Abstract
Iron participates in oxygen transport, energetic, metabolic, and immunologic processes. There are 2 main causes of iron overload: hereditary hemochromatosis which is a primary cause, is a metabolic disorder caused by mutations of genes that control iron metabolism and secondary hemochromatosis caused by multitransfusions, chronic hemolysis, and intake of iron rich food. The most common type of hereditary hemochromatosis is caused by HFE gene mutation. In this study, we analyzed iron metabolism in 100 healthy Polish children in relation to their HFE gene status. The wild-type HFE gene was predominant being observed in 60 children (60%). Twenty-five children (25%), presented with heterozygotic H63D mutation, and 15 children (15%), presented with other mutations (heterozygotic C282Y and S65C mutation, compound heterozygotes C282Y/S65C, C282Y/H63D, H63D homozygote). The mean concentration of iron, the level of ferritin, and transferrin saturation were statistically higher in the group of HFE variants compared with the wild-type group. H63D carriers presented with higher mean concentration of iron, ferritin levels, and transferrin saturation compared with the wild-type group. Male HFE carriers presented with higher iron concentration, transferrin saturation, and ferritin levels than females. This preliminary investigation demonstrates allelic impact on potential disease progression from childhood.
Key Words: children, HFE, iron, ferritin, transferrin saturation
Adequate iron availability is essential to human development and overall health. Iron is indispensable for numerous biological functions such as oxygen transport, cellular respiration, and immune response. Conversely, a high iron concentration is harmful to both cells and tissues.1,2 Hereditary hemochromatosis is an autosomal recessive disorder characterized by enhanced intestinal absorption of dietary iron. The syndrome is a result of genetically determined failure to stop iron entering the circulatory pool when it is not needed. It is associated with pathogenic mutations of at least 5 hemochromatosis genes: HFE (OMIM gene number 613609, phenotype MIM number 235200), TfR2 (OMIM gene number 604720, phenotype MIM number 604250), HJV (OMIM gene number 60837, phenotype MIM number 602390), HAMP (OMIM gene number 606464, phenotype MIM number 613313), and SLC40A1 (OMIM gene number 604653, phenotype MIM number 606069). In addition, it is likely due to a regulatory defect in iron homeostasis.3,4 HFE-related hemochromatosis (OMIM gene number 613609, phenotype MIM number 235200) is the most frequent form of the disease and the most common autosomal recessive disorder in Northern European populations, with a prevalence of 1:200 to 1:250 for homozygosity and a carrier rate of 1:8 to 1:12.5,6 This autosomal recessive disorder is characterized by enhanced intestinal absorption of iron leading to multiple organ damage such as cirrhosis, hepatoma, diabetes mellitus, arthritis, and cardiomyopathy. The symptomatic phenotype preceded by fatigue, arthropathy and impotence, occurs in homozygotic males predominantly between the fourth and sixth decade of life, depending on gene penetrance and multiple concomitant factors.7 Taking into consideration the widespread prevalence of HFE heterozygosity in populations of Northern European descent, H63D heterozygosity is more prevalent (23.6% to 31.1%) than C282Y heterozygosity (8.6% to 11.9%).8 Interestingly, adult heterozygotic HFE genotypes are associated with the progression of some diseases and may present increased serum iron and transferrin saturation.9–11 However, the intensity of iron storage in HFE carriers in the developmental age is still unknown.
The objective of this study was to consider iron metabolism in relation to HFE status in 100 Polish children, living in Poland. Despite the numerous adult studies that have been conducted, this population has not been studied before.12
MATERIALS AND METHODS
One hundred unrelated, that is, nonkindred, children of Polish origin aged from 4 to 18 years (58 boys and 42 girls, average age 11.4±4.19 y), were recruited for the study. There were no chronic disorders, that is, hemochromatosis or hemoglobinopathies in parental families. Parents denied following a vegetarian diet, iron pills administration, and meat consumption was adequate for the patients age. To exclude acute and chronic illnesses, all patients underwent physical examination, laboratory assays which included a full blood count with reticulocytosis and microscopic evaluation, protein C concentration, aspartate and alanine transaminases activity, bilirubin, creatinine levels, hepatitis B virus surface antigen and antibody against hepatitis C virus antibodies. Full blood count with reticulocytosis was performed using SYSMEX XE 2100, whereas, aspartate transaminase, alanine transaminase, bilirubin, creatinine levels, hepatitis B virus surface antigen, antibody against hepatitis C virus antibodies, ferritin, transferrin, and protein C concentration were performed using ARCHITECT CI 8200 (ABBOTT). All reagents used were supplied by the manufacturer.
Iron metabolism was also assessed by measuring iron concentration, ferritin, and transferrin saturation (SYSMEX XE 2100, Architect ci 8200, and Test 1 SDL). Abdominal ultrasonography was also performed and no abnormalities were revealed. Patients underwent genetic testing for HFE mutations using real-time polymerase chain reaction and melting curve analysis methods with the HFE H63D S65C C282Y (TibMolbiol) LightMix in-vitro diagnostics kit and LightCycler 2.0 Instrument (ROCHE). Two fragments of the HFE gene were amplified simultaneously: 163 bp fragment containing 2 polymorphisms: c.187C>G (63 H/D), c.193A>T (65 S/C), and 284 bp fragment containing the c.845G>A (282 C/Y) polymorphism. The genotype results were detected in 2 different optical canals based on melting temperatures. The mutant type 63D allele displayed higher temperature (65°C) than wild-type H63 and S65 allele (57°C) and the mutant 65C allele (52°C). The mutant 282Y allele displayed higher temperature (62°C) than the wild-type C282 allele (56°C). The patients’ genotype profiles were compared with the standards supplied by the manufacturer.
The study was officially approved by the Independent Bioethics Commission for Research in Gdansk NKBBN/409/2013 in accord with the Helsinki Declaration.
Statistical Analyses
Statistical analyses were performed using Statistica 10.0 for Windows (StatSoft 2011). Data are presented as mean±SE and were assessed using Mann-Whitney or nonparametric Kruskal-Wallis unpaired test where appropriate. For the analyses variables were assessed for normality with natural log transformations performed on the serum Fe, ferritin, and Ts variables. Significance for the Mann-Whitney and post hoc tests was set at 0.05.
RESULTS
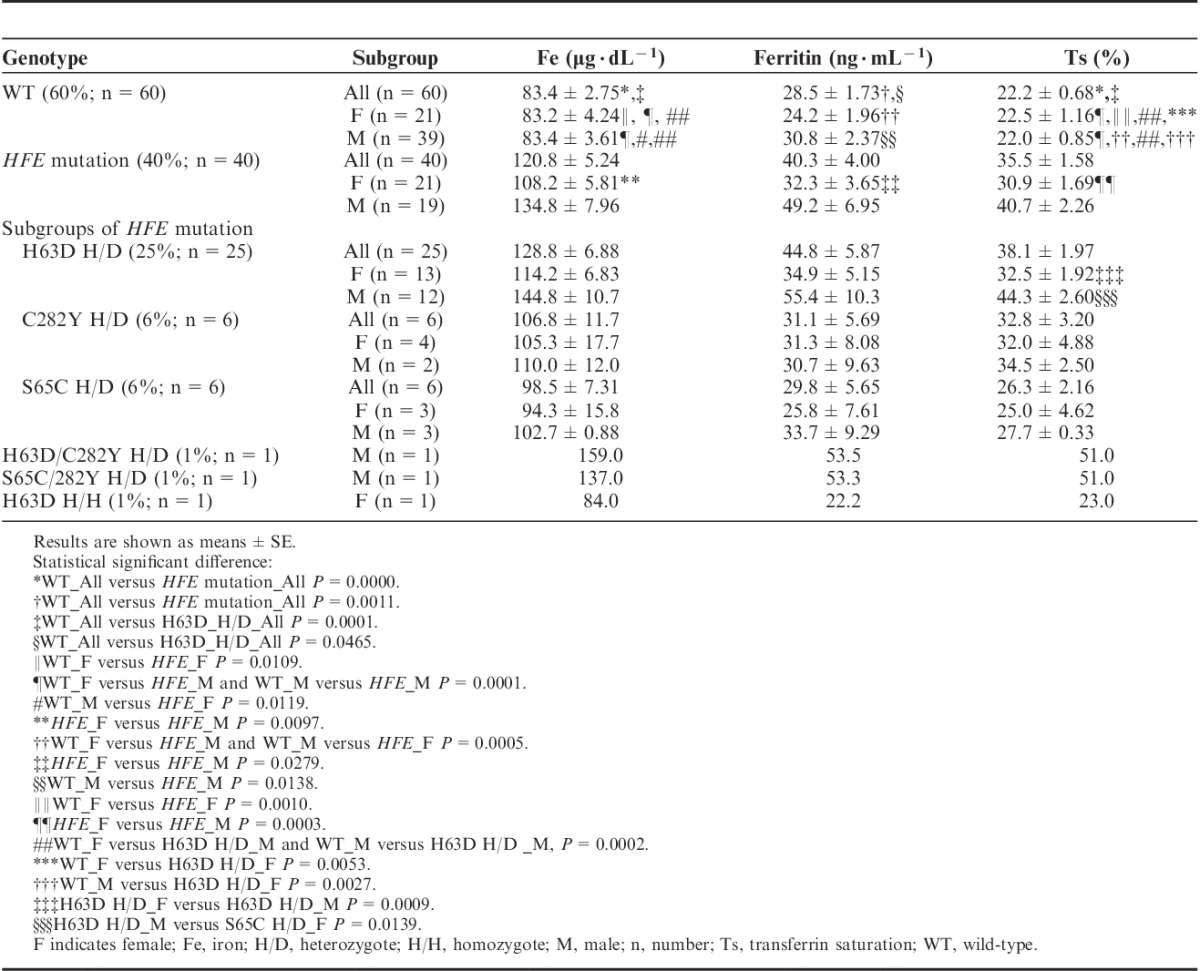
Molecular testing of 100 autochthonous, healthy Polish children, aged 11.4±0.42 years (58 boys and 42 girls) revealed a predominance of the wild-type HFE gene as 60 children (60%), presented with wild-type HFE. 25 children (25%), presented with heterozygotic H63D mutation, 6 children, presented with heterozygotic C282Y mutation and 6 children, presented with heterozygotic S65S mutation. Two boys were compound heterozygotes (C282Y/S65C, C282Y/H63D), whereas only 1 girl was a H63D homozygote (data presented in Table 1). In the entire group of tested children, laboratory assays performed excluded chronic diseases, abdominal ultrasound imaging visualized normal internal organs, and the mean concentration of iron, ferritin, and transferrin saturation were within normal range (mean values: iron 98.3±3.23 μg/dL, ferritin 33.2±1.98 ng/mL, transferrin saturation Ts, 27.5%±1.00%). The wild-type HFE gene group presented with a mean iron concentration of 83.4±2.75 μg/dL, ferritin 28.5±1.73 ng/mL, and transferrin saturation of 22.2%±0.68%. The groups which had other types of mutations (carriers of C282Y, H63D, S65C, compound heterozygotes, and H63 homozygote) had statistically higher mean iron concentration (120.8±5.24 μg/dL), mean ferritin levels (40.3±4.00 ng/mL), and mean transferrin saturation (35.5%±1.58%) than the wild-type group, data presented in Table 1. The group carrying the H63D mutation presented with a mean iron concentration of 128.8±6.88 μg/dL, ferritin levels of 44.8±5.87 ng/mL, and transferrin saturation of 38.1%±1.97%. Transferrin saturation was statistically higher in H63D carriers when compared with those of the wild-type group. All the values were statistically higher in the H63D mutation carriers when compared with those of the wild-type group. Moreover, mean transferrin saturation was statistically nonsignificant higher in the C282Y mutation carriers (Ts, 32.8%±3.20%) compared with that of the wild-type group (Ts, 22.2%±0.85%). Mean transferrin saturation of H63D mutation carriers (Ts, 38.1%±1.97%) was higher than that of the S65C mutation carriers (Ts, 26.3%±2.16%), whereas mean transferrin saturation of S65C mutation carriers (Ts, 26.3%±2.16%) was higher than that of the wild-type (Ts, 22.2%±1.16%). The mean transferrin saturation of the H63D and C282Y mutation carriers was higher than the normal range in children. The entire group of children (100 subjects) was also divided into four sex groups: wild-type males (39 subjects), wild-type females (21 subjects), male carriers of HFE mutation (19 subjects), and female carriers of HFE mutation (21 subjects) to evaluate presumptive iron metabolism variations (data presented in Table 1). There were no statistical differences in age, weight, height, and body mass index between sex groups. There were 11 girls who reported regular menstruation in the wild-type female group and 12 in the HFE group. Mean iron concentration, ferritin concentration, and transferrin saturation were statistically higher in the group of male carriers of HFE mutation (iron, 134.8±7.96 μg/dL; ferritin, 49.2±6.96 ng/mL; Ts, 40.7%±2.26%) than in the group of female carriers (iron, 108.2±5.81 μg/dL; ferritin, 32.3±3.65 ng/mL; Ts, 30.9%±1.69%), wild-type male group (iron, 83.4±3.61 μg/dL; ferritin, 30.8±2.37 ng/mL; Ts, 22.0%±0.85%), and wild-type female group (iron, 83.2±4.24 μg/dL; ferritin, 24.2±1.96 ng/mL; Ts, 22.5%±1.16%). Mean iron concentration and transferrin saturation of females carrying the HFE mutation were statistically higher than in wild-type male and wild-type female groups. The level of ferritin was statistically higher in male carriers of HFE mutation (49.2±6.95 ng/mL) than in female carriers (32.2±3.65 ng/mL), wild-type male group (30.8±2.37 ng/mL), and wild-type female group (24.2±1.96 ng/mL). The entire group of children (100 subjects) was also divided into sex subgroups according to different HFE status. There were 11 groups: wild-type males (39 subjects), wild-type females (21 subjects), male carriers of H63D mutation (12 subjects), female carriers of H63D mutation (13 subjects), male carriers of C282Y mutation (2 subjects), female carriers of C282Y mutation (4 subjects), male carriers of S65C mutation (3 subjects), female carriers of S65C mutation (3 subjects), a single male in the compound heterozygote H63D/C282Y group and S65C/282Y group, and a single female in the H63D homozygote group. Males carrying H63D mutation had statistically higher iron concentration (144.8±10.7 μg/dL) than both wild-type males (83.4±3.62 μg/dL) and females (83.2±4.24 μg/dL). H63D carrier males (55.4±10.3 ng/mL) had statistically nonsignificant higher ferritin concentration than wild-type females (24.2±1.96 ng/mL). Transferrin saturation was statistically higher in both sex groups of H63D carriers (males 44.3%±2.60%; females, 32.5%±1.92%) than in wild-type probands (males, 22.0%±0.85%; females, 22.5%±1.16%), all data are presented in Table 1.
TABLE 1.
Iron Status Parameters in Control and Various HFE Mutation Group
DISCUSSION
In the presented study we attempt to analyze iron metabolism in 100 unrelated, healthy children of Polish origin in relation to their HFE status. The frequency of H63D carriage in our study population was found to be 25%, whereas heterozygosity of C282Y and S65C gene was each 6%. A single patient was found to be a H63D homozygote, whereas 2 boys presented with compound heterozygosity. The prevalence of HFE-hemochromatosis alleles was compatible with other European population reports.5,6,8 Despite the fact that iron status is commonly evaluated in pediatric primary medical care, only deficiency usually elicits diagnostic procedures. Pathologic iron loading in primary hemochromatosis is a prolonged and unpredictable process and without proper treatment may result in multiorgan dysfunction.1–4 Thus early diagnosis, monitoring, and treatment are essential.
The statistical analysis revealed that mean concentration of iron, ferritin levels, and transferrin saturation were statistically higher in the group of various HFE-associated mutations compared with the wild-type group. A statistical difference in iron concentration, level of ferritin, and transferrin saturation was also noted between H63D carrier groups and wild-type groups. Furthermore, mean transferrin saturation of C282Y and S65C carrier groups was higher than in the wild-type group. It is important to note that in all the groups’ mean iron concentration and ferritin levels were within the normal range, whereas transferrin saturation in the various HFE mutation groups, (H63D and C282Y carriers) was elevated. Our data confirms the high sensitivity and diagnostic value of transferrin in detecting iron storage diseases even in childhood.7 Remarkably, the study showed statistically higher iron concentration and transferrin saturation in boys and girls who are carriers of HFE mutation than in wild-type subjects. In addition, boys carrying the HFE mutation presented with higher iron concentration, transferrin saturation, and ferritin level than girls carrying the same mutation. Notably, the presented data emphasizes the varying influence of HFE mutation with regard to sex even in the developmental age.
The impact of the HFE gene mutation on biochemical features of homozygotic and heterozygotic adults seems to be understandable,2,9–11,13 while its impact in childhood still needs to be accurately observed. In contrast, the clinical course of hemochromatosis is unpredictable since reduced gene penetration, possible concomitant environmental and epigenetic factors also play a role in disease development.14,15 All the more, knowing the HFE status of a child seems to be important as it will allow for close observation of affected children. Notable, there are reports which demonstrate that higher blood ferritin but still within the reference range, is associated with increased risk of some morbidities in adults. Zacharski et al16 revealed that 75% of newly diagnosed cancer cases have been diagnosed among patients who presented with mean ferritin levels of >57 ng/mL during follow-up. Similarly, Mainous et al17 reported that higher transferrin saturation correlated with an increased risk of overall all-cause mortality. In addition, a study performed on a young male demonstrated that blood ferritin higher than 150 ng/mL correlates negatively with cardiovascular fitness.18 Altogether, these data indicate that monitoring body iron stores can be recommended as part of a prevention strategy against diseases especially in the HFE mutation group.
Reports considering HFE hemochromatosis in children are uncommon. Researchers are particularly concerned about the validity of screening children or genotyping siblings and spouses. To date, multiple screening programmes: neonatal, descendant, and population have been suggested,11,19–23 but rare phenotypical manifestation, patient stigmatization and economical aspects have derailed progress. On the basis of the data that iron storage is a slow and prolonged process2 and children carrying HFE mutation do not present increased ferritin levels,24 Delatycki et al25 stated that C282Y homozygotes present with iron overload very rarely during the first 2 decades, and the only abnormality they present with is biochemical. However, the risk of iron accumulation cannot be excluded in instances of coexisting circumstances in childhood. Researchers6,26,27 described an association between HFE mutation variants, iron storage and concomitant disease progression. There are also preliminary reports considering the impact of HFE variants and susceptibility to acute lymphoblastic leukemia in childhood.6,28,29 Taking into consideration presented data and cited authors the impact of HFE mutation in childhood still needs further investigation.
CONCLUSIONS
In our study, we demonstrated variations in iron metabolism in relation to various HFE-associated mutations in children of Polish origin. The individual course of hemochromatosis seems to be unique which makes diagnostic procedures, monitoring and proper treatment unquestionable. This preliminary investigation demonstrates allelic impact on potential disease progression from childhood.
Footnotes
Supported by grant DS RIK/2016 funded by Gdansk University of Physical Education and Sport, Faculty of Rehabilitation and Kinesiology. B.K.-H. designed the study and wrote the paper. M.L. performed the research. J.A. designed and performed the research. W.Z., M.M., E.M., and J.J.K. performed the research. E.A.-D. contributed essentials reagents or tools.
The authors declare no conflict of interest.
REFERENCES
- 1.Pietrangelo A. Hereditary hemochromatosis-a new look at an old disease. N Eng J Med. 2004;350:2383–2397. [DOI] [PubMed] [Google Scholar]
- 2.Beutler E. Iron sortage disease: facts, fiction and progress. Blood Cell Mol Dis. 2007;39:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pietrangelo A. Molecular insights into the pathogenesis of hereditary haemochromatosis. Gut. 2004;55:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Gac G, Ferec C. The molecular genetics of hemochromatosis. Eur J Hum Genet. 2005;13:1172–1185. [DOI] [PubMed] [Google Scholar]
- 5.Merryweather-Clarke AT, Pointon JJ, Jouanolle AM, et al. Geography of HFE C282Y and H63D mutations. Genet Test. 2005;4:183–198. [DOI] [PubMed] [Google Scholar]
- 6.Lipshultz SE, Lipsitz SR, Kutok JL, et al. Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer. 2013;119:3555–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szoke D, Panteghanini M. Diagnostic value of transferin. Clin Chim Acta. 2012;413:1184–1189. [DOI] [PubMed] [Google Scholar]
- 8.Zaloumis SG, Allen KJ, Bertalli NA, et al. Natural history of HFE simple heterozygosity for C282Y and H63D: a prospective 12-year study. J Gastroenterol Hepatol. 2015;30:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers V, Sutherland L, Palmer K, et al. Haemochromatosis-associated HFE genotypes in English blood donors: age-related frequency and biochemical expression. J Hepatol. 2003;39:925–931. [DOI] [PubMed] [Google Scholar]
- 10.Datz C, Haas T, Rinner H, et al. Heterozygosity for the C282Y mutation in the hemochromatosis gene is associated with increased serum iron, transferrin saturation, and hemoglobin in young women: a protective role against iron deficiency? Clin Chem. 1998;44:2429–2432. [PubMed] [Google Scholar]
- 11.Cadet E, Capron D, Gallet M, et al. Reverse cascade screening of newborns for hereditary haemochromatosis: a model for other late onset diseases? J Med Genet. 2005;42:390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandok N, Speechley M, Ainsworth PJ, et al. The impact of population-based screening studies on hemochromatosis screening practices. Dig Dis Sci. 2012;57:1420–1422. [DOI] [PubMed] [Google Scholar]
- 13.Cogswell ME, Gallagher ML, Steinberg KK, et al. HFE genotype and transferrin saturation in the United States. Genet Med. 2003;5:304–310. [DOI] [PubMed] [Google Scholar]
- 14.Adams PC, Walker AP, Acton RT. A primer for predicting risk of disease in HFE-linked hemochromatosis. Genet Test. 2001;5:311–316. [DOI] [PubMed] [Google Scholar]
- 15.Beutler E, Felitti VJ, Koziol JA, et al. Penetrance of the 845G6A (282Y) HFE hereditary hemochromatosis mutation in the USA. Lancet. 2002;359:211–218. [DOI] [PubMed] [Google Scholar]
- 16.Zacharski LR, Chow BK, Howes PS, et al. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst. 2008;100:996–1002. [DOI] [PubMed] [Google Scholar]
- 17.Mainous AG, III, Gill JM, Carek PJ. Elevated serum transferrin saturation and mortality. Ann Fam Med. 2004;2:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mainous AG, III, Wells BJ, Everett CJ, et al. Association of ferritin and lipids with C-reactive protein. Am J Cardiol. 2004;93:559–562. [DOI] [PubMed] [Google Scholar]
- 19.Deugnier Y, Jouanolle AM. Screening for hereditary HFE hemochromatosis. Presse Med. 2007;36:1292–1294. [DOI] [PubMed] [Google Scholar]
- 20.El-Serag HB, Inadomi JM, Kowdley KV. Screening for hereditary hemochromatosis in siblings and children of affected patients. Ann Int Med. 2000;132:261–269. [DOI] [PubMed] [Google Scholar]
- 21.Allen K, Williamson R. Screening for hereditary hemochromatosis should be implemented now. BJM. 2000;320:183–184. [PMC free article] [PubMed] [Google Scholar]
- 22.Adams PC. Implications of genotyping of spouses to limit investigation of children in genetic hemochromatosis. Clin Genet. 1998;53:176–178. [DOI] [PubMed] [Google Scholar]
- 23.Basset M, Dunn C, Battese K, et al. Acceptance of neonatal genetic screening for hereditary hemochromatosis by informed parents. Genet Test. 2001;5:317–320. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins MR, Ettinger AS, Hernandez-Avila M, et al. Variants in iron metabolism genes predict higher blood lead levels in young children. Environ Health Perspect. 2008;116:1261–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delatycki MB, Powell LW, Allen KJ. Hereditary hemochromatosis genetic testing of at-risk children; what is the appropriate age? Genet Test. 2004;8:98–103. [DOI] [PubMed] [Google Scholar]
- 26.Sharma V, Panigrahi I, Dutta P, et al. HFE mutation H63D predicts risk of iron over load in thalassemia intermedia irrespective of blood transfusions. Indian J Pathol Microbiol. 2007;50:82–85. [PubMed] [Google Scholar]
- 27.Kaczorowska-Hac B, Maciejka-Kapuscinska L, Milosz-Bartoszewicz E, et al. Co-existence of β thalassemia trait and hemochromatosis in 5-year-old girl of Polish origin. JPHO. 2013;35:239–240. [DOI] [PubMed] [Google Scholar]
- 28.Dorak MT, Mackay RK, Relton CL, et al. Hereditary hemochromatosis gene (HFE) variants are associated with birth weight and childhood leukemia risk. Pediatr Blood and Cancer. 2009;53:1242–1248. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-López R, Donoso M, Fernández-Cavada M, et al. Diagnostic utility of HFE variants in Spanish patients: association with HLA alleles and role in susceptibility to acute lymphoblastic leukemia. Gene. 2013;514:31–35. [DOI] [PubMed] [Google Scholar]


