 | 10.8 (0.31) | <.001 |
| Sex | |||||
| Male | 24 (46) | 25 (50) | .70 | 13 (68) | .14 |
| Female | 28 (54) | 25 (50) | 6 (32) | ||
| Anatomic location | |||||
| Trunk | 51 (98) | 39 (78) | .002 | 19 (100) | <.001 |
| Extremities | 1 (2) | 11 (22) | 0 | ||
| No. of evaluations | |||||
| 2 | 15 (29) | 19 (38) | .45 | 1 (5) | <.001 |
| 3 | 12 (23) | 7 (14) | 0 | ||
| 4 | 10 (19) | 6 (12) | 18 (95) | ||
| 5 | 8 (15) | 7 (14) | 0 | ||
| ≥6 | 7 (14) | 11 (22) | 0 | ||
| Lesion area at baseline, mean (SD), mm2e,f | 11.0 (8.2) | 7.9 (6.0) | .03 | 5.8 (4.5) | .006 |
| Dermoscopic pattern | |||||
| Globular | 22 (42) | 19 (38) | .14 | 9 (47) | .01 |
| Reticular | 7 (14) | 16 (32) | 2 (11) | ||
| Homogeneous | 7 (14) | 4 (8) | 8 (42) | ||
| Reticular and globular | 16 (31) | 11 (22) | 0 | ||
Abbreviations: HCB, Hospital Clinic, University of Barcelona; MSKCC, Memorial Sloan Kettering Cancer Center; SONIC, Study of Nevi in Children.
Unless otherwise indicated, data are expressed as number (percentage) of nevi. Percentages have been rounded and may not total 100.
Unless otherwise specified, calculated as χ2 tests.
Compares the MSKCC and HCB cohorts.
Compares the MSKCC and SONIC cohorts.
Comparisons were calculated as 2-sample, 2-sided t tests for the equality of means.
Lesion size at baseline stratified by age is statistically nonsignificant (P = .57).
Histopathologic Evaluation
Of the 121 PGN, 7 (5.8%) underwent biopsy. One of these 7 PGN was incidentally found to have one of the fastest growth rates of our sample. Histopathologic examination for all 7 lesions revealed compound melanocytic nevi with mild to moderate cytologic atypia. Reasons for biopsy included patient anxiety, rapid growth as perceived by the evaluating physician, and physician-perceived focal change in globules.
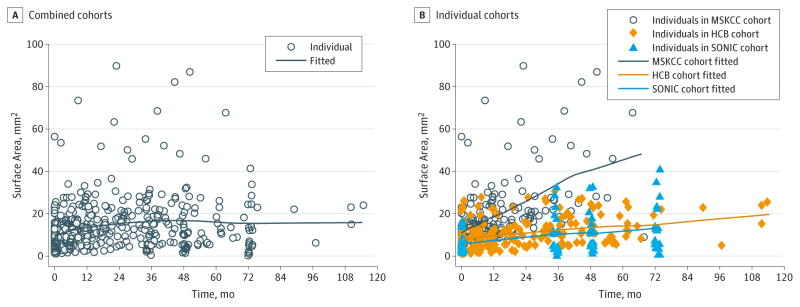
Nevus Growth Dynamics
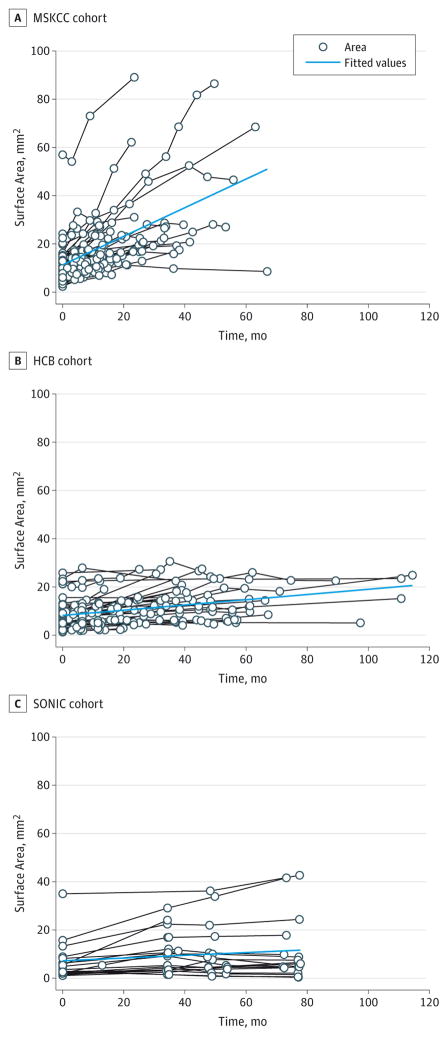
The mean growth rate for all nevi combined was 0.25 (95%CI 0.14–0.36) mm2/mo (P < .001); the null value refers to no growth (Figure 1A). To assess growth rates by cohort, a time × cohort interaction term was included in the model. The MSKCC PGN grew at a mean rate of 0.52 (95% CI, 0.43–0.61) mm2/mo (P < .001), whereas the Barcelona and SONIC PGN grew at slower rates of 0.12 (95%CI, 0.03–0.21 [P = .007]) and 0.14 (95% CI, 0.02–0.26 [P = .02]) mm2/mo, respectively (Figure 1B). No difference in the rate of growth was observed by central dermoscopic pattern or by age when dichotomized to younger than 30 years and 30 years or older. The growth trajectory of each PGN by cohort can be visualized in Figure 2. In a random subset of 20 study lesions, interrater reliability for lesion area measurements was found to be very high (intraclass correlation coefficient, 0.76; see the Bland-Altman plot in the eFigure in the Supplement).
Figure 1. Smoothed Growth Curves of Peripheral Globular Nevi.
The fitted line is created from the predicted value from the regression models. HBC indicates Hospital Clinic, University of Barcelona; MSKCC, Memorial Sloan Kettering Cancer Center; and SONIC, Study of Nevi in Children.
Figure 2. Individual Nevus Growth Trajectories by Cohort.
The fitted line is created from the predicted value from the regression models. HBC indicates Hospital Clinic, University of Barcelona; MSKCC, Memorial Sloan Kettering Cancer Center; and SONIC, Study of Nevi in Children.
The range of growth rates for all nevi varied from −0.47 to 2.26mm2/mo. By the last follow-up, 26 nevi (21.5%)had stabilized or had started to decrease in size. Of these, the median time to growth cessation was at least 58.6 months. The median follow-up time for lesions that continued growing was 19.0 months. Exclusion of nevi that had stabilized and/or decreased in size did not affect the growth rate curves.
Morphologic Changes
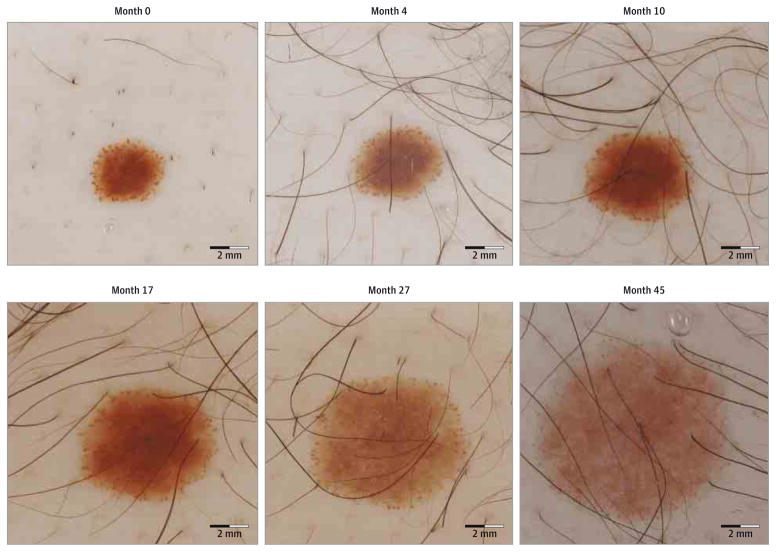
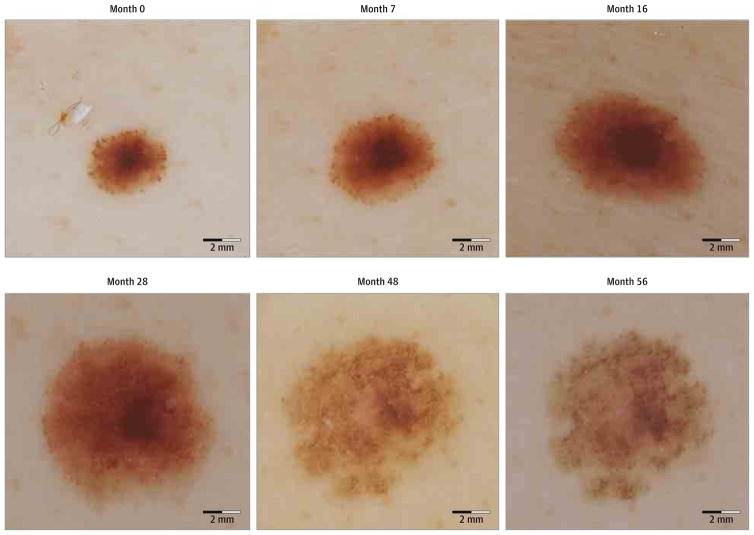
Peripheral globular nevi from all cohorts were examined for morphologic evolution. All growing lesions displayed symmetric enlargement over time. In 91 PGN(75.2%), the density of peripheral globules decreased over time. All nevi that stabilized or eventually started to decrease in size by the last evaluation (n = 26) had decreased density (n = 14) or complete disappearance (n = 12) of peripheral globules. The growth patterns and morphologic changes of 2 representative examples of PGN as they evolved over time are shown in Figure 3 and Figure 4.
Figure 3. Sequential Dermoscopic Images of an Enlarging Peripheral Globular Nevus With a Homogeneous Central Component.
An increase in surface area is seen during 45 months of follow-up. The nevus enlarged in a symmetric manner and displayed a decrease in the density of peripheral globules over time.
Figure 4. Sequential Dermoscopic Images of a Stabilized Peripheral Globular Nevus With a Homogeneous Central Component.
After an initial increase in surface area, the size of the nevus stabilized. By the last evaluation, nearly all of the peripheral globules had disappeared.
Discussion
Change in the size of melanocytic lesions is a sensitive yet non-specific marker for melanoma. Even in populations at high risk for melanoma, studies have shown that fewer than 3% of all changing lesions prove to be melanoma.13,14 Reliance on change alone to determine the management of melanocytic lesions, particularly in younger individuals, may be driving alarming rates of biopsy of benign lesions. For example, in individuals 19 years or younger, a recent study15 estimated that in the United States from 2009 through 2013, 1035 nevi were removed for every 1 melanoma identified. Because PGN are most frequently identified in younger groups, recognition of this dynamic nevus subset as a benign growing pattern is important for optimizing management decisions.6
We report the growth rates of PGN from 3 distinct participant cohorts. Our results validate previous studies that found that PGN are strongly associated with growth.2,3 In a study of 1612 melanocytic lesions followed up with SDDI,3 the relative risk for enlargement was 28-fold higher (95%CI, 20-fold to 39-fold higher) for nevi with compared with nevi without a peripheral rim of globules. In a separate study of serial dermoscopic images of 1862 melanocytic lesions,2 75 lesions were excised because of substantial changes over time. Twenty-five percent of these lesions had a peripheral globular pattern, but none were found to be melanomas. The results from our study corroborate the findings of these studies; more than 95% of our lesions increased in size at some point during the observation period.
Serial digital dermoscopic imaging involves the capture and assessment of successive dermoscopic images of skin lesions to assess for change. This technique most frequently uses short-term (3–4 months) or long-term (6–12 months) monitoring intervals. Although we are uncertain how long our study nevi had been present before the initiation of photographically assisted follow-up, our finding of a median time to growth cessation of 58.6 months for PGN has important clinical implications. First, if a physician is inclined to monitor a PGN until the nevus stops growing, the follow-up duration is likely to be lengthy. Second, patients harboring these nevi should receive tailored counseling regarding the expected size evolution of these nevi to facilitate skin self-examination, prevent unnecessary skin biopsy, and possibly mollify patient anxiety. Although follow-up traditionally is performed in the office setting, Wu et al16 recently showed that patients are capable of acquiring follow-up dermoscopic images and electronically submitting them to a teledermoscopist for evaluation.
Nevus growth is a dynamic process influenced by a complex interplay of genetic and environmental factors. In this study, we found that the growth rate of PGN was highest in the MSKCC cohort. Differences in the growth rate may have been secondary to underlying variations in the genetic and environmental makeup of the 3 cohorts. However, we believe this finding was primarily the result of differences between cohorts in the selection of nevi for inclusion in the study. In contrast to the SONIC and HCB cohorts, the MSKCC cohort included PGN that were identified as new or changing based on the MSKCC’s method for selecting lesions that undergo SDDI (the Methods section provides a detailed explanation of selection by cohort). Selection bias therefore may have accounted for the differences in growth rate between cohorts. If PGN are incidentally found(not selected based on the fact that they are new or changing) and followed up, their growth rate may be more similar to those of the SONIC and HCB cohorts, whereas if PGN are clinically identified as new or changing and then dermoscopically monitored, their growth rate may more closely resemble that of the MSKCC cohort.
Studies have identified growing melanocytic nevi as significantly more likely to have a BRAF (OMIM 164757) V600E mutation or to exhibit a peripheral globular pattern than nevi that exhibit no change in size.17,18 In addition, Zalaudek et al18 found that 10 of 11 PGN (91%) harbor the BRAF V600E mutation, which is significantly higher than the prevalence of BRAF V600E mutations in other dermoscopically defined nevus subsets.19 These findings suggest that BRAF, a protein kinase and downstream effector of ras, may play an important role in the early phases of nevogenesis and in the rapid growth dynamics of PGN. Indeed, transduction of melanocytes in cell cultures with vectors expressing BRAF V600E leads to enhanced melanocyte proliferation.20
A more complete understanding of the life cycle of nevi may shed important insights into the biological features of melanocytes. Although nevi proliferate before undergoing senescence, malignant melanocytes are thought to over come the molecular mediators that induce growth arrest.21 We were careful to use the term growth cessation as opposed to senescence. Although some may consider stability in nevus size to be synonymous with clinically defined senescence, true biological senescence cannot be ascertained without molecular or histologic evidence of growth cessation, which was not evaluated in this study. Nonetheless, PGN are an interesting group of melanocytic lesions to study in this respect because their peripheral rim of globules is thought to disappear over time, which has been considered an in vivo marker of growth abrogation.22 Our study supports these findings because all nevi that had stabilized and/or decreased in size had decreased density or complete disappearance of peripheral globules when compared with baseline.
The biological determination of these dynamic lesions as benign was not established through histopathologic examination and limits our conclusions. However, lesions that did undergo biopsy were diagnosed as benign nevi, and those that did not undergo biopsy grew symmetrically and did not reveal any melanoma-specific dermoscopic structures. In addition, the difference in the selection of nevi by cohort limits comparisons between cohorts. However, heterogeneity among the 3 cohorts also strengthens our study, which is the first, to our knowledge, to longitudinally track PGN originating from participants of various age groups and melanoma-risk profiles.
Establishing normal measures of nevus evolution represents an important step toward improving the diagnostic accuracy of physicians in selecting lesions that require biopsy. In this respect, we stress that although our study represents an important step toward better elucidating the normal growth dynamics of this unique subset of growing nevi, we do not yet suggest that physicians use the discrete growth rate values listed herein to dictate management decisions because our sample size was relatively small and we had significant variation in growth rates between cohorts. Future studies that investigate growth dynamics of larger subsets of nevi and melanomas are necessary to validate our findings and to help guide physicians in differentiating normal from abnormal growth.
Conclusions
By studying the growth trajectories of PGN, we have validated the observation that these nevi are strongly associated with growth. Although change in a melanocytic lesion remains a potential sign of melanoma, we must recognize that not all growth is associated with malignancy, particularly in patients who are young. Although further studies that explore management decisions of melanocytic neoplasms are necessary, a more complete understanding of their life cycle and growth patterns may one day help to prevent unnecessary excisions by identifying benign lesions more accurately and differentiating them from malignant ones.8
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by award R01-AR049342 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (Memorial Sloan Kettering Cancer Center); by grants 03/0019, 05/0302, and 06/0265 from Fondo de Investigaciones Sanitarias, Spain (Melanoma Unit of the Hospital Clinic, University of Barcelona); by the Centro de Investigación Biomédica en Red de Enfermedades Raras of the Instituto de Salud Carlos III, Spain; by the Agencia de Gestio d’Ajuts Universitaris i de Recerca 2009 Suport a grups de recerca 1337 of the Catalan Government, Spain; by contract LSHC-CT-2006-018702 from the European Commission under the Sixth Framework Programme (Melanoma Genetics Consortium); and by grant CA83115 from the National Cancer Institute of the US National Institutes of Health.
Footnotes
Conflict of Interest Disclosures: None reported.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional Contributions: Toni Burbidge, MD, Division of Dermatology, University of Calgary, Calgary, Alberta, Canada, made editorial contributions. She did not receive any compensation for this role.
Author Contributions: Ms Bajaj and Dr Marghoob had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Dusza, Malvehy, Puig, Yagerman, Marghoob.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Bajaj, Dusza, Marchetti, Wu, Fonseca, Malvehy, Yagerman, Liebman, Marghoob.
Critical revision of the manuscript for important intellectual content: Bajaj, Dusza, Marchetti, Fonseca, Kose, Brito, Carrera, Martins de Silva, Malvehy, Puig, Liebman, Scope, Halpern, Marghoob.
Statistical analysis: Bajaj, Dusza, Wu.
Obtained funding: Puig, Halpern.
Administrative, technical, or material support: Bajaj, Fonseca, Kose, Brito, Yagerman, Marghoob.
Study supervision: Fonseca, Puig, Marghoob.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Terushkin V, Scope A, Halpern AC, Marghoob AA. Pathways to involution of nevi: insights from dermoscopic follow-up. Arch Dermatol. 2010;146(4):459–460. doi: 10.1001/archdermatol.2010.20. [DOI] [PubMed] [Google Scholar]
- 2.Kittler H, Pehamberger H, Wolff K, Binder M. Follow-up of melanocytic skin lesions with digital epiluminescence microscopy: patterns of modifications observed in early melanoma, atypical nevi, and common nevi. J Am Acad Dermatol. 2000;43(3):467–476. doi: 10.1067/mjd.2000.107504. [DOI] [PubMed] [Google Scholar]
- 3.Kittler H, Seltenheim M, Dawid M, Pehamberger H, Wolff K, Binder M. Frequency and characteristics of enlarging common melanocytic nevi. Arch Dermatol. 2000;136(3):316–320. doi: 10.1001/archderm.136.3.316. [DOI] [PubMed] [Google Scholar]
- 4.Pellacani G, Scope A, Ferrari B, et al. New insights into nevogenesis: in vivo characterization and follow-up of melanocytic nevi by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61(6):1001–1013. doi: 10.1016/j.jaad.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161(6):1270–1277. doi: 10.1111/j.1365-2133.2009.09374.x. [DOI] [PubMed] [Google Scholar]
- 6.Zalaudek I, Schmid K, Marghoob AA, et al. Frequency of dermoscopic nevus subtypes by age and body site: a cross-sectional study. Arch Dermatol. 2011;147(6):663–670. doi: 10.1001/archdermatol.2011.149. [DOI] [PubMed] [Google Scholar]
- 7.Zalaudek I, Hofmann-Wellenhof R, Kittler H, et al. A dual concept of nevogenesis: theoretical considerations based on dermoscopic features of melanocytic nevi. J Dtsch Dermatol Ges. 2007;5(11):985–992. doi: 10.1111/j.1610-0387.2007.06384.x. [DOI] [PubMed] [Google Scholar]
- 8.Grichnik JM. Age-dependent differences in growth curves for distinct melanocytic nevus subsets. Arch Dermatol. 2011;147(6):731–732. doi: 10.1001/archdermatol.2011.134. [DOI] [PubMed] [Google Scholar]
- 9.Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67(1):e17–e27. doi: 10.1016/j.jaad.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geller AC, Oliveria SA, Bishop M, Buckminster M, Brooks KR, Halpern AC. Study of health outcomes in school children: key challenges and lessons learned from the Framingham Schools’ Natural History of Nevi Study. J Sch Health. 2007;77(6):312–318. doi: 10.1111/j.1746-1561.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 11.Scope A, Dusza SW, Marghoob AA, et al. Clinical and dermoscopic stability and volatility of melanocytic nevi in a population-based cohort of children in Framingham school system. J Invest Dermatol. 2011;131(8):1615–1621. doi: 10.1038/jid.2011.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J AmStat Assoc. 1979;74:829–836. [Google Scholar]
- 13.Banky JP, Kelly JW, English DR, Yeatman JM, Dowling JP. Incidence of new and changed nevi and melanomas detected using baseline images and dermoscopy in patients at high risk for melanoma. Arch Dermatol. 2005;141(8):998–1006. doi: 10.1001/archderm.141.8.998. [DOI] [PubMed] [Google Scholar]
- 14.Robinson JK, Nickoloff BJ. Digital epiluminescence microscopy monitoring of high-risk patients. Arch Dermatol. 2004;140(1):49–56. doi: 10.1001/archderm.140.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Oliveria SA, Selvam N, Mehregan D, et al. Biopsies of nevi in children and adolescents in the United States, 2009 through 2013. JAMA Dermatol. 2015;151(4):447–448. doi: 10.1001/jamadermatol.2014.4576. [DOI] [PubMed] [Google Scholar]
- 16.Wu X, Oliveria SA, Yagerman S, et al. Feasibility and efficacy of patient-initiated mobile teledermoscopy for short-term monitoring of clinically atypical nevi. JAMA Dermatol. 2015;151(5):489–496. doi: 10.1001/jamadermatol.2014.3837. [DOI] [PubMed] [Google Scholar]
- 17.Loewe R, Kittler H, Fischer G, Faé I, Wolff K, Petzelbauer P. BRAF kinase gene V599E mutation in growing melanocytic lesions. J Invest Dermatol. 2004;123(4):733–736. doi: 10.1111/j.0022-202X.2004.23402.x. [DOI] [PubMed] [Google Scholar]
- 18.Zalaudek I, Guelly C, Pellacani G, et al. The dermoscopical and histopathological patterns of nevi correlate with the frequency of BRAF mutations. J Invest Dermatol. 2011;131(2):542–545. doi: 10.1038/jid.2010.332. [DOI] [PubMed] [Google Scholar]
- 19.Marchetti MA, Kiuru MH, Busam KJ, et al. Melanocytic naevi with globular and reticular dermoscopic patterns display distinct BRAF V600E expression profiles and histopathological patterns. Br J Dermatol. 2014;171(5):1060–1065. doi: 10.1111/bjd.13260. [DOI] [PubMed] [Google Scholar]
- 20.Michaloglou C, Vredeveld LC, Soengas MS, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436(7051):720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 21.Ross AL, Sanchez MI, Grichnik JM. Nevus senescence. ISRN Dermatol. 2011;2011:642157. doi: 10.5402/2011/642157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zalaudek I, Manzo M, Savarese I, Docimo G, Ferrara G, Argenziano G. The morphologic universe of melanocytic nevi. Semin Cutan Med Surg. 2009;28(3):149–156. doi: 10.1016/j.sder.2009.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.