Abstract
Brucella spp. infection results in compromised Type1 (Th1) cellular immune response. Several reports have described an immunomodulatory function for Brucella major outer membrane protein Omp25. However, the mechanism by which Omp25 modulates macrophage dysfunction has not been defined. Herein, we reported that Omp25-deficient mutant of Brucella suis exhibited an enhanced ability to induce interleukin (IL)-12 whereas ectopic expression of Omp25 protein inhibited TLR agonists-induced IL-12 p70 production through suppression of both IL-12 p40 and p35 subunit expression in THP-1 cells. In addition, Omp25 significantly upregulated miR-155, -23b and -21-5p, as well as the immunomodulator molecule programmed death-1 (PD-1) in monocyte/macrophages. The upregulation of miR-155 and -23b correlated temporally with decreased TAB2 levels, IκB phosphorylation and IL-12 p40 levels by targeting TAB2 and il12B 3′ untranslated region (UTR), respectively, while miR-21-5p increase directly led to the reduction of lipopolysaccharide (LPS)/R848-induced IL-12 p35 protein by targeting il12A 3′UTR. Consistent with this finding, reduction of miR-155 and -23b attenuated the inhibitory effects of Omp25 on LPS/R848-induced IL-12 p40 expression at both transcriptional and posttranscriptional levels, while reduction of miR-21-5p attenuated the inhibitory effects of Omp25 on LPS/R848-induced IL-12 p35 expression at the posttranscriptional level, together significantly enhanced IL-12 p70 production upon LPS/R848 stimulation. We also found that blocking PD-1 signaling decreased the expression of miR-155, -23b and -21-5p induced by Omp25 and enhanced IL-12 production in monocyte/macrophages. Altogether, these data demonstrate that Brucella Omp25 induces miR-155, -23b and -21-5p to negatively regulate IL-12 production at both transcriptional and posttranscriptional levels via regulation of PD-1 signaling, which provides an entirely new mechanism underlying monocyte/macrophages dysfunction during Brucella spp. infection.
Keywords: cytokine, Omp25, miRNA, macrophage, signaling
Introduction
Brucella spp. is an intracellular pathogen that resides mainly in monocyte/macrophages and causes disease in humans and livestocks (1–3). Resistance to Brucella spp. relies on cell-mediated immunity, which involves the activation of antigen-presenting cells (macrophages and dendritic cells) and the subsequent activation of antigen-specific CD4+ and CD8+ T cells (4, 5). In this process, the production of T helper type 1 (Th1) cytokines and an adequate Th1 immune response are critical for the clearance of Brucella infection (6, 7). Studies on experimental and human brucellosis indicate that interleukin (IL)-12 is the principal cytokine active against Brucella infection (8–10). However, Brucella has evolutionarily developed diverse evasion strategies to interfere the host’s innate and adaptive immunity in order to establish a long-term infection (11). Disturbances of IL-12 production and Th1 response have been described in patients with chronic brucellosis and are associated with poor outcome, but relative mechanisms remain largely unknown (5, 12).
The major outer membrane protein Omp25 is highly conserved across Brucella species, biovars, and strains (13). Several reports have shown that administration of Omp25 DNA vaccine or recombinant Omp25 is protective against the virulent Brucella melitensis or Brucella abortus challenge in mice (14, 15), which makes Omp25 as a viable vaccine target. On the other hand, however, Omp25 has also been reported to be involved in virulence of B. melitensis, B. abortus, and Brucella ovis (16). Brucella species lacking Omp25 have been shown to be attenuated in mice as well as cattle (17, 18). Live Brucella spp. fail to induce tumor necrosis factor alpha (TNF-α) (19), whereas Omp25 null mutants of Brucella suis (Δomp25 B. suis) exhibit the ability to activate human macrophages to secrete TNF-α, suggesting that Omp25 is involved in inhibition of TNF-α production during infection of human macrophages (20). Similarly, the presence of Omp25 in wild-type (WT) Brucella spp. is correlated with the unusual absence of TNF-α and IL-12 in human dendritic cells, which prevents human dendritic cell maturation and antigen presentation (5). Whereas treatment of Brucella-susceptible mice with IL-12 increases primary and secondary immunity (8), the molecular mechanism(s) by which Brucella Omp25 inhibits IL-12 production has not been defined.
In the present work, we evaluated the effects of Omp25 on the Toll-like receptor 4 and 7/8 agonist lipopolysaccharide (LPS)/R848-induced IL-12 expression in monocyte/macrophages (M/MΦs). We found that ectopic expression of Omp25 protein inhibited TLR agonists-induced IL-12 p70 production through suppression of both IL-12 p40 and p35 subunit expression in THP-1 cells. MiR-155, -23b and -21-5p were upregulated in Omp25-expressing cells, as well as in WT B. suis-infected human M/MΦs, and were identified to be involved in the inhibition of LPS/R848-induced IL-12 through inhibiting the expression of IL-12 p35 and p40 at transcriptional and posttranscriptional levels, respectively. We also found that Omp25 induced programmed death-1 (PD-1) expression that is inversely associated with IL-12 production in human M/MΦs. Blocking of PD-1 signaling decreased miR-155, miR-21-5p and miR-23 levels and enhanced IL-12 production.
Materials and Methods
Cell Isolation and Culture
Peripheral blood mononuclear cells (PBMCs) were prepared from healthy adult donors by centrifugation over Ficoll-Histopaque (Sigma, St. Louis, MO, USA) as described (21). All the donors had signed an informed consent before their blood was used in this study. M/MΦs cells were enriched from PBMCs using Ficoll-Percoll gradients (GE Healthcare) and further purified by anti-CD14 magnetic beads with column purification according to the manufacturer’s instructions (Miltenyi Biotec; purity of cells >95%). The enriched cells were cultured in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum (FBS, Hyclone).
The human monocytic cell line THP-1 and HEK-293 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). THP-1 were maintained in RPMI 1640 medium and HEK-293 cells were maintained in Dulbecco’s minimum essential medium, both supplemented with 10% FBS at 37°C with 5% CO2.
Bacterial Strains and Recombinant Adenovirus Preparation
Brucella suis 1330 (ATCC 23444) and its derived mutants were cultured in tryptic soy broth at 37°C. Omp25-deficient mutant (Δomp25 B. suis) were obtained using suicide plasmid PCVD442 carrying omp25 gene interrupted by kanamycin resistance gene as previously described (20). In this study, all live Brucella experiments were performed in biosafety level 3 facilities according to standard procedures. Either deleted omp25 gene or expressed Omp25 protein was omp25, not omp25b, omp25c, omp23d, or omp22 (22). The native omp25 gene (GenBank No. U39397.1) was amplified by PCR from B. suis 1330 using omp25 specific Primers with Flag tag encoding sequence (Table S1 in Supplementary Material), then omp25 gene was inserted into the vector pShuttle-CMV (Clontech) to construct the adenoviral shuttle vector pShuttle-CMV-Omp25. The linearized pShuttle-CMV-Omp25 and pShuttle-CMV were transformed into BJ5183-AD-1 by electroporation to recombine with pAd-Easy-1, followed by amplification and extraction of the recombinant vector named pAd-Omp25 and pAd-Blank. The recombinant adenovirus named recombinant adenoviruses expressing Omp25 protein (rAd-Omp25) and rAd-Blank were obtained by transferring the linearized pAd-Omp25 and pAd-Blank to HEK-293 cells. The titer of rAd-Omp25 and rAd-Blank were measured by the method of TCID50 as described in previous studies (23).
Brucella Infection and Intracellular Survival Assay
Human THP-1 cells were cultured with 100 nM 1,25-dihydroxyvitamin D3 (VD3, Sigma) for 72 h before Brucella infection as previously described (24). THP-1 cells and human M/MΦs were infected with B. suis as previously described (24, 25). Briefly, 1 × 106 cells/ml or 2 × 105 cells/ml of VD3-treated THP-1 cells or human M/MΦs were cultured in RPMI 1640 medium with 10% heat-inactivated FBS and infected with B. suis 1330 or mutated B. suis strains at a multiplicity of infection of 50 for 1 h at 37°C in a 5% CO2 atmosphere. At the end of the incubation time (time 0 p.i.), cells were washed three times with sterile PBS to remove uninternalized bacteria, and then cells were cultured in standard medium with 50 µg/ml of gentamicin (Sigma) and 50 µg/ml of streptomycin (Sigma) to kill remaining extracellular bacteria.
To monitor Brucella survival in cells, infected cells (5 × 105 cells/well) were washed with PBS and lysed in 0.2% Triton X-100 (Sigma). Serial dilutions of lysates were rapidly plated on tryptic soy agar plates to count and calculate the number of intracellular viable bacteria in CFU per well.
MiRNA Target Predictions
Sequences of predicted mature human miRNAs were obtained from miRBase.1 Il12A and il12B sequences were from the National Center for Biotechnology Information Nucleotide database.2 MiRNAs predicted to bind to 3′ untranslated regions (UTRs) of il12A and il12B genes were selected by target analysis through the prediction algorithms Targetscan human and RNAhybrid.
Quantitative PCR
MicroRNAs and IL-12 p40 or p35 subunit mRNA was quantified by quantitative polymerase chain reaction (Q-PCR). Total cellular RNA was isolated by TRIZOL according to the manufactory’s protocol (26). RNA concentration and purity were measured using a NanoDrop spectrophotometer (Thermo), and equal amounts of RNA (10 ng for miRNA or mRNA) were used for Q-PCR analysis. MiRNA and mRNA levels were detected by SYBR Green kit using Bio-Rad IQ5 Real-time system and calculated using RNU6B and β-actin as endogenous control, respectively, following the 2−ΔΔCt method. Primers used in this assay were presented in Table S1 in Supplementary Material.
Western Blotting Analysis
Cells were lysed in RIPA plus protease/phosphorylase inhibitors (Sigma) on ice. The cytosol and nuclear fraction were isolated using NE-PER Nuclear and Cytoplasmic Extraction Reagents according to the manufactory’s instruction (Thermo). Protein from each extract were separated on 4–12% SDS-PAGE and transferred to polyvinyl difluoride membranes (Millipore). After blocking with TBS plus 0.05% Tween 20 containing 5% non-fat milk for 1 h, the membranes were incubated with specific primary antibodies at 4°C overnight. Primary antibodies include anti-IL-12 p40/p35 (Santa Cruz), anti-TAB2, anti-phospho-IκB, anti-IκB, anti-p65, anti-TRAF6, anti-IRAK1, anti-IRAK2, anti-Histone H3 (Cell Signaling Technology), anti-FLAG M2 (Sigma), anti-Omp25, and anti-β-actin (Tianjin Sungene Biotech) antibodies. HRP-conjugated anti-mouse IgG or anti-rabbit IgG (Santa Cruz) were used as secondary antibodies. Pierce® Fast Western Blot Kit, ECL Substrate (Thermo) was used for chemiluminescent detection.
Luciferase Reporter Assays
Human il12A or il12B promoter sequence was cloned into pGL3 basic vector (Promega) to construct the promoter report vector pGL-il12A, pGL-il12B using specific primers (Table S1 in Supplementary Material). The pGL3 basic vector served as a control. THP-1 cells were transfected with a mixture of pGL-il12A, pGL-il12B, or NF-κB activity reporter plasmid and pRL-TK-renilla-luciferase plasmid (Promega, for normalization) using Amaxa nucleofection technology (Human monocyte Nucleofector Kit, Lonza). Cells were then infected with rAd-Blank or rAd-Omp25. At 24 h post infection, luciferase activities were determined via Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions.
The full-length 3′ UTR of the human il12A gene (GenBank accession no. NM_000882) or il12B gene (GenBank accession no. NM_002187) was amplified from human THP-1 cDNA using specific primers (Table S1 in Supplementary Material) and cloned into pMIR-REPORT Luciferase vector (Ambion). The segment (base pairs 984–1450) of the il12A 3′UTR containing the mutated miR-21-5p target sequence (ATAAGCT to TATTCGA) and the segment (base pairs 1030–2347) of the il12B 3′UTR containing the mutated miR-23b target sequence (AATGTGA to TTACACT) were also cloned into the pMIR-REPORT Luciferase vector (Ambion). The primers for mutants were also showed in Table S1 in Supplementary Material. HEK-293 cells were transfected with the indicated luciferase reporter plasmid (500 ng), pRL-TK-Renilla-luciferase plasmid (100 ng), and the indicated miRNA mimics (final concentration, 10 or 50 nM) by Lipofectamine 2000 (Invitrogen). At 48 h post transfection, cellular extracts were prepared and measured luciferase activities by the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s instructions.
Flow Cytometry and Cytokine Detection
Cell surface molecules and IL-12 expression in monocyte/macrophages were determined by flow cytometric analysis as described previously (27, 28). Briefly, 1 × 106 cells were infected with WT B. suis, Δomp25 B. suis, rAd-Omp25 or rAd-Blank for 24 or 48 h to harvest the cells for immune staining. Cells were first stained for cell surface molecules using APC-anti-human CD14 Ab and PE-anti-PD-1 or PE-anti-PD-L1 Ab (Biolegend) in staining buffer and were then treated with fixation and permeabilization buffer (Biolegend) for intracellular staining according to the manufacturer’s instruction. Stained cells were analyzed by flow cytometry (BD Accuri C6) and further analyzed on flowJo V7.6 software as previous (29). Commercial IL-12 p70 ELISA kits (Biolegend) were used to measure the levels of IL-12 p70 in cell-free culture supernatants according to the manufacturer’s instructions.
Statistical Analysis
All experiments were performed at least three times, and results are representative of three independent experiments. Prism software (GraphPad Software) was used for statistical analysis. Data were presented as means ± SEM. Statistical comparisons of the results were made using ANOVA followed by Bonferroni post hoc test or performed by unpaired Student’s t-test. Pearson’s correlation coefficients were calculated to examine the correlations between PD-1+ or PD-L1+ cells with IL-12+ cells. P < 0.05 or less was considered significant.
Results
Omp25-Deficient Mutant of B. suis Exhibit an Enhanced Ability to Induce IL-12 in Infected THP-1 Cells
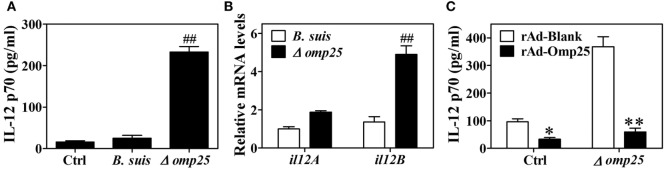
Omp25 is an outer membrane protein on the surface of Brucella, and Omp25-deficient mutants have been shown to exhibit enhanced ability to secret TNF-α compared with WT Brucella (5, 20). Since IL-12 is also an important cytokine for clearance of intracellular pathogens, we investigated the effects of Omp25 on the expression of IL-12. We first confirmed the presence of Omp25 in WT B. suis-infected cells and the absence of Omp25 in the corresponding Omp25-deficient mutant (Δomp25 B. suis)-infected cells (Figure S1 in Supplementary Material). Next, we observed the intracellular survival of WT B. suis and Δomp25 B. suis, and compared the production of IL-12 in WT B. suis- and Δomp25 B. suis-infected human THP-1 cells. As would be expected, the numbers of intracellular viable bacteria were similar for WT and Δomp25 B. suis infection (Figure S2 in Supplementary Material), but Δomp25 B. suis-infected cells produced more IL-12, ranging from 210 to 256 pg/ml, than that of WT (Figure 1A, P < 0.01). Similarly, the level of IL-12 p40 mRNA was higher in the Δomp25 B. suis-infected cells than that in WT B. suis-infected cells (Figure 1B), suggesting that the absence of Omp25 might improve B. suis’s ability to induce IL-12. To further confirm whether Omp25 play a key role in inhibition of IL-12 production, THP-1 cells were infected with recombinant adenoviruses rAd-Omp25 to express Omp25, and then infection of Δomp25 B. suis and measured the production of IL-12. Results showed that the IL-12 p70 production of rAd-Omp25-infected cells was significantly lower than that of control blank adenoviruses (rAd-Blank)-infected cells upon Δomp25 B. suis infection (Figure 1C), in which cells were infected with rAd-Omp25 to complement Omp25 (Figure S1 in Supplementary Material). These results indicated that the presence of Omp25 inhibited B. suis’s ability to induce IL-12.
Figure 1.
The Δomp25 Brucella suis mutant induces interleukin (IL)-12 production in human THP-1 cells. (A) THP-1 cells were infected with wild-type (WT) B. suis, Omp25 mutant (Δomp25) or were uninfected (Ctrl) as described in Section “Materials and Methods,” and cells were cultured for 24 h in complete culture medium. The concentrations of IL-12 p70 in cell supernatants were determined by ELISA. (B) THP-1 cells were infected as in (A) and cultured for 6 h, followed by quantitative polymerase chain reaction detection of il12A (p35) and il12B (p40) mRNAs levels in THP-1 cells. (C) THP-1 cells were infected with 100 multiplicity of infection of recombinant adenoviruses expressing Omp25 protein (rAd-Omp25) or rAd-Blank for 24 h and then infected with Δomp25 for another 24 h or uninfected (Ctrl), followed by ELISA detection of IL-12 p70 in cell supernatants. Each value represents means ± SEMs of three independent experiments. ##P < 0.01 versus WT B. suis-infected cells. *P < 0.05, **P < 0.01 versus rAd-Blank-infected cells in same treatment.
Omp25 Inhibits LPS/R848-Induced IL-12 Production in THP-1 Cells
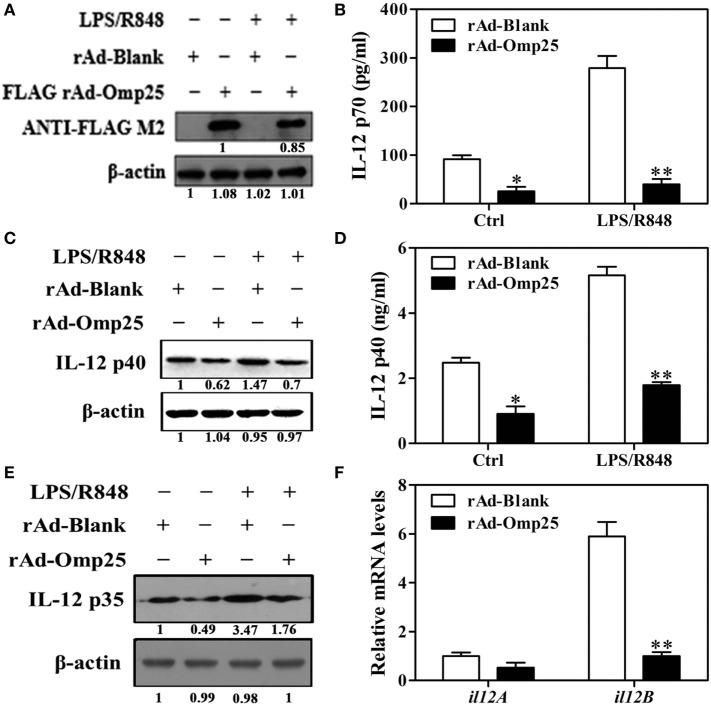
To investigate the direct effects of Omp25 protein on IL-12 p70 production in macrophage, we examined the LPS/R848-induced IL-12 p70 production in THP-1 cells infected with rAd-Omp25 or control blank adenoviruses (rAd-Blank). We found that Omp25 was stably expressed in the THP-1 cells infected with rAd-Omp25 (Figure 2A), which inhibited IL-12 p70 production induced either by LPS/R848 or recombinant adenoviruses themselves (Figure 2B). We also observed that the expression of IL-12 p35 and p40 subunit induced by LPS/R848 was lower in rAd-Omp25-infected cells compared to that in rAd-Blank-infected cells (Figures 2C–E). Similarly, upon LPS/R848 stimulation, the il12B (p40) mRNA level of rAd-Omp25-infected cells was lower than that of rAd-Blank-infected cells, but il12A (p35) mRNA level was not significantly difference between these two cells (Figure 2F). These results indicated that Omp25 can directly inhibit the production of LPS/R848-induced IL-12 p70 through suppressing p40 subunit expression at both protein and mRNA levels, yet suppressing p35 subunit only at protein level in THP-1 cells. Our data also suggested that the expression of both p35 and p40 subunits are regulated at the posttranscriptional level in Omp25-expressed cells.
Figure 2.
Omp25 inhibits lipopolysaccharide (LPS)/R848-induced interleukin (IL)-12 expression in THP-1 cells. (A) Expression of Omp25 in THP-1 cells infected with recombinant adenoviruses expressing Omp25 protein (rAd-Omp25) or rAd-Blank. THP-1 cells were infected with 100 multiplicity of infection of rAd-Omp25 or rAd-Blank for 24 h and then treated with LPS/R848 or PBS for 24 h, followed by western blot detection. (B) Omp25 inhibits LPS/R848-induced IL-12 p70 secretion in THP-1 cells. Cells were infected and treated as in (A), and supernatant IL-12 p70 were measured by ELISA. (C–E) Omp25 inhibits LPS/R848-induced IL-12 p35 and p40 in THP-1 cells. Cells were infected and treated as in (A); and the levels of IL-12 p40 (C,D) or IL-12 p35 (E) were detected by ELISA and western blot. (F) Omp25 decreases il12B (p40) mRNAs in THP-1 cells. THP-1 cells were infected rAd-Omp25 or rAd-Blank for 24 h and stimulated by LPS/R848 for 6 h followed by quantitative polymerase chain reaction detection of il12A and il12B mRNA levels. The results are means ± SEMs of three independent experiments. *P < 0.05, **P < 0.01 versus rAd-Blank-infected cells in same treatment.
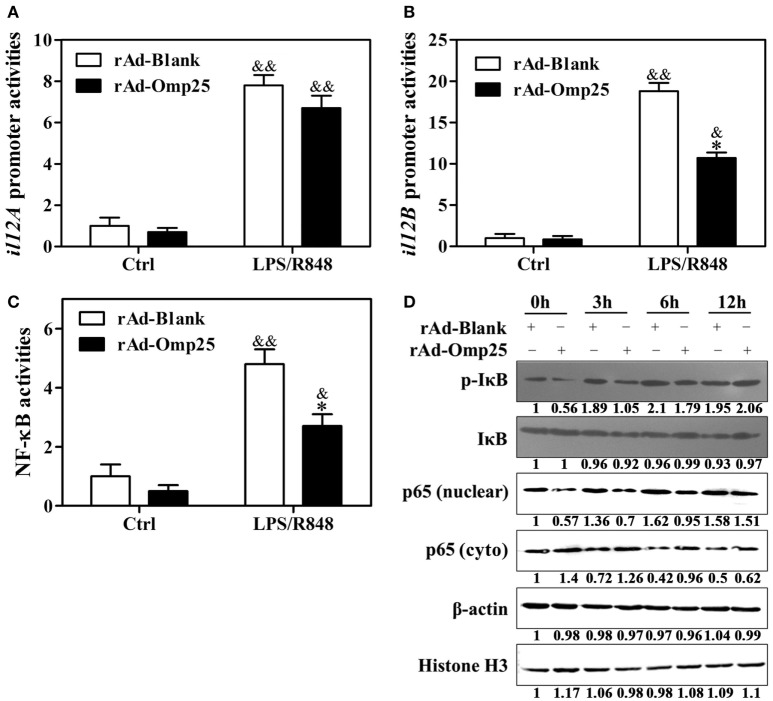
Omp25 Inhibits the Activation of NF-κB Pathway to Attenuate IL-12 p40 but Not IL-12 p35 Transcription
To investigate the effects of Omp25 on the transcription of p35 and p40 subunit, we examined the promoter activities of il12A and il12B and NF-κB transcriptional activities in rAd-Omp25-infected cells and rAd-Blank-infected cells. Omp25 expression did not appear to affect IL-12 p35 transcription (Figure 3A), but did inhibit IL-12 p40 transcription (Figure 3B). Upon LPS/R848 treatment, the transcriptional activities of NF-κB were lower in rAd-Omp25-infected cells than that in rAd-Blank-infected cells (Figure 3C). In line with the changes of NF-κB activities, the phosphorylation levels of IκB and the levels of nuclear NF-κB p65 unit were lower in rAd-Omp25-infected cells than that in rAd-Blank-infected cells at 0, 3, and 6 h after LPS/R848 treatment (Figure 3D). Given the key roles of NF-κB p65 unit in the transcription of il12B, these results further demonstrated that Omp25 inhibits the expression of p40 subunit but not p35 subunit at the transcriptional level.
Figure 3.
Omp25 inhibits the activation of NF-κB pathway to attenuate interleukin-12 p40 transcription. (A–C) THP-1 cells were infected with rAd-Blank or recombinant adenoviruses expressing Omp25 protein (rAd-Omp25) for 24 h and then treated with lipopolysaccharide (LPS)/R848 for another 24 h. The promoter relative activities of il12A (A) and il12B (B) and the transcriptional relative activities of NF-κB (C) were measured. The results are means ± SEMs of three independent experiments. *P < 0.05 versus rAd-Blank infected cells; &P < 0.05, &&P < 0.01 versus control (Ctrl). (D) THP-1 cells were infected with 100 multiplicity of infection rAd-Blank or rAd-Omp25 for 24 h. Western blotting was used to examine the distribution of cytoplasmic and nuclear p65 at indicated times following treatment with LPS/R848 for another 0, 3, 6, and 12 h; similar treatments applied to p-IκB and IκB.
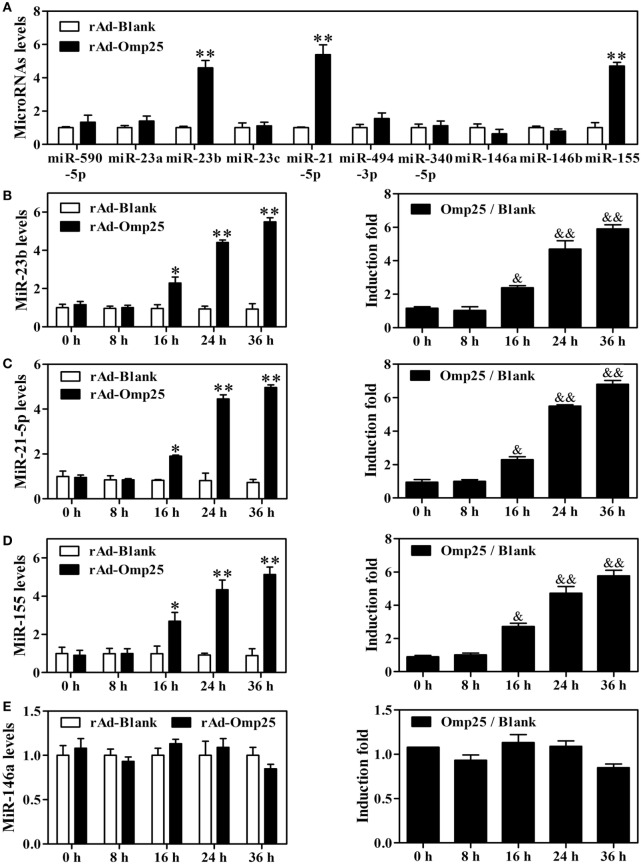
Omp25 Upregulates miR-21-5p, miR-23b, and miR-155 in THP-1 Cells
To investigate the potential role of microRNAs in regulating IL-12 p40 and p35 in Omp25-expressed cells, we used multiple prediction algorithms, including Targetscan human, miRanda, and RNA hybrid, to search for conserved microRNAs whose target sites exist in the il12A and il12B 3′ UTRs of human and other mammals. In total, seven miRNA–mRNA target duplexes were predicted by these algorithms (Figure S3 in Supplementary Material), including miR-590-5p, miR-21-5p, miR-340-5p, miR-23a, miR-23b, miR-23c, and miR-494-3p. To determine which miRNAs might be regulated by Omp25 in THP-1 cells, we examined the expression profile of these miRNAs, as well as some other miRNAs that have been reported to regulate NF-κB signaling, such as miR-146a, miR-146b, and miR-155. Q-PCR profiling of 10 miRNAs revealed that miR-21-5p, miR-23b and miR-155 were higher in rAd-Omp25-infected cells than that in control rAd-Blank-infected cells (Figure 4A). This result suggested that these three miRNAs might be involved in the regulation of IL-12 expression in Omp25-expressed-THP-1 cells.
Figure 4.
Omp25 upregulates miR-155, miR-23b and miR-21-5p expression in THP-1 cells. (A) Effects of Omp25 expression on the expression profiles of microRNAs in THP-1 cells. THP-1 cells were infected with recombinant adenoviruses expressing Omp25 protein (rAd-Omp25) or rAd-Blank for 24 h, and indicated miRNAs were measured by Quantitative PCR and normalized to the expression of RNU6B in each sample. (B–E) The relative expression of miR-23b (B), miR-21-5p (C), miR-155 (D) and miR-146a (E) levels were analyzed by quantitative polymerase chain reaction. Omp25/Blank represents the fold changes in RNA levels as calculated by comparing the value of rAd-Omp25-infected cells to that of rAd-Blank-infected cells in parallel. The results are means ± SEMs of three independent experiments. *P < 0.05, **P < 0.01 versus rAd-Blank-infected cells; &P < 0.05, &&P < 0.01 versus 0 h uninfected cells.
To identify the regulatory roles of miR-21-5p, miR-23b, and miR-155 in the inhibitory effects of Omp25 in THP-1 cells, we first examined the characteristics of miR-21-5p, miR-23b and miR-155 in THP-1 cells after expression of Omp25. A detailed time-course experiment showed that miR-23b, miR-155 and miR-21-5p levels were significantly increased at 16, 24 and 36 h as shown in the Figures 4B–D left panels after infecting rAd-Omp25, respectively. To further understand the kinetics of these miRNAs in the process of Omp25 expression, the fold changes of miRNAs expression were calculated by comparing the value of Omp25-expressing cells to that of control cells without Omp25 in parallel. MiR-23b showed an average increase of 2.4-fold after 16 h, rising to 5.9-fold by 36 h (Figure 4B right panel). MiR-21-5p expression showed increases of 2.3-fold by 16 h of infection and further increased at 24 and 36 h after infection (Figure 4C right panel). MiR-155 increased 2.7-fold by 16 h of infection and further increased by 4.72-fold and 5.77-fold at 24 and 36 h, respectively (Figure 4D right panel). However, no significant change was observed for the expression of miR-146a, which served as a negative control (Figure 4E). These results confirmed that the expressions of miR-21-5p, miR-23b and miR-155 are induced by Omp25, consistent with the original screen data shown in Figure 4A.
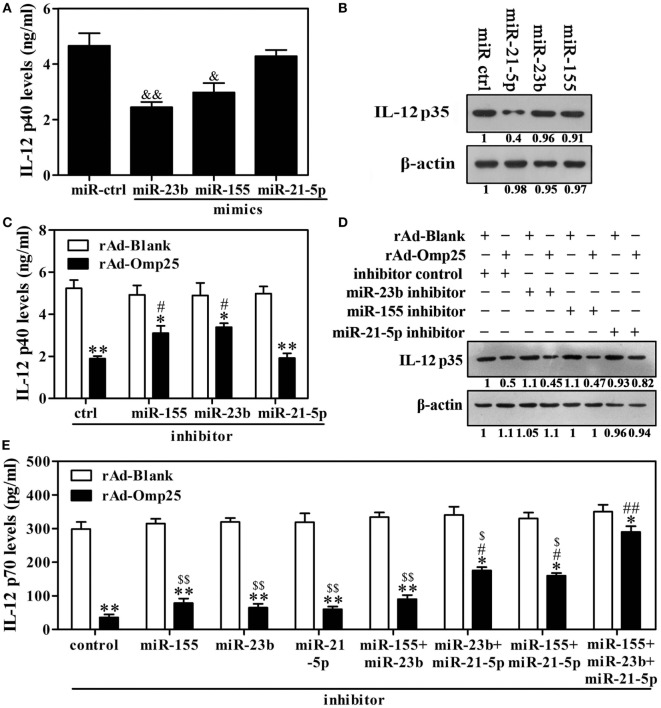
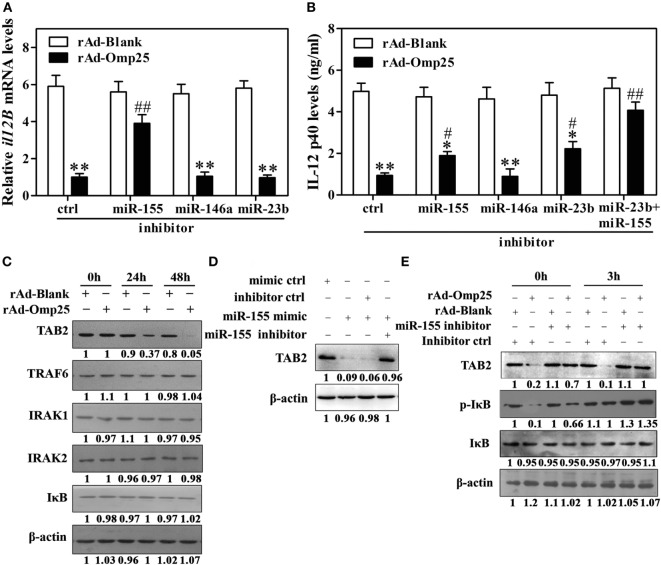
Downregulation of miR-23b, miR-21-5p, and miR-155 Blocks Omp25 Inhibitory Effect on LPS/R848-Induced IL-12 p70 Production
Given above results showing that Omp25 inhibits LPS/R848-induced IL-12 expression and upregulates miR-21-5p, miR-23b and miR-155 in THP-1 cells, we hypothesized that miR-21-5p, miR-23b and miR-155 likely played important roles in the Omp25 inhibition of LPS/R848-induced IL-12 p70 production. To test this hypothesis, we transfected cells with miR-21-5p mimics, miR-23b mimics or miR-155 mimics and stimulated the transfected cells with LPS/R848 for 24 h. IL-12 p40 was significantly decreased in the cells transfected with miR-23b mimics or miR-155 mimics, but not in the miR-21-5p mimics-transfected cells (Figure 5A). IL-12 p35 was significantly decreased in the cells transfected with miR-21-5p mimics, but not in the miR-23b or miR-155 mimics-transfected cells (Figure 5B). After treatment with miR-21-5p, miR-23b or miR-155 inhibitors, the expression of the respective miRNAs was downregulated over 2.5-fold, compared with control-transfected cells (Figure S4 in Supplementary Material). Inhibition of miR-23b or miR-155 induction suppressed the Omp25-induced reduction of IL-12 p40, while having no discernible effect on the Omp25-induced reduction of IL-12 p35 (Figures 5C,D). Inhibition of miR-21-5p induction suppressed the Omp25-induced reduction of IL-12 p35, while having no discernible effect on the Omp25-induced reduction of IL-12 p40 (Figures 5C,D). Consequently, inhibition of miR-21-5p, miR-23b or miR-155 induction did not significantly suppress the Omp25-induced reduction of IL-12 p70, but inhibition of both miR-21-5p and miR-23b induction, or inhibition of both miR-21-5p and miR-155 induction, significantly improved LPS/R848-induced IL-12 p70 production in the rAd-Omp25-infected THP-1 cells (Figure 5E). Notably, in cells transfected with a mix of miR-21-5p, miR-23b and miR-155 inhibitors, the inhibitory effects of Omp25 on LPS/R848-induced IL-12 p70 production were further attenuated, resulting in a level of IL-12 p70 that was higher than other inhibitor-treated cell after rAd-Omp25 infection (Figure 5E). These results demonstrated that downregulation of miR-21-5p, miR-23b, and miR-155 can block the inhibitory effects of Omp25 on LPS/R848-induced IL-12 p70.
Figure 5.
Downregulation of miR-23b and miR-21-5p partly blocks Omp25’s ability to inhibit interleukin (IL)-12 p70 that is induced by lipopolysaccharide (LPS)/R848. (A,B) THP-1 cells were transfected with control mimics (miR ctrl), miR-21-5p mimics, miR-23b mimics or miR-155 mimics and then treated with LPS/R848 for 24 h, the IL-12 p40 and IL-12 p35 levels were examined by ELISA (A) and Western blot (B), respectively. (C,D) THP-1 cells were infected with recombinant adenoviruses expressing Omp25 protein (rAd-Omp25) or rAd-Blank and transfected with miR-21-5p inhibitor, miR-23b inhibitor, miR-155 inhibitor, or inhibitor control for 24 h and then were treated with LPS/R848 for another 24 h. The IL-12 p40 or IL-12 p35 levels were examined by ELISA (C) or Western blot (D). (E) THP-1 cells were infected with rAd-Omp25 or rAd-Blank and transfected with miR-23b inhibitor, miR-21-5p inhibitor, or miR-155 inhibitor, respectively, or a mixture of two inhibitors, or a mixture of three inhibitors for 24 h, then treated with LPS/R848 for another 24 h, and the supernatants were harvested to measure IL-12 p70 secretion by ELISA. The results are means ± SEMs of three independent experiments. &P < 0.05, &&P < 0.01 versus miR ctrl; *P < 0.05, **P < 0.01 versus rAd-Blank-infected cells; #P < 0.05, ##P < 0.01 versus rAd-Omp25-infected cells with inhibitor control;$ P < 0.05, $$P < 0.01 versus rAd-Omp25-infected cells with a mixture of miR-21-5p, miR-23b and miR-155 inhibitor.
Omp25-Induced miR-21-5p and miR-23b Inhibit the Expression of IL-12 p35 and p40 in the Posttranscriptional Level, Respectively
Inhibition of miR-21-5p and -23b attenuates the inhibition effects of Omp25 on LPS/R848-induced IL-12 p35 and p40 expression, respectively. To confirm the possibility that IL-12 p35 and p40 were regulated posttranscriptionally by these Omp25-inducible miRNAs, we constructed reporter plasmids encoding the complete WT 3′UTR of the human il12A and il12B mRNA downstream of the firefly luciferase gene (il12A or il12B WT-3′UTR); parallel plasmids containing mismatches in the predicted miR-21-5p or -23b binding sites (miR-21-5p or 23b MT-3′UTR) of the 3′UTR region were also made (Figure S5A in Supplementary Material). In HEK-293 cells transfected with the reporter plasmids together with the indicated miRNA mimics and an internal control, pRL-TK-Renilla-luciferase, we observed that miR-21-5p and miR-23b reduced the expression of luciferase from il12A and il12B WT-3′UTR compared with either control miRNA mimics transfections (Figure S5B in Supplementary Material). However, miR-21-5p and miR-23b did not reduce luciferase expression from their respective MT-3′UTR plasmids containing specific mutations in their cognate binding sites (Figure S5B in Supplementary Material). These experiments validated the regulatory potential of miR-21-5p and miR-23b via binding with the il12A and il12B WT-3′UTR, respectively, and further confirmed that Omp25-induced miR-21-5p and miR-23b inhibit the expression of IL-12 p35 and p40 induced by LPS/R848 at the posttranscriptional level, respectively.
Omp25-Induced miR-155 Expression Inhibits the Transcription of il12B via Targeting to TAB2
MiR-155 is an NF-κB transactivational target and involved in a negative feedback loop through downregulation of TAB2 or other genes. Our data suggested that miR-155 might be an additional regulator at the transcriptional level in rAd-Omp25-infected cells. We thus hypothesized that miR-155 likely plays a role in Omp25’s inhibition of IL-12 p40 transcription by targeting some key molecules involved in the activation of NF-κB pathway. Indeed, we found that miR-155 inhibitor markedly decreased the suppression of Omp25 to the transcription of il12B compared with the inhibitor control, but miR-146a and miR-23b inhibitors did not affect the transcription of il12B (Figure 6A). Consequently, miR-155 inhibitor attenuated the suppression of Omp25 on IL-12 p40 production, and together with miR-23b inhibitor, it further blocked the inhibitory effects of Omp25 on the production of IL-12 p40 in THP-1 cells (Figure 6B). We also found that TAB2 was decreased in rAd-Omp25-infected cells compared with rAd-Blank-infected cells, but no change was observed for TRAF6, IRAK1, IRAK2, or IκB (Figure 6C). MiR-155 mimics decreased the level of TAB2 protein expression, suggesting that TAB2 is the target of miR-155 in THP-1 cells (Figure 6D). To further identify the regulatory roles of miR-155 in TAB2 in Omp25-expressed cells, cells were transfected with miR-155 inhibitors or an inhibitor control and infected with rAd-Omp25 or rAd-Blank, followed by stimulation with LPS/R848 for 0 and 3 h. In the presence of inhibitor control, the levels of phospho-IκB and TAB2 were decreased in rAd-Omp25-infected cells compared with rAd-Blank-infected cells, but the reduction of phospho-IκB and TAB2 were partly reversed in the presence of miR-155 inhibitor (Figure 6E). These results suggested that Omp25-induced miR-155 inhibits the transcription of il12B via targeting TAB2, and downregulation of miR-155 and -23b can block the suppression of Omp25 on IL-12 p40 expression at transcriptional and posttranscriptional levels, likely by targeting TAB2 and il12B 3′UTR, respectively.
Figure 6.
Omp25-induced miR-155 expression inhibits the transcription of il12B via targeting to TAB2. (A,B) THP-1 cells were infected with recombinant adenoviruses expressing Omp25 protein (rAd-Omp25) or rAd-Blank and transfected with miR-155, miR-146a or miR-23b inhibitor for 24 h, and cell were then incubated with lipopolysaccharide (LPS)/R848 for another 6 or 24 h, and the il12B mRNA and interleukin-12 p40 levels were examined by quantitative polymerase chain reaction and ELISA, respectively. (C) THP-1 cells were infected with rAd-Omp25 or rAd-Blank for 24 or 48 h, then lysed and analyzed by western blotting. (D) THP-1 cells were transfected with mimics control (ctrl), miR-155 mimics or miR-155 mimics and miR-155 inhibitor and treated with LPS/R848 for 24 h. Cell lysates were analyzed by western blotting to detect TAB2. (E) THP-1 cells were infected with rAd-Omp25 or rAd-Blank and transfected with miR-155 inhibitor or inhibitor control for 24 h, and cells were then treated with LPS/R848 for another 3 h. Cells were harvested for western blot analysis of TAB2, p-IκB, and IκB levels. The results are means ± SEMs of three independent experiments. *P < 0.05, **P < 0.01 versus rAd-Blank infected cells with same miRNA inhibitors. #P < 0.05, ##P < 0.01 versus rAd-Omp25 infected cells with inhibitor control.
Wild-type B. suis Induces Higher Levels of miR-21-5p, miR-23b and miR-155 than the Omp25-Deficient Mutant of B. suis
Now that Omp25 can directly induce miR-21-5p, miR-23b and miR-155 in monocytic THP-1 cells, we suspected if WT B. suis could induce a higher level of these miRNAs than the Omp25-deficient mutant. Indeed, we found that the levels of miR-21-5p, miR-23b and miR-155 were higher in the human M/MΦs infected with WT B. suis than that in the M/MΦs infected with Omp25-deficient mutant (Δomp25 B. suis) (Figure 7A). Upon stimulation with LPS/R848, Δomp25 B. suis-infected human M/MΦs produced more IL-12 than WT B. suis-infected human M/MΦs (Figure 7B). These results further demonstrated that Omp25 plays a key role in the dysfunction of Brucella-infected monocyte/macrophages.
Figure 7.
Wild-type (WT) Brucella suis induces higher levels of miR-21-5p, miR-23b and miR-155 compared to Omp25-deficient mutant of B. suis. (A) Human M/MΦs were infected with WT B. suis or Δomp25 mutant for 0, 6, 12, and 24 h, and the levels of miR-21-5p, miR-23b and miR-155 were analyzed by quantitative polymerase chain reaction. (B) Human M/MΦs were infected with WT B. suis or Δ omp25 mutant for 24 h, and cells were then stimulated with lipopolysaccharide (LPS)/R848 for another 24 h in complete culture medium. The concentrations of interleukin (IL)-12 p70 in cell supernatants were determined by ELISA as stated above. The results are means ± SEMs of three independent experiments. #P < 0.05, ##P < 0.01 versus WT B. suis-infected cells at same time point.
Omp25-Induced PD-1 Expression Is Inversely Associated with IL-12 Production in Human Monocyte/Macrophages
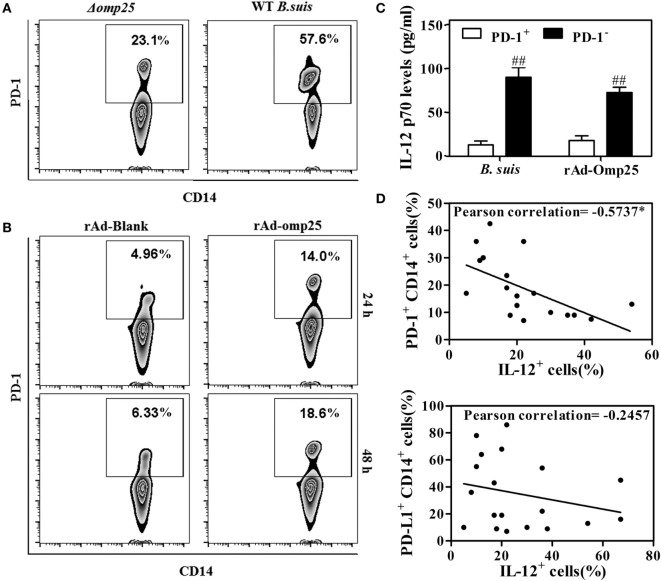
Several studies have showed that PD-1 is upregulated in monocyte/macrophages and associated with dysfunction during different infections (30, 31). To determine whether PD-1 is also upregulated and involved in the regulation of IL-12 production in M/MΦs during Brucella infection, we examined PD-1 expression on the M/MΦs infected with WT or Δomp25 B. suis, or on the M/MΦs infected with rAd-Omp25 or rAd-Blank. Results showed more PD-1 positive CD14+ M/MΦs in WT B. suis-infected cells compared to that of Δomp25 B. suis-infected cells (Figure 8A). Similarly, the percentage of PD-1+ cells in rAd-Omp25-infected M/MΦs was higher than that in rAd-Blank-infected cells (Figure 8B). In contrast to the suppression of IL-12 production in PD-1+ cells during LPS/R848 stimulation, the IL-12 p70 production of PD-1− cells was significantly greater (Figure 8C). Notably, Omp25 significantly increased PD-1 expression in M/MΦs, as well as decreased of IL-12 production.
Figure 8.
Omp25-induced programmed death-1 (PD-1) expression is inversely associated with interleukin (IL)-12 production in human M/MΦs. (A,B) M/MΦs were infected with wild-type (WT) Brucella suis, Δomp25 B. suis, recombinant adenoviruses expressing Omp25 protein (rAd-Omp25), or rAd-Blank, and PD-1+ CD14+ M/MΦs were analyzed by flow cytometry. (C) The M/MΦs were infected with WT B. suis or rAd-Omp25, PD-1 positive and negative cells were isolated and further stimulated with lipopolysaccharide (LPS)/R848. The levels of IL-12 p70 in cell supernatants were determined by ELISA. ##P < 0.01 versus PD-1+ cells. (D,E) M/MΦs derived from different healthy donors were infected with different doses of B. suis, followed by LPS/R848 stimulation. The expression of PD-1/PD-L1 and IL-12 were analyzed, and their associations were evaluated using Pearson correlation analysis as shown by negative line trends. *P < 0.05 demonstrated that PD-1 expression is inversely associated with IL-12 production.
To determine whether the upregulated PD-1 expression on the surface of M/MΦs correlated with intracellular IL-12 production during Brucella infection, M/MΦs derived from different donors were infected with different doses of Brucella and stimulated with LPS/R848, then the expression of PD-1, PD-L1, and IL-12 were determined and compared by Pearson correlation analysis. As shown in Figure 8D, the upregulation of PD-1 in M/MΦs was inversely associated with IL-12 production (r = −0.5737, P = 0.0102) (representative dot plot experiment in inset). Interestingly, although the expression of PD-L1 was also upregulated in infected M/MΦs, it did not significantly correlate with IL-12 production (Figure 8E).
Blocking PD-1 Signaling Decreases miRNAs Levels and Enhances IL-12 Production
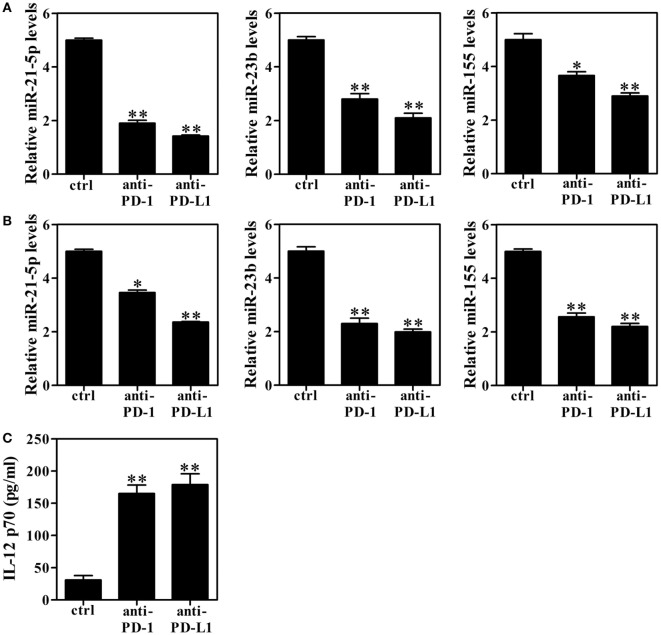
Because PD-1+ cells displayed impaired IL-12 production, we next sought to define the relationship between PD-1 and miRNAs (miR-21-5p, miR-23b and miR-155) upregulation as a potential mechanism underlying Omp25-mediated IL-12 inhibition. As an initial approach, we investigated whether blockade of the PD-1 pathway affected Omp25-induced miRNAs expression in M/MΦs. THP-1 cells and M/MΦs isolated from healthy donors were infected with WT B. suis and preincubated with anti-PD-1, anti-PD-L1, or control IgG Abs overnight followed by stimulation with LPS/R848. Incubation of THP-1 cells or healthy M/MΦs with anti-PD-1 or anti-PD-L1 Ab resulted in significant suppression of miR-21-5p, miR-23b and miR-155 compared with the control IgG (Figures 9A,B). Omp25-induced IL-12 inhibition was also reduced by either anti-PD-1 or anti-PD-L1 treatment (Figure 9C). Together, these data suggested that modulation of PD-1 signaling can significantly improve the dysfunction of Brucella-infected M/MΦs and promote IL-12 production.
Figure 9.
Blocking programmed death-1 (PD-1) signaling decreases miRNAs levels and enhances interleukin (IL)-12 production. (A,B) THP-1 cells and M/MΦs isolated from healthy donors were infected with wild-type Brucella suis and preincubated with anti-PD-1, anti-PD-L1, or control IgG Abs (ctrl) overnight and then stimulated with lipopolysaccharide (LPS)/R848. Real-time PCR was used to detect the relative changes of miR-21-5p, miR-23b and miR-155 in THP-1 cells (A) and M/MΦs (B). (C) The levels of IL-12 p70 in M/MΦs were detected with ELISA. *P < 0.05, **P < 0.01 versus control IgG Abs (ctrl).
Discussion
Despite extensive work in the field of Brucella-induced innate and adaptive immunity dysfunction (5, 20, 32, 33), the molecular mechanisms of Omp25-mediated monocyte/macrophages dysfunction, including the potential regulatory role of miRNA involved in this process, remains incompletely understood. In this study, we demonstrated the important roles and the underlying mechanisms of Brucella Omp25 in inhibiting TLR agonists-induced IL-12 production in monocyte/macrophages. In Omp25-expressing cells, the upregulation miR-21-5p, miR-23b, miR-155 and PD-1 correlated with decreased IL-12 p35 and p40 levels, resulting in a decreased IL-12 p70 production following LPS/R848 stimulation. In this process, miR-21-5p and miR-23b appeared to directly target the 3′UTR of il12A and il12B that downregulated the expression of IL-12 p35 and p40 subunits at the posttranscriptional level, while miR-155 directly targets the 3′ UTR of TAB2 to downregulate the expression of IL-12 p40 subunit at the transcriptional level via inhibiting the activation of NF-κB pathway (Figure 10). Consistently, WT B. suis-infected M/MΦs exhibited an increased level of PD-1 expression and induced higher levels of miR-21-5p, miR-23b and miR-155 than the Δomp25 B. suis mutant, which correlated with the degree of IL-12 inhibition. By contrast, blocking PD-1 signaling decreased miRNAs levels and further enhanced IL-12 production.
Figure 10.
Model of Brucella Omp25-mediated inhibition of interleukin (IL)-12 production in human monocyte/macrophages. Brucella Omp25 inhibits TLR agonist-induced IL-12 p35 and p40 subunits at both the transcriptional and posttranscriptional levels via activating programmed death-1 (PD-1) signaling and upregulating miR-21-5p, miR-23b and miR-155, together resulting in a decreased IL-12 production in human monocyte/macrophages.
MicroRNAs are regulators of inflammation in various cell types, including monocyte/macrophages (33, 34). Numerous miRNAs have been found to be upregulated or downregulated in pathogen-infected or in activated immune cells. In B. melitensis-infected mouse macrophage cell line RAW264.7 cells, microRNA expression profile identified 57 differentially expressed miRNAs between mock- and Brucella-infected cells (35). MiR-146a is the first miRNA identified to negatively regulate TLR4 signaling in human monocytic cells stimulated with LPS (36). MiR-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting tumor necrosis factor receptor-associated factor 6 (TRAF6) and IL-1 receptor–associated kinase 1 and 2 (IRAK1 and IRAK2) (37). MiR-146a is also found to represses mycobacteria-induced inflammatory response and facilitate bacterial replication via targeting IRAK1 and TRAF6 (38). However, in B. melitensis-infected RAW264.7 cells (35) and Omp25-expressing THP-1 cells, miR-146a is not upregulated, and miR-146a inhibitor does not decrease the suppression of Omp25 to the transcription of il12B compared with the inhibitor control. MiR-155 is induced in macrophages in response to both bacterial and viral infection that activates TLR4, TLR2, TLR3, or TLR9 (39, 40). MiR-155 have been shown to target transforming growth factor-β-activated kinase 1-binding protein 2 (TAB2) and Pellino-1, adaptors in the signaling complex that activates IκB kinase β, which suggests that miR-155 is part of a negative feedback loop to dampen inflammatory responses (41). In Omp25-expressing THP-1 cells, we found that miR-155 was upregulated and TAB2 were decreased, while TRAF6, IRAK1, and IRAK2 did not change. Consequently, miR-155 inhibitor decreased the suppression of Omp25 on the transcription of il12B compared with the inhibitor control and miR-146a inhibitor. MiR-21-5p has been identified to inhibit IL-12 p35 expression at posttranscriptional level by targeting the 3′UTR of il12A in previous studies (42), and miR-23b has been reported to promote the tolerogenic properties of dendritic cells through inhibiting Notch1/NF-kappa B signaling (43). In this study, we found that miR-21-5p and miR-23b were upregulated and correlated with gradually decreasing IL-12 p35 and p40 levels in Omp25-expressing THP-1 cells. Downregulation of miR-23b and miR-21-5p partly blocked Omp25 inhibitory effect on LPS/R848-induced IL-12 p70 production. Our results not only confirmed that miR-21-5p inhibits IL-12 p35 expression at posttranscriptional level by targeting to the 3′ UTR of il12A but also found that miR-23b inhibits IL-12 p40 expression at posttranscriptional level by targeting to the 3′ UTR of il12B. However, other predicted miRNAs that also target the 3′UTR of il12A and il12B, including miR-590-5p, miR-340-5p, miR-23a, miR-23c and miR-494-3p, were not involved in the inhibition of IL-12 production in Omp25-expressing THP-1 cells. In addition, our data showed that the expression of miR-21-5p, miR-23b and miR-155 is downregulated in Δomp25 B. suis infection compared with that WT B. suis infection. These results demonstrate an important role of these miRNAs in the regulation of IL-12 expression in Brucella-infected monocyte/macrophages.
Programmed death-1 signaling has been suggested to be involved in the inhibition of some proinflammatory cytokines production, resulting in compromised Type1 (Th1) cellular immune response (31, 44, 45). However, the roles and molecular mechanism(s) of PD-1-mediated monocyte/macrophages dysfunction have not been defined in Brucella infection. In the present study, WT B. suis infection and Omp25 expression significantly increased PD-1 expression, which is inversely correlated with IL-12 production in monocyte/macrophages; anti-PD-1 and anti-PD-L1 treatment blocked the IL-12 inhibition induced by WT B. suis. Furthermore, we defined the relationship between PD-1 expression and miRNAs (miR-21-5p, miR-23b and miR-155) and demonstrated that PD-1 signaling is critical for upregulation of miR-21-5p, miR-23b and miR-155 in Omp25-expressing cells as well as WT B. suis-infected cells.
In summary, in this work, we have provided strong evidence for the roles of microRNAs (miR-155, -23b and -21-5p) and PD-1 signaling in regulating Omp25-induced IL-12 inhibition in Brucella-infected monocyte/macrophages and demonstrated the regulation patterns of different molecules in this process. Our findings would help further understand the mechanism(s) of Brucella-induced monocyte/macrophages dysfunction and provide new targets for the development of novel therapeutic or vaccination approaches.
Author Contributions
YH, DT, BC, and S-LL designed the experiments and wrote the paper. BC, WL, YC, XW, QD, and XZ performed the experiments. YH, BC, and HZ analyzed the data. WL contributed the human blood. YH, DT, and HZ contributed reagents/materials/analysis tools.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank other members of Tong laboratory and Zhang laboratory for insightful suggestion and discussion and thank Dr. Yingfen Lei of Fourth Military Medical University for assistance in bacterial performance.
Funding. This work was supported by the National Natural Science Foundation of China (31372411, 31672535), NIH grants to S.-L. Liu (AI112381 and AI107095), Key project of Shaanxi Province Science and Technology Innovation Team (2013KCT-28), Young talent development program of Northwest A&F University (2452017023), and the Fundamental Research Funds for the Central Universities (2452016116).
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2017.00708/full#supplementary-material.
References
- 1.Kohler S, Foulongne V, Ouahrani-Bettache S, Bourg G, Teyssier J, Ramuz M, et al. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc Natl Acad Sci U S A (2002) 99(24):15711–6. 10.1073/pnas.232454299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galinska EM, Zagorski J. Brucellosis in humans – etiology, diagnostics, clinical forms. Ann Agric Environ Med (2013) 20(2):233–8. [PubMed] [Google Scholar]
- 3.Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: a re-emerging zoonosis. Vet Microbiol (2010) 140(3–4):392–8. 10.1016/j.vetmic.2009.06.021 [DOI] [PubMed] [Google Scholar]
- 4.Skendros P, Boura P. Immunity to brucellosis. Rev Sci Tech (2013) 32(1):137–47. 10.20506/rst.32.1.2190 [DOI] [PubMed] [Google Scholar]
- 5.Billard E, Dornand J, Gross A. Brucella suis prevents human dendritic cell maturation and antigen presentation through regulation of tumor necrosis factor alpha secretion. Infect Immun (2007) 75(10):4980–9. 10.1128/IAI.00637-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan Y, Cheers C. Endogenous interleukin-12 is involved in resistance to Brucella abortus infection. Infect Immun (1995) 63(4):1387–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitry MA, De Trez C, Goriely S, Dumoutier L, Akira S, Ryffel B, et al. Crucial role of gamma interferon-producing CD4+ Th1 cells but dispensable function of CD8+ T cell, B cell, Th2, and Th17 responses in the control of Brucella melitensis infection in mice. Infect Immun (2012) 80(12):4271–80. 10.1128/IAI.00761-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sathiyaseelan J, Goenka R, Parent M, Benson RM, Murphy EA, Fernandes DM, et al. Treatment of Brucella-susceptible mice with IL-12 increases primary and secondary immunity. Cell Immunol (2006) 243(1):1–9. 10.1016/j.cellimm.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 9.Pasquali P, Adone R, Gasbarre LC, Pistoia C, Ciuchini F. Effect of exogenous interleukin-18 (IL-18) and IL-12 in the course of Brucella abortus 2308 infection in mice. Clin Diagn Lab Immunol (2002) 9(2):491–2. 10.1128/CDLI.9.2.491-492.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee IK, Olsen SC, Bolin CA. Effects of exogenous recombinant interleukin-12 on immune responses and protection against Brucella abortus in a murine model. Can J Vet Res (2001) 65(4):223–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Zapata M, Matias MJ, Prieto A, Jonde MA, Monserrat J, Sanchez L, et al. Human brucellosis is characterized by an intense Th1 profile associated with a defective monocyte function. Infect Immun (2010) 78(7):3272–9. 10.1128/IAI.01385-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kariminia A, Kavoossy G, Khatami S, Zowghi E, Ardestani SK. Study of interleukin-10 and interleukin-12 productions in response to lipopolysaccharides extracted from two different Brucella strains. Comp Immunol Microbiol Infect Dis (2002) 25(2):85–93. 10.1016/S0147-9571(01)00029-7 [DOI] [PubMed] [Google Scholar]
- 13.Cloeckaert A, Verger JM, Grayon M, Vizcaino N. Molecular and immunological characterization of the major outer membrane proteins of Brucella. FEMS Microbiol Lett (1996) 145(1):1–8. 10.1111/j.1574-6968.1996.tb08547.x [DOI] [PubMed] [Google Scholar]
- 14.Commander NJ, Spencer SA, Wren BW, MacMillan AP. The identification of two protective DNA vaccines from a panel of five plasmid constructs encoding Brucella melitensis 16M genes. Vaccine (2007) 25(1):43–54. 10.1016/j.vaccine.2006.07.046 [DOI] [PubMed] [Google Scholar]
- 15.Goel D, Rajendran V, Ghosh PC, Bhatnagar R. Cell mediated immune response after challenge in Omp25 liposome immunized mice contributes to protection against virulent Brucella abortus 544. Vaccine (2013) 31(8):1231–7. 10.1016/j.vaccine.2012.12.043 [DOI] [PubMed] [Google Scholar]
- 16.Caro-Hernandez P, Fernandez-Lago L, de Miguel MJ, Martin-Martin AI, Cloeckaert A, Grillo MJ, et al. Role of the Omp25/Omp31 family in outer membrane properties and virulence of Brucella ovis. Infect Immun (2007) 75(8):4050–61. 10.1128/IAI.00486-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edmonds MD, Cloeckaert A, Elzer PH. Brucella species lacking the major outer membrane protein Omp25 are attenuated in mice and protect against Brucella melitensis and Brucella ovis. Vet Microbiol (2002) 88(3):205–21. 10.1016/S0378-1135(02)00110-4 [DOI] [PubMed] [Google Scholar]
- 18.Edmonds MD, Cloeckaert A, Booth NJ, Fulton WT, Hagius SD, Walker JV, et al. Attenuation of a Brucella abortus mutant lacking a major 25 kDa outer membrane protein in cattle. Am J Vet Res (2001) 62(9):1461–6. 10.2460/ajvr.2001.62.1461 [DOI] [PubMed] [Google Scholar]
- 19.Caron E, Peyrard T, Kohler S, Cabane S, Liautard JP, Dornand J. Live Brucella spp. fail to induce tumor necrosis factor alpha excretion upon infection of U937-derived phagocytes. Infect Immun (1994) 62(12):5267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jubier-Maurin V, Boigegrain RA, Cloeckaert A, Gross A, Alvarez-Martinez MT, Terraza A, et al. Major outer membrane protein Omp25 of Brucella suis is involved in inhibition of tumor necrosis factor alpha production during infection of human macrophages. Infect Immun (2001) 69(8):4823–30. 10.1128/IAI.69.8.4823-4830.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y, Lei Y, Zhang H, Zhang M, Dayton A. Interleukin-12 treatment down-regulates STAT4 and induces apoptosis with increasing ROS production in human natural killer cells. J Leukoc Biol (2011) 90(1):87–97. 10.1189/jlb.1210674 [DOI] [PubMed] [Google Scholar]
- 22.Salhi I, Boigegrain RA, Machold J, Weise C, Cloeckaert A, Rouot B. Characterization of new members of the group 3 outer membrane protein family of Brucella spp. Infect Immun (2003) 71(8):4326–32. 10.1128/IAI.71.8.4326-4332.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed LJ, Muench H. A simple method of estimating fifty percent endpoint. Am J Hyg (1938) 27:493–7. [Google Scholar]
- 24.Caron E, Gross A, Liautard JP, Dornand J. Brucella species release a specific, protease-sensitive, inhibitor of TNF-alpha expression, active on human macrophage-like cells. J Immunol (1996) 156(8):2885–93. [PubMed] [Google Scholar]
- 25.Barrionuevo P, Delpino MV, Velasquez LN, Garcia Samartino C, Coria LM, Ibanez AE, et al. Brucella abortus inhibits IFN-gamma-induced FcgammaRI expression and FcgammaRI-restricted phagocytosis via toll-like receptor 2 on human monocytes/macrophages. Microbes Infect (2011) 13(3):239–50. 10.1016/j.micinf.2010.10.020 [DOI] [PubMed] [Google Scholar]
- 26.Kumar N, Lin M, Zhao X, Ott S, Santana-Cruz I, Daugherty S, et al. Efficient enrichment of bacterial mRNA from host-bacteria total RNA samples. Sci Rep (2016) 6:34850. 10.1038/srep34850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoenlaub L, Elliott A, Freches D, Mitchell WJ, Zhang G. Role of B cells in host defense against primary Coxiella burnetii infection. Infect Immun (2015) 83(12):4826–36. 10.1128/iai.01073-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blaho VA, Buczynski MW, Dennis EA, Brown CR. Cyclooxygenase-1 orchestrates germinal center formation and antibody class-switch via regulation of IL-17. J Immunol (2009) 183(9):5644–53. 10.4049/jimmunol.0901499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Lei Y, Zhang H, Hou L, Zhang M, Dayton AI. MicroRNA regulation of STAT4 protein expression: rapid and sensitive modulation of IL-12 signaling in human natural killer cells. Blood (2011) 118(26):6793–802. 10.1182/blood-2011-05-356162 [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Huang X, Chung CS, Chen Y, Hutchins NA, Ayala A. Contribution of programmed cell death receptor (PD)-1 to Kupffer cell dysfunction in murine polymicrobial sepsis. Am J Physiol Gastrointest Liver Physiol (2016) 311(2):G237–45. 10.1152/ajpgi.00371.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho HY, Choi EK, Lee SW, Jung KO, Seo SK, Choi IW, et al. Programmed death-1 receptor negatively regulates LPS-mediated IL-12 production and differentiation of murine macrophage RAW264.7 cells. Immunol Lett (2009) 127(1):39–47. 10.1016/j.imlet.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 32.Ma QL, Liu AC, Ma XJ, Wang YB, Hou YT, Wang ZH. Brucella outer membrane protein Omp25 induces microglial cells in vitro to secrete inflammatory cytokines and inhibit apoptosis. Int J Clin Exp Med (2015) 8(10):17530–5. [PMC free article] [PubMed] [Google Scholar]
- 33.Liu G, Abraham E. MicroRNAs in immune response and macrophage polarization. Arterioscler Thromb Vasc Biol (2013) 33(2):170–7. 10.1161/ATVBAHA.112.300068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma X, Becker Buscaglia LE, Barker JR, Li Y. MicroRNAs in NF-kappaB signaling. J Mol Cell Biol (2011) 3(3):159–66. 10.1093/jmcb/mjr007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng K, Chen DS, Wu YQ, Xu XJ, Zhang H, Chen CF, et al. MicroRNA expression profile in RAW264.7 cells in response to Brucella melitensis infection. Int J Biol Sci (2012) 8(7):1013–22. 10.7150/ijbs.3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A (2006) 103(33):12481–6. 10.1073/pnas.0605298103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou J, Wang P, Lin L, Liu X, Ma F, An H, et al. MicroRNA-146a feedback inhibits RIG-I-dependent type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J Immunol (2009) 183(3):2150–8. 10.4049/jimmunol.0900707 [DOI] [PubMed] [Google Scholar]
- 38.Li S, Yue Y, Xu W, Xiong S. MicroRNA-146a represses mycobacteria-induced inflammatory response and facilitates bacterial replication via targeting IRAK-1 and TRAF-6. PLoS One (2013) 8(12):e81438. 10.1371/journal.pone.0081438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci U S A (2007) 104(5):1604–9. 10.1073/pnas.0610731104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Androulidaki A, Iliopoulos D, Arranz A, Doxaki C, Schworer S, Zacharioudaki V, et al. The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity (2009) 31(2):220–31. 10.1016/j.immuni.2009.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceppi M, Pereira PM, Dunand-Sauthier I, Barras E, Reith W, Santos MA, et al. MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc Natl Acad Sci U S A (2009) 106(8):2735–40. 10.1073/pnas.0811073106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu TX, Munitz A, Rothenberg ME. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J Immunol (2009) 182(8):4994–5002. 10.4049/jimmunol.0803560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng J, Jiang HY, Li J, Tang HC, Zhang XM, Wang XR, et al. MicroRNA-23b promotes tolerogenic properties of dendritic cells in vitro through inhibiting Notch1/NF-kappaB signalling pathways. Allergy (2012) 67(3):362–70. 10.1111/j.1398-9995.2011.02776.x [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Ma CJ, Ni L, Zhang CL, Wu XY, Kumaraguru U, et al. Cross-talk between programmed death-1 and suppressor of cytokine signaling-1 in inhibition of IL-12 production by monocytes/macrophages in hepatitis C virus infection. J Immunol (2011) 186(5):3093–103. 10.4049/jimmunol.1002006 [DOI] [PubMed] [Google Scholar]
- 45.Porichis F, Hart MG, Zupkosky J, Barblu L, Kwon DS, McMullen A, et al. Differential impact of PD-1 and/or interleukin-10 blockade on HIV-1-specific CD4 T cell and antigen-presenting cell functions. J Virol (2014) 88(5):2508–18. 10.1128/JVI.02034-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.