Abstract
Background/Aims
Thyroid disease is a common comorbidity in individuals with Down syndrome (DS) but historical studies have multiple limitations. We assessed thyroid abnormalities in a large cohort of children with Down syndrome.
Methods
Retrospective review of records from patients from a single institution. Calculated prevalence of common thyroid abnormalities and possible associations with common comorbidities.
Results
Among 508 patients, 120 (24%) had a thyroid-related diagnosis, the majority having subclinical hypothyroidism. A Kaplan-Meier estimate projects that 50% have thyroid disorder by adulthood, with 20% of hypothyroidism diagnosed before age 6 months. When tested, approximately 50% had positive antithyroid antibodies, though this was 100% in overt hypothyroidism. There was no association between congenital or acquired hypothyroidism and common comorbidities
Conclusion
Thyroid disease in Down syndrome is more common and occurs earlier than in the general population, and is often transient. Thyroid disease is unrelated to gender, obesity, or other comorbidities. Apart from overt hypothyroidism, much of hypothyroidism in DS appears unrelated to autoimmunity and we recommend checking of anti-thyroid antibodies only in select cases. Additional screens for thyroid disease at routine 6–8 week and 4-month well-child visits will detect early cases of hypothyroidism who passed their newborn screen.
Keywords: Down syndrome, thyroid diseases, congenital heart malformation, congenital hypothyroidism, screening
Introduction
Down syndrome is the most common autosomal aneuploidy with national birth prevalence of 14.57 per 10,000, or 1 in 691, live births[1]. It is well established that patients with Down syndrome (DS) are at increased risk for the development of thyroid hormone abnormalities, with reported prevalence between 8–49%, depending on the definition of abnormality [2–18]. This includes all forms of thyroid disease: congenital hypothyroidism; acquired subclinical hypothyroidism; acquired overt hypothyroidism; and hyperthyroidism. The wide range is due to small patient numbers in historical studies, and though some recent studies have greater numbers, they lack diagnostic detail. Many studies also lack information related to possible associations of thyroid disease with other comorbidities, or were too small to reach statistical significance. The current American Academy of Pediatrics (AAP) guidelines on the care of patients with DS, based mainly on expert opinion, recommend screening for thyroid dysfunction at birth, 6 months, 12 months, and annually thereafter[19]. The absence of a solid evidence base has made criteria for diagnosis and treatment decisions, in cases of subclinical hypothyroidism in particular, a hotly debated topic in the pediatric endocrinology literature.
The goals of this study are to better characterize the timing and prevalence of thyroid abnormalities in children with DS, to compare age at diagnosis between different types of thyroid disease, and to reexamine the screening recommendations. This study also investigates biochemical thyroid status, autoimmunity, treatment decisions, and possible related comorbidities. Of particular interest was evaluation of the prevailing wisdom that most thyroid disease in DS is both permanent and related to autoimmunity[20].
Methods
The Child Development and Rehabilitation Center at Oregon Health and Science University (OHSU) sees pediatric patients with DS primarily from Oregon and Washington in a multidisciplinary team setting. A retrospective review of the electronic medical record (EMR) revealed 508 eligible patients seen in the DS Clinic between the clinic opening in November 2007 through January 2015. Fifty-seven additional patients with DS and a thyroid-related ICD-9 visit code were identified in the OHSU Pediatric Endocrine Clinic (PEC), though 2 were ultimately not diagnosed with thyroid disease. Data from PEC patients were added to DS Clinic data to increase power in association analyses but were not used in prevalence calculations. Study data were collected and managed using REDCap electronic tools hosted at OHSU. REDCap (Research Electronic Data Capture) is a secure, web-based application that supports data capture for research studies, providing 1) interfaces for validated data entry; 2) audit trails for tracking data manipulation and export procedures; and 3) automated export for data downloads. Approval for this study was obtained from the OHSU Institutional Review Board.
Demographic data included gender, ethnicity, and primary language at home. Ethnicity was determined by patient report, was retrieved from the EMR, and was assessed in the study because of possible differences in rates of autoimmunity or other comorbidities. Historic health information included karyotype, history and type of thyroid disease, age and thyroid function tests (TFTs) at time of diagnosis, presence and type of antithyroid antibodies, treatment, history and type of cardiac disease and surgical interventions, and other notable comorbidities. Anthropometric data at visits included height, weight, and body mass index (BMI) as well as height, weight, and BMI percentile for age. Height and weight percentiles were based on the Down syndrome growth charts from Cronk et al[21]. BMI percentiles were based on Center for Disease Control growth charts for patients older than age 2 years[22].
Thyroid disease was characterized as congenital hypothyroidism, subclinical hypothyroidism, overt hypothyroidism, isolated hyperthyrotropinemia, unknown hypothyroidism, and hyperthyroidism according to the criteria in Table 2 as defined by the American Thyroid Association[23]. Although there are potentially differences in treatment practices with mildly elevated TSH levels, biochemical evidence of subclinical hypothyroidism was used to define cases where available. In cases where diagnostic data was unavailable, classification was made based on abnormal TFTs, if available, within 6 months before starting treatment. All “unknown” patients on levothyroxine were counted among those with “acquired hypothyroidism.”
Table 2.
Diagnostic Criteria for Types of Thyroid Disease
| Thyroid Disease | Diagnostic Criteriaa |
|---|---|
| Congenital Hypothyroidism | Diagnosed by newborn screen, or as stated in the electronic medical record |
| Subclinical Hypothyroidism | “High” TSH with normal free T4 at diagnosis or while off medication, or as stated in the medical record |
| Overt Hypothyroidism | “High” TSH with low free T4 at any time |
| Isolated Hyperthyrotropinemia | “High” diagnostic TSH without diagnostic free T4 |
| Unknown Hypothyroidism | Diagnosis in electronic medical record of non-specific hypothyroidism and/or prescribed levothyroxine. No diagnostic thyroid function tests available. |
| Hyperthyroidism | Diagnosis in electronic medical record of Graves’ disease, hyperthyroidism, or prescribed methimazole |
Representative reference ranges after one month of age are TSH 0.5–5.0 uIU/ml and Free T4 0.8–1.8 ng/dl, however classification of “high” and “low” was assessed for individual laboratory normal ranges.
For comparisons between two groups, including percentage of patients tested for autoimmunity, percentage of those testing positive, and differences in levothyroxine doses, a two-sided t-test was used. Logistic regression was used to evaluate associations between thyroid disease and age, gender, ethnicity, presence of cardiac disease, BMI percentile, weight and weight percentile, and presence of gastrointestinal anomalies (Hirschprung, duodenal atresia, and other gastrointestinal atresias). Because of collinearity, regression was repeated with cardiac surgery or cardiopulmonary bypass in place of cardiac disease. The Wald tests was used to compare individual variables for significant associations. Linear regression was used to evaluate differences in weight-based levothyroxine dose between subclinical and overt hypothyroidism. Within each subgroup, analysis was done with chi-squared comparison, except when groups were smaller than 5, in which case Fisher’s exact test was used. All statistical analyses were performed using STATA 14.1 software (StataCorp, College Station TX).
Results
Prevalence and Summary Statistics
Five hundred sixty-five patients were included in the study: 508 patients in the DS Clinic and 57 patients in the PEC (Table 1). There was a slight male predominance (54%) and a high number of patients identified as Hispanic/Latino(a) (31%). The cohort is similar to published cohorts of patients with DS with regards to karyotype and common co-morbidities (Table 1). Patients in the PEC were older and less likely to have a reported history of cardiac disease, though rates of cardiac surgery were not different between the clinics. The PEC had a higher percentage of patients with leukemia/myeloproliferative disease and type I diabetes.
Table 1.
Demographics and Comorbid Diagnoses for Children seen in Down Syndrome Clinic and Pediatric Endocrine Clinic.
| Down Syndrome Clinic | Pediatric Endocrine Clinic | |||
|---|---|---|---|---|
| n = 508 | % | n = 57 | % | |
| Age, Mean and Range (years) | 6.5 | 11.4a | ||
| 0.05–26 | 0.2–28 | |||
| Gender | ||||
| Male | 282 | 55.5 | 25 | 44 |
| Female | 226 | 44.5 | 32 | 56 |
| Ethnicity (n=494) | ||||
| Non-Hispanic/Latino | 336 | 68.0 | 43 | 80 |
| Hispanic/Latino | 158 | 32.0 | 11 | 20 |
| Karyotype (n= 448) | ||||
| 47, XX/47, XY | 420 | 93.8 | 14 | |
| 46 XX, t(14;21)/46 XY, t(14;21) | 6 | 1.3 | ||
| Other (mosaic, other translocations) | 22 | 4.9 | ||
| Unknown | 60 | 43 | ||
| Primary Language at Home (n = 506) | ||||
| English | 372 | 73.5 | 44 | 79 |
| Spanish | 109 | 21.5 | 10 | 18 |
| Other | 25 | 4.9 | 2 | 4 |
| Congenital Cardiac Disease | ||||
| No | 160 | 31.5 | 27 | 47b |
| Yes | 348 | 68.5 | 30 | 52 |
| Required Surgery | 145 | 41.6c | 13 | 43c |
| Visual Impairment (including astigmatism, strabismus) | 312 | 61.4 | 24 | 42b |
| Hearing Impairment (including pressure equalization tubes) | 270 | 53.2 | 24 | 42 |
| Sleep Apnea | 154 | 30.3 | 14 | 25 |
| Gastrointestinal Atresia (duodenal, anal, other) | 27 | 5.3 | 5 | 9 |
| Seizure Disorder | 26 | 5.1 | 3 | 5 |
| Autism Spectrum Disorder | 25 | 4.9 | 3 | 5 |
| Undescended Testicles (one or both; males only) | 21 | 7.5 | ||
| Celiac Disease | 19 | 3.7 | 4 | 7 |
| Hirschsprung | 14 | 2.8 | 2 | 4 |
| Leukemia/Myeloproliferative Disorder | 15 | 3.0 | 5 | 9b |
| Type I Diabetes | 4 | 0.8 | 4 | 7b |
two-sided t-test, p < 0.05
χ2 p-value < 0.05 comparing Down Syndrome Clinic and Pediatric Endocrine Clinic, cardiac disease and visual impairment are more common in Down Syndrome Clinic, leukemia and type 1 diabetes are more common in Pediatric Endocrine Clinic.
Percent of patients requiring cardiac surgery is taken only from patients with cardiac disease.
There were 422 patients without a thyroid-related diagnosis who had TSH and FT4 recorded within 6 months of a visit, resulting in 648 TSH and FT4 measurements total. This excludes any children diagnosed with thyroid disease based on TFTs at the visit in question. Mean TSH was 3.30 uIU/ml (range 0.04–9.66). Mean FT4 was 1.04 ng/dl (range 0.6–2.0).
As shown in Table 3, 120 (24%) patients in the DS Clinic had documented history of a thyroid-related diagnosis: 2% congenital hypothyroidism; 10% subclinical hypothyroidism; 1% overt hypothyroidism; 4.5% with isolated hyperthyrotropinemia; 4.3% unknown hypothyroidism; and 1.6% hyperthyroidism. There was no difference in rates of any type of thyroid disease between males and females; mean serum TSH and free T4 at diagnosis are shown in Table 3. There was a statistically significant difference in TSH and mean FT4 between those with overt and subclinical hypothyroidism (p < 0.005). All those with hyperthyroidism were diagnosed with Graves’ disease and treated with methimazole with good response to therapy.
Table 3.
Prevalence of Different Types of Thyroid Disease.a
| Thyroid Disease | Down Syndrome Clinic, n | Ped Endo Clinic, n | Age at Dx | Mean TSH at Dx, mIU/ml (range) | Mean FT4 at Dx, ng/dl (range) |
|---|---|---|---|---|---|
| Congenital Hypothyroidism | 10 (2%) | 4 | 1.5 m (n=13) |
52.7 (15.66–109.5) (n=8) |
Total T4: b 6.95 (3.9–9.8) (n=8) |
| Subclinical Hypothyroidism | 52 (10%) | 24 | 5 yr 5 m | 11.6 (5.31–72.7) |
1.03 (0.6–1.8) |
| Overt Hypothyroidism | 5 (1%) | 4 | 7 yr 11 mo (n= 9) |
52.1 (21.6–150) (n = 9) |
0.43 (0.1–0.75) (n= 9) |
| Isolated Hyper-thyrotropinemia | 23 (4.5%) | 5 | 2 yr 5 mo (n = 24) |
13.5 (5.55–45.4) (n = 15) |
|
| Unknown Hypothyroidism | 22 (4.3%) | 10 | 4 yr 4 m (n=23) |
||
| Hyperthyroidism | 8 (1.6%) | 8 | 8 yr 11 m (n=16) |
0.02 (0.008–0.1) (n = 15) |
3.64 (1.0–6.5) (n=15) |
| Total | 120 (24%) | 55 |
Diagnostic data for age, mean TSH, and mean free thyroxine (FT4) at diagnosis are taken from all patients for whom the data was available.
Total T4 values from newborn screen, instead of free T4, are reported for congenital hypothyroidism cases.
For all patients, the median age of diagnosis with any thyroid disease was 4 years 10 months. For any acquired hypothyroidism, there was no statistical difference in age of diagnosis between those with subclinical and overt hypothyroidism. Among those diagnosed with hypothyroidism outside of newborn screening, 11 patients (7.5% of all acquired hypothyroidism) were diagnosed before the 6 month screening recommended by the AAP, although indications for testing earlier were not determined. For hyperthyroidism, median age at diagnosis was just under 9 years. Odds of developing thyroid disease increased by 10% per year with increasing age. Using a Kaplan-Meier estimate for the development of thyroid disease, it is estimated that 25% of patients with Down syndrome will carry a diagnosis of thyroid dysfunction by age 7.5 years and projected that up to 50% will by the time they reach adulthood (Figure 1). This projection agrees with our clinical data, where at a mean age of 7 years at the time of the visit, 24% of patients had thyroid disease.
Figure 1.
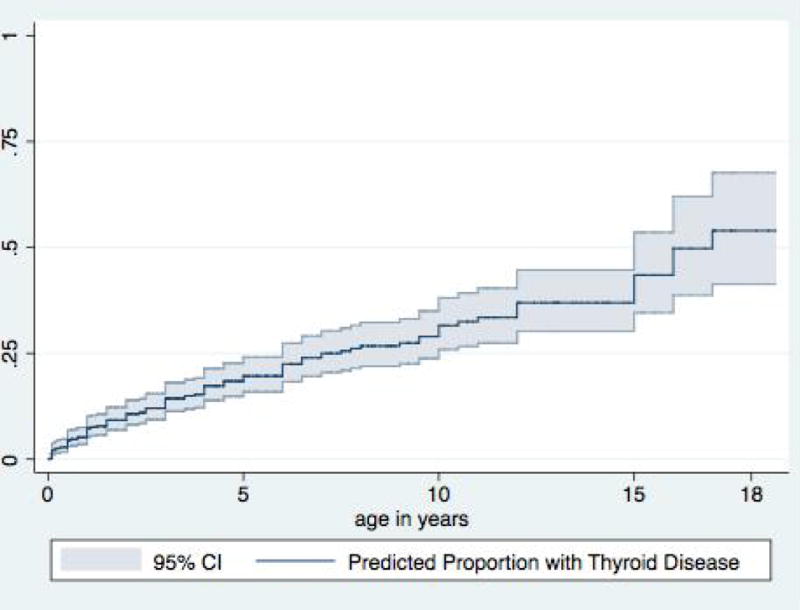
Kaplan-Meier curve showing prevalence of any type of thyroid disease in pediatric Down syndrome population.
Associations with Comorbidities
Logistic regression for odds of developing any thyroid disease and congenital hypothyroidism or acquired hypothyroidism separately, showed no relationship to gender, history of cardiac disease or cardiac surgery, need for cardiopulmonary bypass, BMI weight or height percentile, or presence of gastrointestinal anomalies. There was an increased risk for thyroid disease in those with type I diabetes, though this was weighted to patients seen in the PEC.
Autoimmunity and Differences between Subclinical and Overt Hypothyroidism
A total of 99 patients had antithyroid antibodies tested, with 46 (46%) having any positive antibody (Table 4). Half of patients with positive antibodies had only one antibody present and 6 (13%) (all with hyperthyroidism) had more than two. There were no differences in rates of antibody positivity between males and females (χ2 p-value = 0.2). Of those with diagnosis of acquired hypothyroidism, 62 had antibodies tested and 29 (47%) were positive. It was unclear in EMR documentation whether antibody positivity impacted diagnostic decisions. Of those with positive antibodies, 15/29 with acquired hypothyroidism and 7/15 with hyperthyroidism developed antibody positivity before age 8 years, including two patients with anti-thyroperoxidase (antiTPO) antibodies detected at 10 and 14 months respectively.
Table 4.
Rates of Anti-Thyroid Antibody Testing and Antibody Positivity by Diagnosis.
| Diagnosis | Antibodies Tested | Antibodies Positive |
|---|---|---|
| Subclinical | 37/76 (49%) | 17/37 (46%) |
| Overt | 5/9 (55%) | 5/5(100%)a |
| Isolated TSH | 12/28 (43%) | 4/12 (33%)a |
| Unknown hypothyroidism | 8/32 (25%) | 3/8 (38%) |
| Hyperthyroidism | 15/16 (94%) | 15/15 (100%) |
| No thyroid dysfunction | 22/390 (5.6%) | 2/22 (10%) |
Statistically significant difference in proportions of positive antibodies between subclinical and overt hypothyroidism and isolated hyperthyrotropinemia and overt hypothyroidism. p-value = 0.02 and 0.01. No difference in antibody positivity between subclinical hypothyroidism and isolated hyperthyrotropinemia.
Patients were equally likely to be tested for antibodies across all forms of acquired hypothyroidism. However amongst those tested, patients with overt hypothyroidism had twice the rate of antibody positivity (100% vs 46%). For the population without thyroid dysfunction, 22 were tested for antithyroid antibodies with only 2/22 having any positive test.
Thyroid hormone dosing
Patients with TSH > 11 mIU/ml appeared to require 0.5 mcg/kg/day higher dose of levothyroxine when controlling for gender, age, and weight (95% CI 0.2–0.8 mcg/kg, p-value = 0.001).
Transient Cases
Of note, 18 patients with a diagnosis of hypothyroidism (1/14 congenital, 10/76 subclinical, 4/28 isolated hyperthyrotropinemia, and 3/32 unknown) were not on therapy and had normal TSH and FT4 at their most recent screening. There was one case of remission in the patients diagnosed with Graves’ disease. There were no significant differences in rates of non-treatment between diagnoses. The highest recorded diagnostic TSH for all transient cases was 13.6 uIU/ml. Almost half (8/18) were diagnosed at or before age 3 years. Of these, 4 had measurements of antithyroid antibodies in the EMR, with 2 having positive antibodies. For those diagnosed after age 3 years, 5/6 tested had positive antibodies. As a sensitivity analysis, repeat analyses of differences in antibody testing and positivity between types of hypothyroidism, as discussed above, did not change conclusions when excluding those with transient hypothyroidism.
Discussion
Prevalence and Summary Statistics
This study confirms the previously reported high prevalence of thyroid disease in patients with Down syndrome. The majority of our patients have mild, subclinical hypothyroidism or hyperthyrotropinemia without measurement of free T4. Unlike the general population, there is no female predominance for any type of thyroid disorder, including hyperthyroidism. When looking at all types of thyroid disease, both treated and untreated, up to 50% of patients with DS are expected to have a diagnosis of thyroid disease by adulthood.
Our study appears to confirm a relatively high prevalence of congenital hypothyroidism in children with DS. All patients were diagnosed by newborn screen, most often with a primary total T4 less than the 10th percentile and elevated TSH [24]. The majority of these cases are presumed to be permanent. The prevalence in the Down Syndrome clinic population of 1:50 (2%) as shown in Table 3 is similar to rates seen in other studies [3, 6, 25]. TSH by NBS blood spot was available for 8/14 and suggested milder disease with only moderately elevated TSH, though all but one were still on levothyroxine replacement at mean age 6 years at follow-up. Although an association between congenital hypothyroidism and gastrointestinal anomalies has been suggested previously, in this larger study no patients with congenital hypothyroidism had duodenal atresia, anal atresia, or Hirschsprung disease [3, 26].
The high rate of diagnosis after NBS but before age 6 months suggests a nonimmune thyroid dysfunction in infancy. This early dysfunction is also supported by the finding that more than half of patients diagnosed with hypothyroidism are diagnosed before age 5 years. This early dysfunction could be a mild form of congenital hypothyroidism that escapes detection by NBS, as suggested in earlier, smaller studies, though more conclusive studies would be needed to confirm this hypothesis[25, 27]. In congenital hypothyroidism, it is well established that every month without thyroid hormone supplementation worsens developmental outcomes at school age [28, 29]. If an additional screening for hypothyroidism were added at the routine well-child visits at 6–8 weeks and at 4 months for children with Down syndrome, this could improve the developmental trajectory of some patients without adding additional physician visits for the family.
Autoimmunity and Differences between Subclinical and Overt Hypothyroidism
Rates of autoimmunity in those without thyroid disease, as indicated by antithyroid antibody positivity, were lower than previously reported. This may be sampling bias as those with minor abnormalities in TFTs may have been more likely to be labeled as hypothyroid if they had positive antibodies. Rates of positivity in our study population for antiTPO were much higher than for antithyroglobulin (antiTG). In the general population, antibody positivity for either is approximately 10%, though antiTPO is more likely to be associated with biochemical thyroid disease[30]. Although a previous study showed no cases of antibody positivity in early childhood in Down syndrome, we found almost half of patients with positive antibodies developed them before age 8 years[8]. There was also no difference in antibody positivity between males and females. When sorted by degree of TSH elevation or FT4 depression, antibody positivity did not impact levothyroxine requirements, though higher diagnostic TSH seemed to be associated with antibody positivity and all patients with low FT4 had evidence of autoimmunity when measured.
Some authors have argued that patients with Down syndrome have a non-pathological shift in the normal range of TSH and that perhaps this leads to over-diagnosis of subclinical hypothyroidism [31, 32]. Indeed, Meyerovich et al reported a normal TSH reference range for healthy patients with Down syndrome to be 1.5–8.9 uIU/ml [32]. Our data showed a lower upper limit of normal with 2.5 standard deviations above the mean for TSH of 7.1 uIU/ml. Diagnostic TSH in subclinical hypothyroidism overlapped with this range but mean TSH was higher in in those labeled as having hypothyroidism. However, even in cases where TSH was clearly elevated, FT4 was maintained in the normal range in all but 9 of 83 patients in whom it was measured. TSH also did not appear to be greatly affected by obesity as seen in the general population[33]. When tested, much of subclinical hypothyroidism does not appear to be autoimmune mediated, though the rates of antibody positivity are higher in all groups than reported for the US healthy adult population[30].
Transient Cases
Thirteen percent of patients with history of hypothyroidism were not receiving treatment and had normal TFTs, despite high rates of antibody positivity when tested. Similar high rates of normalization of TSH elevation are also found in the general population and have been reported in those with DS, though typically associated with absence of autoimmune disease[34, 35]. In congenital hypothyroidism, the disease is also more likely to be transient in patients with DS [36]. Combined, these factors suggest that TSH elevation may be transient in a significant portion of patients including those diagnosed in the first year of life. Patients may be mislabeled as having permanent hypothyroidism and will be subject to more frequent laboratory testing and physician follow-up.
It has been suggested in one randomized controlled trial of patients with DS that treatment in the first two years of life results in small benefits in growth and gross motor development at 24 months, but there was no difference at follow-up at age 10 years [37, 38]. The idea that there could be benefit and little harm may result in physicians being more likely in this vulnerable population to treat transient TSH elevations without waiting for a confirmatory second test. This bias towards treatment increases the rate of recorded diagnoses of thyroid disease in the DS population. The interpretation becomes self-perpetuating and is flawed, as the increased rate of diagnosis is likely due to the application of a different diagnostic treatment threshold compared to the general population rather than a true difference in disease rates. We posit that this greater likelihood of diagnosing a normal patient with thyroid disease may be mitigated by delaying diagnosis of subclinical hypothyroidism until more than one sample over 2–3 months suggests sustained TSH elevation, though greater caution and clinical judgement may be required in children under the age of 3 years.
There are multiple limitations to the current study. All patients were seen in specialty clinics that potentially draw more complex patients, although rates of common co-morbidities were similar to other published cohorts[11, 12]. Diagnosis of thyroid disease was made by outside providers in most cases, including decisions regarding interpretation of TFTs. Results for TSH and FT4 came from multiple institutions. Although there was broad overlap between the normal ranges from different laboratories, the reported means should be interpreted with caution as laboratories do not share the same reference standards. There was a moderate amount of missing diagnostic data including TFTs for the 32 patients classified as “unknown hypothyroidism.” Finally, a relatively small number of patients were screened for antithyroid antibodies, particularly among those labeled as not having thyroid disease.
Conclusion
We believe our data support the conclusion that high rates of hypothyroidism are evidence of inherent dysfunction of the thyroid axis in patients with Down syndrome. Given the significant number of patients diagnosed before age 6 months, we recommend additional screening of TSH and FT4 at both a 6–8 weeks and 4 months. While patients would otherwise be detected at the currently recommended 6 month screen, with these additional tests most, if not all, patients, with early hypothyroidism not detected on NBS would be started on critical therapy earlier. Approximately 100 patients would need to be screened to detect one case of infantile hypothyroidism. Although compliance with routine screening is low, the additional points of testing could theoretically improve rates of any testing being done in the first year[10].
We also encourage recognition that all hypothyroidism is potentially transient. Particularly in patients diagnosed before the age of 3 years, we recommend management similar to that for congenital hypothyroidism: consideration of a structured trial off therapy with recheck of TSH and FT4 in 4 weeks in patients who have not had a rise in TSH > 10 uIU/ml on treatment or required increases in levothyroxine dose after initiation of therapy [24, 39].
It would appear that most, if not all, cases of severe hypothyroidism with low FT4 and cases of hyperthyroidism are secondary to autoimmune thyroid disease. However, autoimmunity was not predictive of thyroid disease severity in the absence of significant elevations in TSH and may not be useful in the diagnosis of acquired hypothyroidism in Down syndrome in cases with a single test showing mild TSH elevation. Repeat testing of TSH and FT4 in 2–3 months should be considered before decision is made to treat what could be a transient biochemical finding.
Acknowledgments
Dr. Pierce conceptualized and designed the study and data collection instruments, performed data collection, drafted the initial manuscript, and approved the final manuscript as submitted. Dr. Pierce had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. LaFranchi provided critical feedback in the development of the study, reviewed and revised the manuscript and approved the final manuscript as submitted. Dr. Pinter assisted with the conceptualization and design of the study, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Salary support for M Pierce provided in part by the National Institutes of Health Training Grant T32HD007497. REDCap Database managed by the Oregon Clinical and Translational Research Institute and supported by the National Center for Advancing Translational Science of National Institutes of Health under grant 1 UL1 RR024140 01.
The authors would like to acknowledge Daniel Pinter for assistance with data collection and Katrina Ramsey, MPH for advise on statistical analysis. Ms. Ramsey is supported by the Oregon Clinical and Translational Research Institute, with funding by the National Center for Advancing Translational Science of National Institutes of Health under grant 1 UL1 RR024140 01.
Abbreviations
- AAP
American Academy of Pediatrics
- BMI
body mass index
- DS
Down syndrome
- EMR
electronic medical record
- NBS
newborn screen
- OHSU
Oregon Health and Science University
- PEC
Pediatric Endocrine Clinic
- TFT
thyroid function test
- TG
thyroglobulin
- TPO
thyroperoxidase
- TSH
thyrotropin
- FT4
free thyroxine
Footnotes
PES Membership: Both Drs. Pierce and LaFranchi are members of PES
The authors have no financial relationships relevant to this article to disclose. The authors have no conflicts of interest to this article to disclose.
References
- 1.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, Correa A, National Birth Defects Prevention N Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 2.Claret C, Goday A, Benaiges D, Chillaron JJ, Flores JA, Hernandez E, Corretger JM, Cano JF. Subclinical hypothyroidism in the first years of life in patients with Down syndrome. Pediatr Res. 2013;73:674–678. doi: 10.1038/pr.2013.26. [DOI] [PubMed] [Google Scholar]
- 3.Cutler AT, Benezra-Obeiter R, Brink SJ. Thyroid function in young children with Down syndrome. Am J Dis Child. 1986;140:479–483. doi: 10.1001/archpedi.1986.02140190089034. [DOI] [PubMed] [Google Scholar]
- 4.Dinani S, Carpenter S. Down’s syndrome and thyroid disorder. J Ment Defic Res. 1990;34(Pt 2):187–193. doi: 10.1111/j.1365-2788.1990.tb01528.x. [DOI] [PubMed] [Google Scholar]
- 5.Gibson PA, Newton RW, Selby K, Price DA, Leyland K, Addison GM. Longitudinal study of thyroid function in Down’s syndrome in the first two decades. Arch Dis Child. 2005;90:574–578. doi: 10.1136/adc.2004.049536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruneiro de Papendieck L, Chiesa A, Bastida MG, Alonso G, Finkielstain G, Heinrich JJ. Thyroid dysfunction and high thyroid stimulating hormone levels in children with Down’s syndrome. J Pediatr Endocrinol Metab. 2002;15:1543–1548. doi: 10.1515/jpem.2002.15.9.1543. [DOI] [PubMed] [Google Scholar]
- 7.Iughetti L, Predieri B, Bruzzi P, Predieri F, Vellani G, Madeo SF, Garavelli L, Biagioni O, Bedogni G, Bozzola M. Ten-year longitudinal study of thyroid function in children with Down’s syndrome. Horm Res Paediatr. 2014;82:113–121. doi: 10.1159/000362450. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson B, Gustafsson J, Hedov G, Ivarsson SA, Anneren G. Thyroid dysfunction in Down’s syndrome: relation to age and thyroid autoimmunity. Arch Dis Child. 1998;79:242–245. doi: 10.1136/adc.79.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loudon MM, Day RE, Duke EM. Thyroid dysfunction in Down’s syndrome. Arch Dis Child. 1985;60:1149–1151. doi: 10.1136/adc.60.12.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGowan S, Jones J, Brown A, Reynolds L, Leyland K, Charleton P, Rahim M, Mansor M, Ritha S, Donaldson M, Scottish Down Syndrome Thyroid Screening G Capillary TSH screening programme for Down’s syndrome in Scotland, 1997–2009. Arch Dis Child. 2011;96:1113–1117. doi: 10.1136/archdischild-2011-300124. [DOI] [PubMed] [Google Scholar]
- 11.Roizen NJ, Magyar CI, Kuschner ES, Sulkes SB, Druschel C, van Wijngaarden E, Rodgers L, Diehl A, Lowry R, Hyman SL. A community cross-sectional survey of medical problems in 440 children with Down syndrome in New York State. J Pediatr. 2014;164:871–875. doi: 10.1016/j.jpeds.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Schieve LA, Boulet SL, Boyle C, Rasmussen SA, Schendel D. Health of children 3 to 17 years of age with Down syndrome in the 1997–2005 national health interview survey. Pediatrics. 2009;123:e253–260. doi: 10.1542/peds.2008-1440. [DOI] [PubMed] [Google Scholar]
- 13.Mihci E, Akcurin G, Eren E, Kardelen F, Akcurin S, Keser I, Ertug H. Evaluation of congenital heart diseases and thyroid abnormalities in children with Down syndrome. Anadolu Kardiyol Derg. 2010;10:440–445. doi: 10.5152/akd.2010.143. [DOI] [PubMed] [Google Scholar]
- 14.Moosa S, Segal DG, Christianson AL, Gregersen NE. Thyroid dysfunction in a cohort of South African children with Down syndrome. S Afr Med J. 2013;103:966–970. doi: 10.7196/samj.7111. [DOI] [PubMed] [Google Scholar]
- 15.Pueschel SM, Pezzullo JC. Thyroid dysfunction in Down syndrome. Am J Dis Child. 1985;139:636–639. doi: 10.1001/archpedi.1985.02140080106045. [DOI] [PubMed] [Google Scholar]
- 16.Tuysuz B, Beker DB. Thyroid dysfunction in children with Down’s syndrome. Acta Paediatr. 2001;90:1389–1393. doi: 10.1080/08035250152708770. [DOI] [PubMed] [Google Scholar]
- 17.Rubello D, Pozzan GB, Casara D, Girelli ME, Boccato S, Rigon F, Baccichetti C, Piccolo M, Betterle C, Busnardo B. Natural course of subclinical hypothyroidism in Down’s syndrome: prospective study results and therapeutic considerations. J Endocrinol Invest. 1995;18:35–40. doi: 10.1007/BF03349694. [DOI] [PubMed] [Google Scholar]
- 18.Murphy J, Philip M, Macken S, Meehan J, Roche E, Mayne PD, O’Regan M, Hoey HM. Thyroid dysfunction in Down’s syndrome and screening for hypothyroidism in children and adolescents using capillary TSH measurement. J Pediatr Endocrinol Metab. 2008;21:155–163. doi: 10.1515/jpem.2008.21.2.155. [DOI] [PubMed] [Google Scholar]
- 19.Bull MJ, Committee on G Health supervision for children with Down syndrome. Pediatrics. 2011;128:393–406. doi: 10.1542/peds.2011-1605. [DOI] [PubMed] [Google Scholar]
- 20.Dallas JS. Hypothyroidism. In: Lipfshitz F, editor. Pediatric Endocrinology. New York: Marcel Dekker; 2003. pp. 359–406. [Google Scholar]
- 21.Cronk C, Crocker AC, Pueschel SM, Shea AM, Zackai E, Pickens G, Reed RB. Growth charts for children with Down syndrome: 1 month to 18 years of age. Pediatrics. 1988;81:102–110. [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention NCfHS. CDC growth charts. United States: 2000. [Google Scholar]
- 23.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM, American Thyroid Association Task Force on Thyroid Hormone R Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670–1751. doi: 10.1089/thy.2014.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leger J, Olivieri A, Donaldson M, Torresani T, Krude H, van Vliet G, Polak M, Butler G, Espe Pes Slep Jspe Apeg Appes I, Congenital Hypothyroidism Consensus Conference G European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. Horm Res Paediatr. 2014;81:80–103. doi: 10.1159/000358198. [DOI] [PubMed] [Google Scholar]
- 25.Purdy IB, Singh N, Brown WL, Vangala S, Devaskar UP. Revisiting early hypothyroidism screening in infants with Down syndrome. J Perinatol. 2014;34:936–940. doi: 10.1038/jp.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaruratanasirikul S, Patarakijvanich N, Patanapisarnsak C. The association of congenital hypothyroidism and congenital gastrointestinal anomalies in Down’s syndrome infants. J Pediatr Endocrinol Metab. 1998;11:241–246. doi: 10.1515/jpem.1998.11.2.241. [DOI] [PubMed] [Google Scholar]
- 27.van Trotsenburg AS, Vulsma T, van Santen HM, Cheung W, de Vijlder JJ. Lower neonatal screening thyroxine concentrations in down syndrome newborns. J Clin Endocrinol Metab. 2003;88:1512–1515. doi: 10.1210/jc.2002-021303. [DOI] [PubMed] [Google Scholar]
- 28.Fisher DA, Foley BL. Early treatment of congenital hypothyroidism. Pediatrics. 1989;83:785–789. [PubMed] [Google Scholar]
- 29.Klein AH, Meltzer S, Kenny FM. Improved prognosis in congenital hypothyroidism treated before age three months. J Pediatr. 1972;81:912–915. doi: 10.1016/s0022-3476(72)80542-0. [DOI] [PubMed] [Google Scholar]
- 30.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 31.Prasher V, Haque MS. Misdiagnosis of thyroid disorders in down syndrome: time to reexamine the myth? Am J Ment Retard. 2005;110:23–27. doi: 10.1352/0895-8017(2005)110<23:MOTDID>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Meyerovitch J, Antebi F, Greenberg-Dotan S, Bar-Tal O, Hochberg Z. Hyperthyrotropinaemia in untreated subjects with Down’s syndrome aged 6 months to 64 years: a comparative analysis. Arch Dis Child. 2012;97:595–598. doi: 10.1136/archdischild-2011-300806. [DOI] [PubMed] [Google Scholar]
- 33.Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316:165–171. doi: 10.1016/j.mce.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Wasniewska M, Salerno M, Cassio A, Corrias A, Aversa T, Zirilli G, Capalbo D, Bal M, Mussa A, De Luca F. Prospective evaluation of the natural course of idiopathic subclinical hypothyroidism in childhood and adolescence. Eur J Endocrinol. 2009;160:417–421. doi: 10.1530/EJE-08-0625. [DOI] [PubMed] [Google Scholar]
- 35.Selikowitz M. A five-year longitudinal study of thyroid function in children with Down syndrome. Dev Med Child Neurol. 1993;35:396–401. doi: 10.1111/j.1469-8749.1993.tb11660.x. [DOI] [PubMed] [Google Scholar]
- 36.Oakley GA, Muir T, Ray M, Girdwood RW, Kennedy R, Donaldson MD. Increased incidence of congenital malformations in children with transient thyroid-stimulating hormone elevation on neonatal screening. J Pediatr. 1998;132:726–730. doi: 10.1016/s0022-3476(98)70369-5. [DOI] [PubMed] [Google Scholar]
- 37.van Trotsenburg AS, Vulsma T, van Rozenburg-Marres SL, van Baar AL, Ridder JC, Heymans HS, Tijssen JG, de Vijlder JJ. The effect of thyroxine treatment started in the neonatal period on development and growth of two-year-old Down syndrome children: a randomized clinical trial. J Clin Endocrinol Metab. 2005;90:3304–3311. doi: 10.1210/jc.2005-0130. [DOI] [PubMed] [Google Scholar]
- 38.Marchal JP, Maurice-Stam H, Ikelaar NA, Klouwer FC, Verhorstert KW, Witteveen ME, Houtzager BA, Grootenhuis MA, van Trotsenburg AS. Effects of early thyroxine treatment on development and growth at age 10.7 years: follow-up of a randomized placebo-controlled trial in children with Down’s syndrome. J Clin Endocrinol Metab. 2014;99:E2722–2729. doi: 10.1210/jc.2014-2849. [DOI] [PubMed] [Google Scholar]
- 39.American Academy of P. Rose SR, Section on E, Committee on Genetics ATA. Brown RS, Public Health Committee LWPES. Foley T, Kaplowitz PB, Kaye CI, Sundararajan S, Varma SK. Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117:2290–2303. doi: 10.1542/peds.2006-0915. [DOI] [PubMed] [Google Scholar]


