Abstract
Eveningness, a preference for later sleep-wake timing, is linked to altered reward function, which may explain a consistent association with substance abuse. Notably, the extant literature rests largely on cross-sectional data, yet both eveningness and reward function show developmental changes. We examined whether circadian preference during late adolescence predicted the neural response to reward two years later. A sample of 93 males reported circadian preference and completed a monetary reward fMRI paradigm at ages 20 and 22. Primary analyses examined longitudinal paths from circadian preference to medial prefrontal cortex (mPFC) and ventral striatal (VS) reward responses. We also explored whether reward responses mediated longitudinal associations between circadian preference and alcohol dependence, frequency of alcohol use, and/or frequency of cannabis use. Age 20 eveningness was positively associated with age 22 mPFC and VS responses to win, but not associated with age 22 reactivity to reward anticipation. Age 20 eveningness was indirectly related to age 22 alcohol dependence via age 22 mPFC response to win. Our findings provide novel evidence that altered reward-related brain function could underlie associations between eveningness and alcohol use problems. Eveningness may be an under-recognized but modifiable risk factor for reward-related problems such as mood and substance use disorders.
Keywords: circadian preference, reward, fMRI, adolescence, alcohol, cannabis
1. INTRODUCTION1
Eveningness, a preference for later sleep-wake timing, shows consistent associations with greater substance use. Eveningness has also been linked to variations in reward function, including increased depression, impulsivity, novelty-seeking, and recently, an altered neural response to monetary reward, which could explain the associations with substance use. Notably, the extant literature is nearly entirely based on cross-sectional data, yet both eveningness and reward function show key developmental changes through adolescence into young adulthood. Longitudinal studies are required for a full understanding of the links among eveningness, reward function, and related disorders, which may inform development of novel prevention approaches for substance abuse and affective disorders, especially as eveningness appears to be modifiable by both behavioral and pharmacological approaches (1–3).
Substantial cross-sectional data indicate that individuals endorsing eveningness and/or reporting later actual sleep timing exhibit altered reward function and increased rates of affective dysregulation. In both adolescent and young adult samples, evening-types (or late circadian preferences) score higher on measures tapping a range of reward-related constructs, such as impulsivity (4, 5), impulsive sensation seeking (6), sensation and novelty seeking (7), and risky decision-making (8). Evening-types also show altered daily rhythms in positive affect, ostensibly a manifestation of the reward system (9, 10). A handful of neuroimaging studies suggest that later sleep timing is associated with altered neural response to reward, at least in adolescents (11, 12).
Likewise, evening-types report greater substance use, including alcohol, cannabis, tobacco, and caffeine (e.g., (13–18), and higher rates and/or more severe depression (19–22). The association with substance use appears to hold whether eveningness is assessed via circadian preference measures or based on reports of actual sleep timing (e.g., (23)). Also, although most relevant studies are cross-sectional, recent longitudinal work suggests that eveningness and/or late sleep timing during adolescence predicts subsequent substance abuse and other risky behavior (24).
Most recently, we reported that extreme evening-types in a sample of 20-year-old males exhibited altered neural response to monetary reward relative to extreme morning-types (25). Specifically, the evening-types exhibited relatively reduced response in the medial prefrontal cortex (mPFC) during reward anticipation and relatively enhanced response in the ventral striatum (VS) during win outcome. Both the mPFC and VS are key regions in the network implicated in reward function (26). Furthermore, these same patterns of mPFC and VS response were correlated with greater alcohol consumption and symptoms of alcohol dependence, respectively, suggesting that the greater alcohol dependence observed among the evening-types may be due, in part, to the altered neural reactivity to reward. That said, the cross-sectional nature of the analyses precluded any determination of directionality, much less causality.
Circadian preference and sleep timing undergo important developmental changes that may be relevant to developmental changes in reward function and/or substance involvement. Beginning with puberty, eveningness increases throughout adolescence, peaking around age 20, before beginning a long, slow shift towards morningness over the rest of the life span (27, 28). This peak in eveningness corresponds with a key developmental period, referred to as late adolescence or emerging adulthood (29).
Propensity for risk-taking remains high during this period (30), which is characterized by peaks in substance use, and subsequently the time of greatest risk for developing an alcohol use disorder (31–33). Notably, key aspects of brain development continue during this period, including in prefrontal cortical areas relevant to reward function (34), while previously increasing reward sensitivity may begin declining (35, 36). Although longitudinal studies of reward-related brain changes during this period remain scarce, a recent developmental study reported that from 10–16 and 12–19 years, neither mPFC or VS exhibited either mean change or stability (37). However, VS response was positively correlated with self-reported reward sensitivity over time, suggesting developmental changes in reward sensitivity are reflected in the corresponding brain circuitry. Given ongoing developmental changes in circadian preference, reward-related brain function, and substance use during the transition from adolescence to young adulthood, studies that can simultaneously address all of these processes are warranted.
In the present analyses, we examined whether a circadian preference for eveningness during late adolescence/emerging adulthood predicted the neural response to reward two years later. To examine this question, we drew on the same study of young males that we previously examined in the aforementioned group comparison of evening-types and morning-types (25), this time employing a continuous measure of circadian preference to include a larger swath of the sample. We again selected the mPFC and VS as key regions-of-interest (ROIs) within the reward circuit, and employed cross-lag panel analyses that accounted for eveningness and brain activation at both ages. In these primary analyses, and consistent with prior findings, we hypothesized that greater eveningness at age 20 would predict lower mPFC response during reward anticipation and greater VS response to win outcome at age 22. We also examined the relative stability of circadian preference and reward-related brain function with the cross-lag models. Lastly, based on evidence that both eveningness and reward function are implicated in substance involvement, including our prior finding in the age 20 sample regarding alcohol consumption and dependence, we also explored mediation models in which associations between age 20 circadian preference and age 22 alcohol dependence, alcohol use, and cannabis use were mediated by age 22 mPFC and/or VS response.
2. METHODS AND MATERIALS
2.1 Participants
Participants were drawn from the Pitt Mother & Child Project (PMCP), an ongoing longitudinal study examining vulnerability and resilience in low-income boys. The initial sample consisted of 310 boys and their families, recruited when boys were 6 to 17 months old through Women, Infants, and Children Nutritional Supplement (WIC) programs serving low-income families in the Pittsburgh metropolitan area (see (38, 39). The current study utilizes data from subsequent laboratory visits when participants were 20 and 22 years of age. These visits each included a clinical interview, self-report measures, and an fMRI scan. Written informed consent was obtained for all participants. The study was approved by the Institutional Review Board of the University of Pittsburgh.
Participants were asked to refrain from using any illegal substances starting 48 h prior to their fMRI scan and all participants were approved for the scan by a registered nurse. A combination of breathalyzer, saliva drug screen, self-report, and clinical judgment were used to determine which participants were approved to scan.
A total of 106 participants had circadian preference data at both age 20 and 22. Of these, a total of 93 participants had valid reward task fMRI data at both ages (5 were excluded because of scheduling limitations, 2 were excluded because of claustrophobia, 2 were excluded because of metal/bullet fragments, 2 were excluded because of inadequate coverage of the ventral striatum, 1 was excluded because of misunderstanding the task, and 1 refused the scan).
2.2 Clinical measures
Circadian preference
We used the Composite Scale of Morningness (40) to assess circadian preference at the age 20 and 22 assessments (age 20 α=0.63; age 22 α=0.65). The score is obtained by the sum of 13 Likert-type items, and ranges from 13 (extreme eveningness) to 55 (extreme morningness). Although we used the CSM score as a continuous measure for the primary analyses, for descriptive purposes we also classified participants by type using the scoring thresholds suggested by Natale and Alzani (41): 13–26=evening-type; 27–41=intermediate-type; 42–55=morning-type.
Alcohol dependence
We used the age 22 administration of the Alcohol Dependence Scale (ADS; (42)), a 25-item questionnaire to assess self-reported alcohol withdrawal symptoms, impaired control over drinking, awareness of a compulsion to drink, increased tolerance to alcohol, and salience of drink-seeking behavior in the past year. A score of 9 or more is highly indicative of current diagnosis of alcohol abuse or dependence (α=0.78 for sample). Because the ADS was administered only to participants reporting current alcohol use at age 20 (n=70 in the present sample), we elected to focus only on age 22 ADS scores in the primary analyses.
Alcohol use
We assessed frequency of alcohol use via the age 20 and 22 administrations of the Lifetime Drinking History (42–45), a structured interview designed to quantify lifetime patterns of the use of alcohol. Participants reported on past year frequency of alcohol (days per month). Based on the skewed distribution of alcohol use data, we used a square-root transformation.
Cannabis use
Although we did not have a measure of cannabis problems parallel to the ADS, we assessed frequency of cannabis use via the age 20 and 22 administrations of the Lifetime Drug Use History (42–45), a structured interview designed to quantify lifetime patterns of the use of various substances. Participants reported on past year frequency of cannabis use (days per month). Based on the bimodal distribution of the cannabis use data, we created an ordinal version of the variable (0=no days per month, 1=1–19 days per month, 3=20+ days per month) with a unimodal distribution (n = 39, 37, and 13, respectively) for use in the analyses.
Tobacco use
We also assessed daily tobacco use given because it is associated with decreased reward-related brain function (46). We used the 8-point Likert-scale tobacco use item from the age 22 administration of the Alcohol and Drug Consumption Questionnaire (47), dichotomizing the item into no daily use (scores = 0–7) and daily use (score=8).
Depressive symptoms
We used the Beck Depression Inventory (BDI) to assess depressive symptomatology at age 20 and 22, given prior reports that depression is associated with both eveningness (19–22) and altered reward-related brain function (48).
Psychopathology
Finally, at age 22, we assessed current and past Axis I clinical disorders using the SCID (49) with a clinically trained staff person trained to reliability with a licensed clinical psychologist. For descriptive purposes, we provide information on Major Depressive Disorder, Dysthymia, Bipolar Disorder, Alcohol Dependence, Substance Dependence, and Antisocial Personality Disorder given their relevance to reward function. At age 22, 11% had a history of Major Depressive Disorder, 1% had a history of Bipolar Disorder, 5% had a history of Dysthmia, 2% had a history of Alcohol Dependence, 2% had a history of non-alcohol Substance Dependence, and 7% had a history of Antisocial Personality Disorder.
2.3 Reward processing task
We employed a slow event-related fMRI card-guessing paradigm designed to examine neural reactivity to anticipation and receipt of monetary reward and loss see (25)). Trials were presented in a pseudorandom order with predetermined outcomes. Each 20-s trial consisted of a 4-s decision phase when participants guessed whether the value of a visually presented card with a possible value of 1–9 was higher or lower than 5, a 6-s anticipation period when the trial type (possible-reward or possible-loss) was displayed, a 1–s outcome period the numerical value of the card and then outcome feedback (win, loss, or no-change) were presented, and a 9-s interstimulus interval. In reward trials participants would win $1 if their guess was correct and there would be no-change in earnings if their guess was incorrect. In loss trials participants would lose 50 cents if their guess was incorrect and there would be no-change in earnings if their guess was correct. Twenty-four trials were presented in one run with 12 reward-anticipation and 12 loss-anticipation trials. Within reward-anticipation trials there were a balanced number of win-outcome and no-change outcome trials.
In the present study we focus on the anticipation and outcome phases of the reward trials, based on evidence that circadian preference is more strongly related to reward, appetitive motivation, and positive affect than to negative affect processes (20), and specifically used the reward anticipation>baseline and reward win>baseline contrasts, in which baseline was defined as the last 3 s of the interstimulus interval. Outcome probabilities were fixed trial-wise to ensure an identical win/loss time series modeling and pattern of outcome experiences for every participant. Each participant was given $10 in earnings. Participants were unaware of the fixed outcome probabilities in the paradigm, and were led to believe their performance would determine net monetary gain.
Neuroimaging data were collected using a 3.0 T Siemens Trio MRI scanner at the University of Pittsburgh. Structural 3D axial MPRAGE images were acquired in the same session (TE=3.29 ms; TR=2200 ms; Flip Angle=9°; Field of View=256 × 192 mm2; Slice-Thickness=1 mm; Matrix: 256 × 256; 192 continuous slices). Mean blood-oxygenation-level-dependent (BOLD) images were then acquired with a gradient echo EPI sequence during 13-min covering 39 axial slices (3.1 mm thick; TR/TE=2000/28 ms; FOV=205×205 mm; matrix 64 × 64; Flip Angle 90°).
2.4 fMRI preprocessing and initial analyses
Neuroimaging data were preprocessed and analyzed with Statistical Parametric Mapping software, Version-8 (SPM8; http://www.fil.ion.ucl.ac.uk/spm). Data for each participant were realigned to the first volume in the time series to correct for head motion. Data sets were then selected for quality based on our standard small-motion correction (<2 mm on average across all frames) and adequate coverage of the ventral striatum (>80%). Realigned images were then coregistered with the subject’s anatomical image, segmented to restrict analyses to gray matter, normalized to standard Montreal Neurological Institute (MNI) template, and spatially smoothed with a Gaussian kernel of 6 mm full-width at half-maximum.
A first-level fixed-effect model was constructed for each participant and scan and predetermined condition effects at each voxel were calculated using a t-statistic, producing a statistical image for the reward-anticipation- minus-baseline and win-outcome-after-reward-anticipation-minus-baseline contrast. First-level contrast images were then included in second-level whole-brain and region of interest (ROI) analyses. The VS ROI was constructed using the WFU Pickatlas Tool (v1.04) and defined as a sphere 20mm in radius, centered on the ventral striatum using Talairach coordinates x=0, y=10, and z=−10, and encompassing the head of the caudate nucleus and ventral areas. The mPFC ROI was constructed using the PickAtlas and defined as a 5393-voxel sphere including medial Brodmann Area (BA) 10 and BA32, key projection targets of midbrain dopamine neurons, which demonstrate connectivity with the ventral striatum, and which have been implicated in evaluating the relative value of rewards and reward-directed behavior (26).
At the second level, we used 1-sample t-tests in the age 20 sample to ascertain the main effect of task (anticipation-baseline and win-baseline contrasts) in each ROI, using a height threshold of p<0.001 and a cluster-level threshold of pFWE<0.05. A single cluster was identified in each of the VS and mPFC ROIs. To focus on voxels within each ROI responsive to the reward task, we created functional masks based on these VS and mPFC clusters (see Figures S1 and S2 in the Supplement), and then employed the Marsbar toolbox to extract the mean activation across each functional cluster in both the age 20 and age 22 datasets. These mean activations across VS and mPFC were subsequently used in all remaining analyses. We examined the distribution of the extracted ROI data, excluding 4 participants with values greater than 3SD from the mean, resulting in a sample of 89 participants. Also, based on prior evidence of circadian rhythms in reward processes, including reward-related brain activation (50, 51), we explored correlations between the extracted ROI data and the time of scan (which ranged from 9AM–4PM). There were no significant correlations between extracted ROI data and the time of scan, and thus time of scan was not used as a covariate in subsequent analyses.
2.5 Main data analyses
Changes in circadian preference, substance outcomes, and reward-related brain function were examined via paired-sample t-tests or the Wilcoxon Signed Ranks Test (cannabis use only). Fisher’s Exact Test (FET) was used to examine changes in distribution of circadian preference types. Hypothesized associations between circadian preference, reward-related brain function, and substance use were initially examined via bivariate Pearson’s correlations in SPSS 22 (IBM Corporation, 2013). Significant relationships were subsequently evaluated using cross-lag panel and mediation analyses in Mplus 7 (52). Cross-lag panel analyses with maximum likelihood estimation tested whether circadian preference at age 20 predicted reward-related brain function in the mPFC and VS at age 22 after adjusting for reward-related brain function at age 20 and circadian preference at age 22. Analyses were performed separately for reward anticipation and win outcome responses. Given prior reports that depressive symptoms are associated with both eveningness (19–22) and reward-related brain function (48), we initially added BDI scores at age 20 and 22 to the cross-lag models. Parameter estimates were generated for autocorrelations (e.g., longitudinal path of one variable on itself), cross-lag relationships (e.g., longitudinal path of one variable on another variable), and concurrent correlations between variables at each assessment point. This model resulted in zero degrees of freedom (a just-identified model), therefore, goodness-of-fit could not be evaluated.
Mediation analyses tested whether associations between circadian preference at age 20 and substance involvement at age 22 were mediated through reward-related brain function in the mPFC and VS at age 22. Analyses were performed separately for alcohol dependence, frequency of alcohol use, and frequency of cannabis use. Parameter estimates were generated for autocorrelations; cross-lag relationships of endogenous age 22 variables on age 20 circadian preference; concurrent correlations between mPFC and VS response at ages 20 and 22; concurrent correlations between circadian preference and substance involvement at age 22; and regressions of age 22 substance involvement on concurrent mPFC and VS response. Model goodness-of-fit was evaluated using absolute indices (i.e., χ2, RMSEA, SRMR) and comparative indices (i.e., CFI, TLI). Indirect effects were calculated using 95% two-tailed, bias-corrected bootstrap confidence intervals (53). In addition, because smoking on the day of the scan may affect brain blood flow and is associated with other substance use, we performed supplemental analyses that included a dichotomous indicator of daily smoking (yes, no) as a covariate in mediation analyses. Including the daily smoking covariate did not substantially alter the results of mediation analyses (see Figures S3 and S4 of the Supplement); results without the daily smoking covariate are reported here.
3. RESULTS
3.1 Sample characteristics (Table 1)
Table 1.
Sample characteristics
| n | Mean ± SD | range | |
|---|---|---|---|
| Composite Scale of Morningness (CSM) | |||
| Age 20 | 89 | 32.98 ± 6.56 | 18–49 |
| Age 22 | 89 | 34.46 ± 7.02 | 14–49 |
| Beck Depression Inventory (BDI) | |||
| Age 20 | 89 | 5.43 ± 5.98 | 0–25 |
| Age 22 | 89 | 4.62 ± 6.10 | 0–29 |
| Alcohol Dependence Scale (ADS) | |||
| Age 20 | 70 | 5.06 ± 3.93 | 0–19 |
| Age 22 | 89 | 4.29 ± 3.72 | 0–14 |
| Alcohol use (days per month) | |||
| Age 20 | 87 | 3.86 ± 4.45 | 0–20 |
| Age 22 | 89 | 4.14 ± 4.23 | 0–17 |
| Cannabis use (days per month) | |||
| Age 20 | 86 | 0 days: n=33 (37.1%); 1–19 days: n=35 (39.3%); 20+days: n=18 (20.2%) | |
| Age 22 | 85 | 0 days: n=36 (42.4%); 1–19 days: n=36 (42.4%); 20+days: n=13 (15.3%) | |
| Daily smoking – Age 22a | Non-daily smoker: n=61 (71.8%); Daily smoker: n=24 (28.2%) | ||
On average, participants fell into the intermediate range of morningness-eveningness, were not depressed, reported low levels of alcohol dependence, and were not regular alcohol users across age 20 and 22. The majority of participants were cannabis users across both time points. The majority (71.8%) were not daily smokers.
3.2 Changes in circadian preference and cannabis use
Circadian preference showed a small but significant shift in the direction of morningness from age 20 to 22, with CSM scores increasing an average of 1.48 points (t(88)= −2.19, p=0.031, 95% CI[−2.93, −0.14]). Although there was a corresponding small shift towards more morning-types and fewer evening-types at age 22 (11 E-types, 64 I-types, 14 M-types) relative to age 20 (16 E-types, 63 I-types, and 10 M-types), this change was not statistically significant (FET=1.59, p=0.47). There was also a statistically-significant decrease in frequency of cannabise use from age 20 to 22 (Z=−2.50, p=0.013). However, there were no statistically significant changes in other substance measures or in reward-related brain function from age 20 to 22 (p’s=0.10–0.85).
3.3 Bivariate correlations (Table 2)
Table 2.
Bivariate correlations between circadian preference, reward-related brain response, and substance involvement
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15c | 16c | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Circadian preference - 20 | 1.00 | 0.56** | 0.15 | −0.02 | 0.10 | −0.03 | −0.04 | −0.26* | 0.06 | −0.23* | − 0.22† | −0.26* | −0.16 | −0.22* | −0.21† | −0.35** |
| 2 Circadian preference - 22 | 1.00 | 0.06 | −0.14 | 0.02 | −0.09 | −0.05 | −0.12 | 0.00 | −0.15 | −0.05 | −0.17 | 0.05 | −0.25* | −0.11 | −0.25* | |
| 3 Anticipation - mPFC - 20 | 1.00 | −0.14 | 0.52** | −0.05 | 0.08 | −0.05 | 0.09 | 0.03 | −0.04 | −0.08 | −0.07 | 0.15 | −0.00 | −0.01 | ||
| 4 Anticipation - mPFC - 22 | 1.00 | −0.10 | 0.65** | 0.19† | 0.52** | 0.07 | 0.23* | 0.05 | 0.07 | 0.13 | −0.00 | −0.02 | 0.02 | |||
| 5 Anticipation - VS - 20 | 1.00 | −0.05 | 0.09 | 0.12 | −0.01 | 0.13 | −0.04 | 0.03 | −0.10 | −0.03 | 0.02 | −0.06 | ||||
| 6 Anticipation - VS - 22 | 1.00 | 0.24* | 0.29** | 0.05 | 0.54** | 0.04 | 0.03 | 0.07 | 0.12 | 0.02 | 0.03 | |||||
| 7 Win - mPFC - 20 | 1.00 | 0.26* | 0.47** | 0.05 | 0.00 | −0.02 | −0.06 | −0.06 | 0.03 | −0.01 | ||||||
| 8 Win - mPFC - 22 | 1.00 | −0.01 | 0.40** | 0.25* | 0.34** | 0.17 | 0.26* | 0.21* | 0.19† | |||||||
| 9 Win - VS - 20 | 1.00 | −0.09 | 0.01 | −0.03 | −0.11 | −0.12 | −0.04 | −0.18† | ||||||||
| 10 Win - VS - 22 | 1.00 | 0.15 | 0.21† | 0.04 | 0.16 | −0.07 | −0.01 | |||||||||
| 11 Alcohol dependence - 20 a | 1.00 | 0.54*** | 0.39*** | 0.43*** | 0.26* | 0.35** | ||||||||||
| 12 Alcohol dependence - 22 | 1.00 | 0.38*** | 0.44*** | 0.30** | 0.33** | |||||||||||
| 13Alcohol use – 20b | 1.00 | 0.61*** | 0.33** | 0.35*** | ||||||||||||
| 14Alcohol use – 22b | 1.00 | 0.27* | 0.37*** | |||||||||||||
| 15 Cannabis use – 20c | 1.00 | 0.86*** | ||||||||||||||
| 16 Cannabis use – 22c | 1.00 |
NOTES: Circadian preference is based on the Composite Scale of Morningness, with higher scores indicating greater morningness.
Alcohol dependence (Alcohol Dependence Scale) was available for n=70 at age 20.
Alcohol use data was square-root transformed prior to analyses.
Given the ordinal nature of the cannabis use variable (0=no use, 1–19 days per month, 2=20+ days per month), Spearman’s correlations are reported for all analyses involving the cannabis use variable.
p>0.05,
p<0.05,
p<0.01,
p<0.001
The results from bivariate correlation analyses, presented in Table 2, revealed that greater evening preference at age-20 was positively associated with age-22 eveningness, mPFC and VS response to reward win outcomes, ADS scores, and the frequency of alcohol and cannabis use. There were no significant cross-sectional associations between age-20 or 22 circadian preference and the other study variables with the exception of positive correlations between greater eveningness at age 22 and more frequent alcohol and cannabis use.
With regard to reward-related brain response, the VS responses to reward win at age 20 and 22 were each significantly cross-sectionally associated with the corresponding mPFC responses, but did not show any other significant cross-sectional or prospective associations with ADS scores or cannabis use. The age-20 mPFC response to win significantly correlated with the age-22 mPFC response, but was unrelated to other study variables at either age. In contrast, the age-22 mPFC response was significantly associated with age-22 ADS, alcohol use, and cannabis use; greater mPFC response to win correlated with higher ADS scores and more frequent use of alcohol and cannabis.
3.4 Cross-lagged Panel Analyses (Figures 1 and 2)
Figure 1.
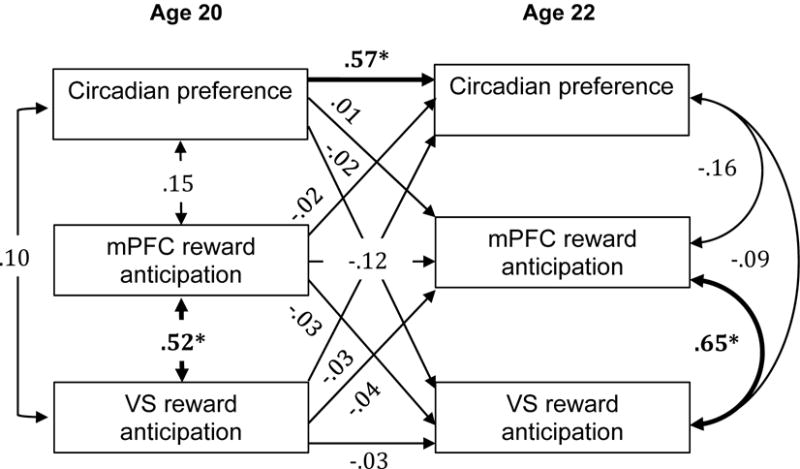
Cross-lag panel analysis with circadian preference and reward anticipation response in the mPFC and VS at ages 20 and 22 years. Numbers indicate standardized beta weights and asterisks indicate statistically significant paths.
Figure 2.
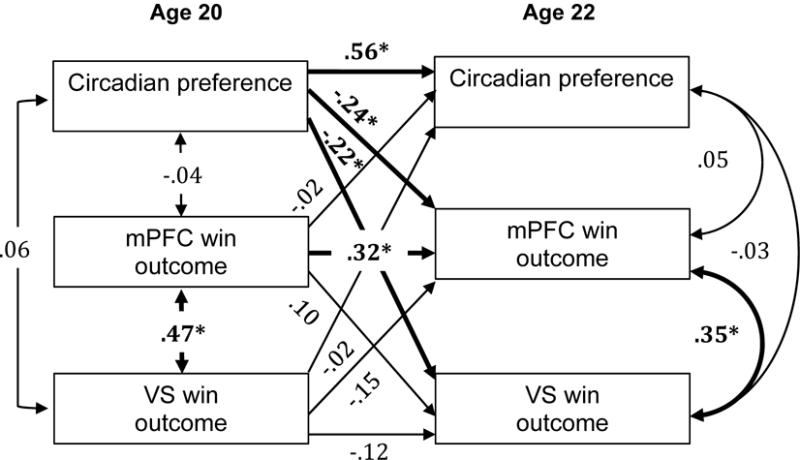
Cross-lag panel analysis with circadian preference and win outcome response in the mPFC and VS at ages 20 and 22 years. Numbers indicate standardized beta weights and asterisks indicate statistically significant paths.
Cross-lag panel analyses, presented in Figures 1 and 2, indicated that greater evening preference at age 20 was associated with greater mPFC and VS response to reward outcome at age 22. These associations were maintained when including BDI scores at age 20 and 22, and thus BDI scores were dropped from the presented models for the sake of parsimony. Cross-lag associations between evening preference at age 20 and response to reward anticipation at age 22 were not significant. There were no cross-sectional associations between circadian preference and brain response at ages 20 or 22.
3.5 Mediation analyses (Figures 3–5)
Figure 3.
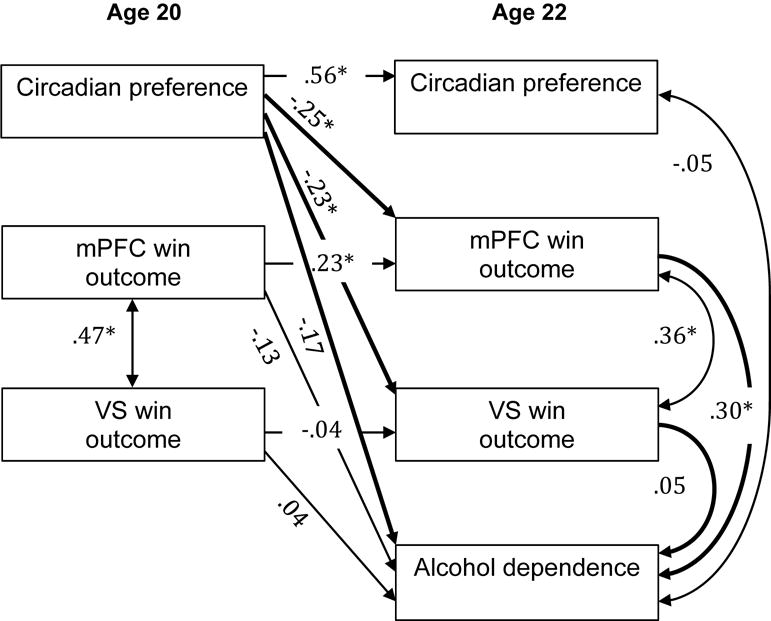
mPFC and VS win outcome response as a mediator of the relationship between circadian preference and alcohol dependence. Numbers indicate standardized beta weights, asterisks indicate statistically significant paths, and bold lines indicate paths of interest for the meditational model. Standardized 95% CI for the indirect effect through mPFC: −0.21, −0.01 (s) and VS: −0.08, 0.03 (ns).
Figure 5.
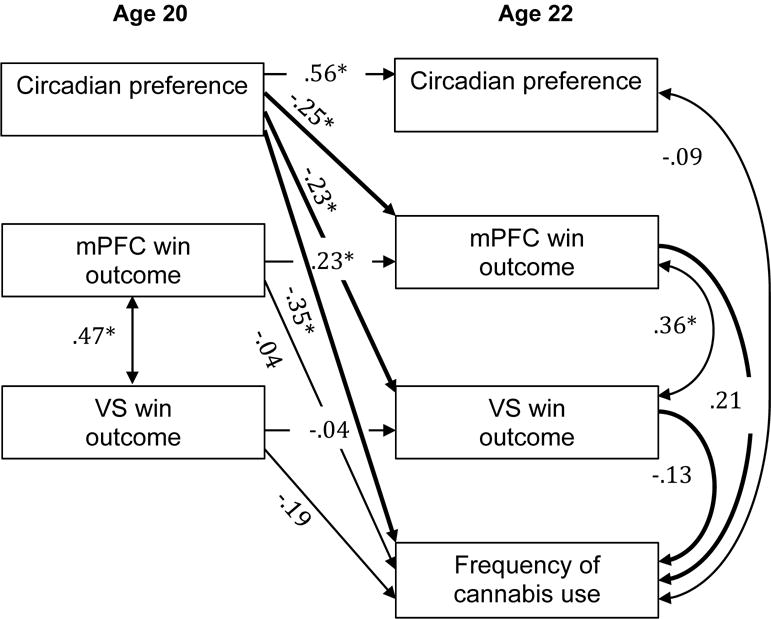
mPFC and VS win outcome response as a mediator of the relationship between circadian preference and frequency of cannabis use. Numbers indicate standardized beta weights, asterisks indicate statistically significant paths, and bold lines indicate paths of interest for the meditational model. Standardized 95% CI for the indirect effect through mPFC: −0.17, 0.00 (ns) and VS: −0.01, 0.11 (ns).
Figure 4.
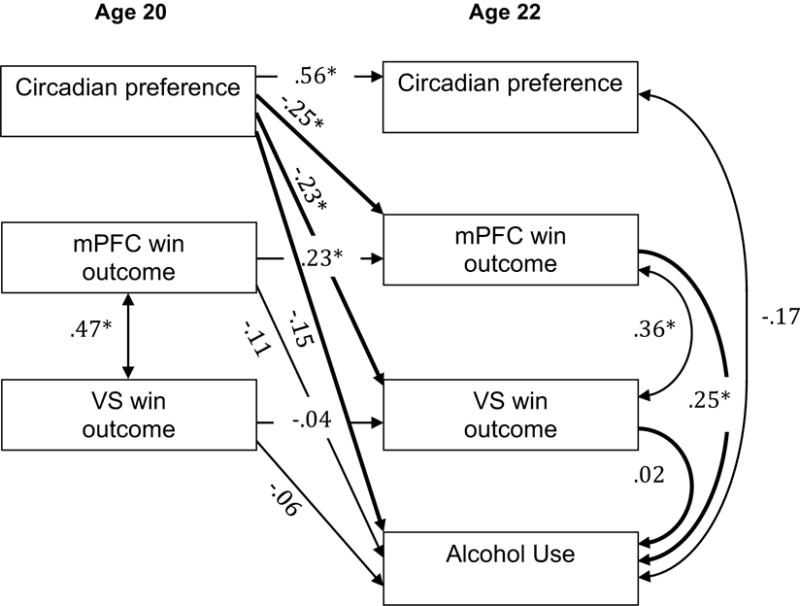
mPFC and VS win outcome response as a mediator of the relationship between circadian preference and alcohol use. Numbers indicate standardized beta weights, asterisks indicate statistically significant paths, and bold lines indicate paths of interest for the meditational model. Standardized 95% CI for the indirect effect through mPFC: −0.18, −0.01 (s) and VS: −0.08, 0.04 (ns).
Mediation analyses were only run for win response because there were no significant cross-lag associations for reward anticipation.
Alcohol dependence
Path analyses for alcohol dependence resulted in excellent model fit [χ2 (8) = 3.55, p > .10; RMSEA = .00 (90% CI: 0.00–0.06); SRMR = .03; CFI = 1.00; TLI = 1.16]; (54). Mediation analyses for alcohol dependence (Figure 3) did not support direct effects of age-20 circadian preference on age-22 alcohol dependence, and thus traditional mediation was not evidenced. The analyses did indicate a significant indirect path from age-20 circadian preference to age-22 alcohol dependence via age-22 win response in the mPFC. The indirect path via age-22 VS response to win was not statistically significant.
We ran two additional path analyses for alcohol dependence. First, we ran a model that included age-22 alcohol use as a covariate (see Figure S5 of the Supplement). Second, we ran a model that included age-20 alcohol dependence in the 70 participants with ADS scores at both ages (specific results available upon request. For both models the above findings held; the indirect path from age-20 circadian preference to age-22 alcohol dependence via age-22 win response in the mPFC remained statistically significant.
Alcohol use
Path analyses for alcohol use resulted in excellent model fit [χ2 (8) = 3.56, p > .10; RMSEA = .00 (90% CI: 0.00–0.06); SRMR = .03; CFI = 1.00; TLI = 1.16]; (54). As for alcohol dependence, mediation analyses for alcohol dependence (Figure 4) did not support direct effects of age-20 circadian preference on age-22 alcohol use, and thus traditional mediation was not evidenced. The analyses did indicate a significant indirect path from age-20 circadian preference to age-22 alcohol use via age-22 win response in the mPFC. The indirect path via age-22 VS response to win was not statistically significant. In contrast to the findings for alcohol dependence, the indirect path from age-20 circadian preference to age-22 alcohol use via age-22 mPFC win response did not remain significant after including age-20 alcohol use.
Cannabis use
Path analyses for cannabis use resulted in excellent model fit [χ2 (8) = 3.55, p > .10; RMSEA = .00 (90% CI: 0.00–0.06); SRMR = .03; CFI = 1.00; TLI = 1.14]; (54). Mediation analyses for cannabis use did not reveal significant associations (Figure 5). This finding remained unchanged after including age-20 cannabis use in the model.
4. DISCUSSION
In the present findings, we report for the first time, that the circadian preference of late adolescent males prospectively predicts their neural response to winning rewards two years later. Specifically, greater eveningness at age 20 predicted greater activation across the mPFC and VS in response to notification of winning a monetary reward at the age 22 assessment. We did not observe a prospective association between age 20 reward-related brain function and age 22 circadian preference. This suggests that the oft-noted cross-sectional associations between circadian preference and reward-related processes and behaviors are not primarily driven by impulsive, reward-seeking behavior, and/or substance use leading to later bedtimes and rise times. Rather, our findings are consistent with a burgeoning animal and human literature supporting sleep and circadian modulation of reward processes and behavior, with relevance to both affective disorders and addiction (reviewed in (55, 56).
We observed longitudinal associations between the age 20 and 22 response to reward within the mPFC, but not the VS. Perhaps mPFC function has more trait-like stability in early adulthood. Our VS findings are consistent with those van Duijvenvoorde and colleagues (37), who found no longitudinal associations between the mPFC and VS responses to the Jackpot Task when it was administered two years apart in a sample aged 10–25 at baseline. In addition, neither mPFC nor VS responses to reward anticipation and outcome showed statistically significant differences from age 20 to 22, consistent with a relative lack of systematic change in reward-related brain function during this time frame.
Based on our conceptual model in which eveningness is hypothesized to lead to substance abuse via effects on the neural processing of reward, we also explored whether the prospective association between circadian preference and reward-related brain function was reflected in measures of alcohol and cannabis involvement. Although full mediation could not be tested because eveningness was not directly linked to later alcohol use, we found indirect effects between eveningness and both alcohol use and symptoms of alcohol dependence through the mPFC response to winning rewards. The finding for alcohol dependence held after accounting for earlier (age 20) symptoms of alcohol dependence, concurrent alcohol use, and concurrent daily smoking. The indirect effect on alcohol use no longer remained statistically significant after including earlier (age 20) alcohol use in the model. Although zero-order correlations suggested that a similar indirect path between eveningness and cannabis use via mPFC response to winning rewards, the indirect path was not statistically significant in the full model. Together, the findings suggest that alcohol dependence may be more sensitive than alcohol or marijuana use to the effects of circadian preference on the mPFC response to winning rewards. This may be due in part to the normative higher levels of alcohol use in this age range, and it is also clinically relevant given that it suggests that circadian-reward pathways may influence alcohol problems more than use per se.
Although our mediation analyses suggest a path in which eveningness disturbs reward-related brain function, and thereby influences symptoms of alcohol dependence, we must approach the findings with caution. Notably, although we satisfied temporal precedence requirements with assessment of our independent variable (circadian preference) occurring prior to assessment of our mediator (reward-related brain function) and dependent variable (alcohol dependence), the mediator and dependent variables were assessed concurrently. Future studies should include at least three prospective assessments to test these pathways, and better yet, employ experimental manipulations to demonstrate causation.
In contrast to our prior report based on a subsample of the age 20 sample, we found that eveningness was associated with increased, rather than decreased mPFC response to reward outcome, and this increased mPFC response was correlated in turn with symptoms of alcohol dependence. That is, our findings differed both in terms of direction of association (positive versus negative association between eveningness and reward activation) and stage of reward processing (outcome versus anticipation). Several possible factors could explain these different patterns of association. First, our prior report looked at cross-sectional, rather than longitudinal associations, and the associations between circadian preference, mPFC reactivity to reward, and alcohol dependence may differ over time. Second, neurodevelopmental changes in the mPFC and/or its connectivity with the VS may underlie these shifting associations with circadian preference and alcohol dependence. Third, our prior analytic approach focused on portions of the mPFC and VS that correlated with eveningness, whereas the current approach focused on task-defined mPFC and VS clusters. In particular, the subregions of the mPFC in the current analyses appear to be somewhat more dorsal and rostral (particularly Brodmann area 9) than those in our prior paper, which extended more into the anterior dorsal and pregenual anterior cingulate (particularly Brodmann area 32).
As noted, we predicted based on our prior work that eveningness would be associated with a lower mPFC response during reward anticipation, but instead found an association with higher mPFC response during reward outcome only. The apparent difference in task phase may be less surprising; in the prior paper the mPFC showed parallel responses during reward anticipation and outcome, but only the anticipation response met statistical criteria for reporting in the paper. Furthermore, although the anticipation and outcome phases conceptually map onto “wanting” and “liking” processes that have been differentially related to different stages of addiction (57), findings based on fMRI studies of monetary reward tasks have not always clearly fit with conceptual predictions (58, 59). However, it is more difficult to reconcile why opposite patterns of mPFC response would be associated with alcohol dependence. The current findings also diverge from another prior paper from our group in which young adults (M age = 27.2) meeting DSM-IV criteria for alcohol dependence exhibited lower mPFC response during the outcome phase of this same monetary reward task in comparison to a group of healthy controls (60). Furthermore, within the alcohol-dependent group, those with a family history of alcohol dependence showed even lower mPFC response. [We] speculated in that paper that the lower mPFC response could reflect compromised modulation of reward processing in a manner that might facilitate alcohol problems. Notably, that paper used a different contrast (win versus loss) during the fMRI analyses. The different contrast, the inclusion of females, and higher prevalence of clinically signficant alcohol dependence could account for the discrepancies with the present findings. Noting another recent finding of increased mPFC response to reward outcome (using a variant of the Balloon Analogue Risk Task) that was correlated with greater alcohol use and trait-level disinhibition ((61), we speculate that this increased mPFC response to reward outcome may represent compromised process of performance monitoring among those with greater eveningness. It is not possible to disentangle based on our design, however, if this compromised performance monitoring contributes to, and/or is a consequence of more severe alcohol dependence.
Also in contrast to our prior report, we did not find a cross-sectional association between circadian preference and reward-related brain function. Notably, the prior analysis focused on a group comparison of extreme evening- and morning-types, whereas the present analysis involved a considerably large sample including the “intermediate-types” that did not express an extreme preference towards morningness or eveningness. The circadian preference of these “intermediate-types” may be relatively more determined by differential rates in the accumulation of homeostatic sleep drive (62) than circadian factors per se, and thus may show distinct associations with reward-related processes. Alternatively, differences in sociocultural (e.g., school or work) or developmental context at age 20 may underlie the disparate results.
Our study has notable limitations. Use of a self-report measure of circadian preference does not allow parsing of circadian, homeostatic, and/or personality contributions to the observed associations. Furthermore, the CSM’s internal consistency fell into the questionable range for this sample. Followup studies should include physiological measures of circadian phase, electroencephalographic measures of homeostatic sleep processes, and/or prospective measures of sleep timing to help disentangle the specific sleep/circadian factors. For example, we are unable to assess whether our findings were influenced by recent sleep loss, which is important to consider given prior evidence that sleep loss influences reward-related brain function (63, 64). Our mediation analyses are limited by the assessment schedule, in which age 22 fMRI and substance assessments occurred concurrently, thus precluding the temporal ordering needed to demonstrate mediation. In addition to longitudinal studies that better sequence assessments of circadian preference, brain function, and substance involvement, experimental studies are needed to best ascertain causality. Another limitation is that the test-retest reliability of the card-guessing task has not been established, and the 2-year timeframe of our study is not appropriate for assessing test-retest reliability given the myriad of developmental and environmental changes occurring during this period. This is a pervasive issue in the neuroimaging field and future work (and funding) should be devoted to establishing test-retest reliabilities of tasks (50, 65). The issue may be slightly mitigated by published evidence that the VS response to a similar reward task showed good test-retest reliability over two weeks (66). Finally, while our all-male, predominantly low-income sample reduced heterogeneity and thus provided greater statistical power, it limits generalizability to females and males and females from non-urban, higher SES backgrounds. Females tend to differ in circadian preference, reward function, and substance involvement (e.g., (67–69).
Although our findings require replication in more generalizable samples (including females), they may have some important implications for prevention and treatment of alcohol problems. Notably, emerging evidence indicates that circadian preference is modifiable via behavioral and pharmacological treatments (1–3), and that circadian-based interventions (e.g., bright light therapy) directly impact reward-related brain regions (70), thus providing novel adjunctive approaches to add to existing prevention and intervention efforts.
Supplementary Material
HIGHLIGHTS.
Eveningness, a preference for later sleep timing, is linked to altered reward function
Eveningness is also linked to substance use
We examined eveningness and reward-related brain function longitudinally
Eveningness at age 20 predicated reward-related brain function at age 22
Eveningness had an indirect effect on alcohol dependence via reward response
Acknowledgments
The authors would like to thank the participants and their parents who participated in this study. We also thank Dr. Aidan Wright in the Department of Psychology at the University of Pittsburgh for providing consultation regarding the CFA and meditational models. This work was supported by grants from the National Institutes of Health, including MH050907 (Shaw), DA026222 (Forbes, Shaw), K01DA032557 (Hasler) and K01MH103511 (Casement).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ABBREVIATIONS: ADS = Alcohol Dependence Scale; BDI = Beck Depression Inventory; CSM = Composite Scale of Mornignness; mPFC = medial prefrontal cortex; VS = ventral striatum
AUTHOR DISCLOSURES
Drs. Hasler, Casement, Sitnick, Shaw and Forbes all reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Corruble E, Frank E, Gressier F, Courtet P, Bayle F, Llorca PM, et al. Morningness-eveningness and treatment response in major depressive disorder. Chronobiology International. 2014;31(2):283–9. doi: 10.3109/07420528.2013.834924. [DOI] [PubMed] [Google Scholar]
- 2.Natale V, Ballardini D, Schumann R, Mencarelli C, Magelli V. Morningness-eveningness preference and eating disorders. Personality and Individual Differences. 2008;45(6):549–53. [Google Scholar]
- 3.Hasler BP, Buysse DJ, Germain A. Shifts Toward Morningness During Behavioral Sleep Interventions Are Associated With Improvements in Depression, Positive Affect, and Sleep Quality. Behavioral sleep medicine. 2015:1–12. doi: 10.1080/15402002.2015.1048452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang JI, Park CI, Sohn SY, Kim HW, Namkoong K, Kim SJ. Circadian preference and trait impulsivity, sensation-seeking and response inhibition in healthy young adults. Chronobiol Int. 2015;32(2):235–41. doi: 10.3109/07420528.2014.965313. [DOI] [PubMed] [Google Scholar]
- 5.Caci H, Robert P, Boyer P. Novelty seekers and impulsive subjects are low in morningness. European Psychiatry. 2004;19(2):79–84. doi: 10.1016/j.eurpsy.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Tonetti L, Pascalis V, Fabbri M, Martoni M, Russo PM, Natale V. Circadian typology and the Alternative Five-Factor Model of personality. Int J Psychol. 2015 doi: 10.1002/ijop.12170. [DOI] [PubMed] [Google Scholar]
- 7.Antúnez JM, Navarro JF, Adan A. Morningness–eveningness and personality characteristics of young healthy adults. Personality and Individual Differences. 2014;68:136–42. [Google Scholar]
- 8.Ponzi D, Wilson MC, Maestripieri D. Eveningness is associated with higher risk-taking, independent of sex and personality. Psychol Rep. 2014;115(3):932–47. doi: 10.2466/19.12.PR0.115c28z5. [DOI] [PubMed] [Google Scholar]
- 9.Hasler BP, Germain A, Nofzinger EA, Kupfer DJ, Krafty RT, Rothenberger SD, et al. Chronotype and diurnal patterns of positive affect and affective neural circuitry in primary insomnia. Journal of Sleep Research. 2012 doi: 10.1111/j.1365-2869.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller MA, Rothenberger SD, Hasler BP, Donofry SD, Wong PM, Manuck SB, et al. Chronotype predicts positive affect rhythms measured by ecological momentary assessment. Chronobiol Int. 2015;32(3):376–84. doi: 10.3109/07420528.2014.983602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forbes EE, Dahl RE, Almeida JR, Ferrell RE, Nimgaonkar VL, Mansour H, et al. PER2 rs2304672 polymorphism moderates circadian-relevant reward circuitry activity in adolescents. Biological Psychiatry. 2012;71(5):451–7. doi: 10.1016/j.biopsych.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holm SM, Forbes EE, Ryan ND, Phillips ML, Tarr JA, Dahl RE. Reward-related brain function and sleep in pre/early pubertal and mid/late pubertal adolescents. Journal of Adolescent Health. 2009;45(4):326–34. doi: 10.1016/j.jadohealth.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adan A. Chronotype and personality factors in the daily consumption of alcohol and psychostimulants. Addiction. 1994;89(4):455–62. doi: 10.1111/j.1360-0443.1994.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 14.Broms U, Kaprio J, Hublin C, Partinen M, Madden PA, Koskenvuo M. Evening types are more often current smokers and nicotine-dependent-a study of Finnish adult twins. Addiction. 2011;106(1):170–7. doi: 10.1111/j.1360-0443.2010.03112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gau SS, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng AT. Association between morningness-eveningness and behavioral/emotional problems among adolescents. Journal of Biological Rhythms. 2007;22(3):268–74. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- 16.Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. Journal of Sleep Research. 2002;11(3):191–9. doi: 10.1046/j.1365-2869.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- 17.Randler C. Differences between smokers and nonsmokers in morningness-eveningness. Social Behavior and Personality. 2008;36(5):673–80. [Google Scholar]
- 18.Urban R, Magyarodi T, Rigo A. Morningness-eveningness, chronotypes and health-impairing behaviors in adolescents. Chronobiology International. 2011;28(3):238–47. doi: 10.3109/07420528.2010.549599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drennan M, Klauber M, Kripke D, Goyette L. The effects of depression and age on the Horne-Ostberg morningness-eveningness score. Journal of Affective Disorders. 1991;23(2):93–8. doi: 10.1016/0165-0327(91)90096-b. [DOI] [PubMed] [Google Scholar]
- 20.Hasler BP, Allen JJB, Sbarra DA, Bootzin RR, Bernert RA. Morningness-eveningness and depression: Preliminary evidence for the role of BAS and positive affect. Psychiatry Research. 2010;176(2–3):166–73. doi: 10.1016/j.psychres.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitamura S, Hida A, Watanabe M, Enomoto M, Aritake-Okada S, Moriguchi Y, et al. Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiology International. 2010;27(9–10):1797–812. doi: 10.3109/07420528.2010.516705. [DOI] [PubMed] [Google Scholar]
- 22.Merikanto I, Kronholm E, Peltonen M, Laatikainen T, Vartiainen E, Partonen T. Circadian preference links to depression in general adult population. J Affect Disord. 2015;188:143–8. doi: 10.1016/j.jad.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 23.Saxvig IW, Pallesen S, Wilhelmsen-Langeland A, Molde H, Bjorvatn B. Prevalence and correlates of delayed sleep phase in high school students. Sleep medicine. 2012;13(2):193–9. doi: 10.1016/j.sleep.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 24.McGlinchey EL, Harvey AG. Risk behaviors and negative health outcomes for adolescents with late bedtimes. Journa of Youth and Adolescence. 2015;44(2):478–88. doi: 10.1007/s10964-014-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasler BP, Sitnick SL, Shaw DS, Forbes EE. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Research: Neuroimaging. 2013;214(3):357–64. doi: 10.1016/j.pscychresns.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, et al. A marker for the end of adolescence. Current Biology. 2004;14(24):R1038–9. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 28.Randler C. Age and gender differences in morningness-eveningness during adolescence. The Journal of genetic psychology. 2011;172(3):302–8. doi: 10.1080/00221325.2010.535225. [DOI] [PubMed] [Google Scholar]
- 29.Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol. 2000;55(5):469–80. [PubMed] [Google Scholar]
- 30.Quinn PD, Harden KP. Differential changes in impulsivity and sensation seeking and the escalation of substance use from adolescence to early adulthood. Dev Psychopathol. 2013;25(1):223–39. doi: 10.1017/S0954579412000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnett JJ. The developmental context of substance use in emerging adulthood. Journal of drug issues. 2005;35(2):235–54. [Google Scholar]
- 32.Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, et al. A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics. 2008;121(Supplement 4):S290–S310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of general psychiatry. 2007;64(7):830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 34.Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral cortex. 2010;20(3):534–48. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- 35.Urošević S, Collins P, Muetzel R, Lim K, Luciana M. Longitudinal changes in behavioral approach system sensitivity and brain structures involved in reward processing during adolescence. Developmental psychology. 2012;48(5):1488. doi: 10.1037/a0027502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Somerville LH, Jones RM, Casey B. A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and cognition. 2010;72(1):124–33. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Duijvenvoorde AC, de Macks ZAO, Overgaauw S, Moor BG, Dahl RE, Crone EA. A cross-sectional and longitudinal analysis of reward-related brain activation: effects of age, pubertal stage, and reward sensitivity. Brain and cognition. 2014;89:3–14. doi: 10.1016/j.bandc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Shaw DS, Gilliom M, Ingoldsby EM, Nagin DS. Trajectories leading to school-age conduct problems. Developmental Psychology. 2003;39(2):189–200. doi: 10.1037//0012-1649.39.2.189. [DOI] [PubMed] [Google Scholar]
- 39.Shaw DS, Winslow EB, Flanagan C. A prospective study of the effects of marital status and family relations on young children’s adjustment among African American and European American families. Child Development. 1999;70(3):742–55. doi: 10.1111/1467-8624.00053. [DOI] [PubMed] [Google Scholar]
- 40.Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. Journal of Applied Psychology. 1989;74(5):728–38. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- 41.Natale V, Alzani A. Additional validity evidence for the composite scale of morningness. Personality and Individual Differences. 2001;30(2):293–301. [Google Scholar]
- 42.Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. Journal of Abnormal Psychology. 1982;91(3):199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- 43.Clark DB, Pollock NK, Mezzich A, Cornelius J, Martin C. Diachronic assessment and the emergence of substance use disorders. Journal of Child and Adolescent Substance Abuse. 2001;10:13–22. [Google Scholar]
- 44.Skinner HA, Horn JL. Alcohol Dependence Scale (ADS) User’s Guide. Toronto: Addiction Research Foundation; 1984. [Google Scholar]
- 45.Day E, Day E, Best D, Cantillano V, Lopez Gaston R, Nambamali A, et al. Measuring the use and career histories of drug users in treatment: reliability of the Lifetime Drug Use History (LDUH) and its data yield relative to clinical case notes. Drug and alcohol review. 2008;27(2):171–7. doi: 10.1080/09595230701829504. [DOI] [PubMed] [Google Scholar]
- 46.Rose EJ, Ross TJ, Salmeron BJ, Lee M, Shakleya DM, Huestis MA, et al. Acute nicotine differentially impacts anticipatory valence-and magnitude-related striatal activity. Biological psychiatry. 2013;73(3):280–8. doi: 10.1016/j.biopsych.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cahalan D, Cisin I, Crossley H. American drinking practices. New Brunswick, N.J.: Center for Alcohol Studies, Rutgers University; 1969. [Google Scholar]
- 48.Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, et al. Healthy adolescents’ neural response to reward: associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49(2):162–72. e1–5. doi: 10.1097/00004583-201002000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.First MB, Gibbon M, Spitzer RL. Structured clinical interview for DSM-IV axis II personality disorders (SCID-II) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 50.Hasler BP, Forbes EE, Franzen PL. Time-of-day differences and short-term stability of the neural response to monetary reward: a pilot study. Psychiatry Research: Neuroimaging. 2014;224(1):22–7. doi: 10.1016/j.pscychresns.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, et al. Nature’s clocks and human mood: the circadian system modulates reward motivation. Emotion. 2009;9(5):705–16. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- 52.Muthé L, Muthén B. Mplus (Version 7.3)[computer software]. 1998–2014. Los Angeles, CA: Muthén & Muthén; 2014. [Google Scholar]
- 53.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; 2013. [Google Scholar]
- 54.Schreiber JB, Nora A, Stage FK, Barlow EA, King J. Reporting structural equation modeling and confirmatory factor analysis results: A review. The Journal of Educational Research. 2006;99(6):323–38. [Google Scholar]
- 55.Hasler BP, Soehner AM, Clark DB. Sleep and circadian contributions to adolescent alcohol use disorder. Alcohol. 2014 doi: 10.1016/j.alcohol.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Logan RW, Williams WP, McClung CA. Circadian rhythms and addiction: Mechanistic insights and future directions. Behavioral neuroscience. 2014 doi: 10.1037/a0036268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward:‘liking’,‘wanting’, and learning. Current opinion in pharmacology. 2009;9(1):65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hommer DW, Bjork JM, Gilman JM. Imaging brain response to reward in addictive disorders. Annals of the New York Academy of Sciences. 2011;1216(1):50–61. doi: 10.1111/j.1749-6632.2010.05898.x. [DOI] [PubMed] [Google Scholar]
- 59.Balodis IM, Potenza MN. Anticipatory reward processing in addicted populations: a focus on the monetary incentive delay task. Biological psychiatry. 2015;77(5):434–44. doi: 10.1016/j.biopsych.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Forbes EE, Rodriguez EE, Musselman S, Narendran R. Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PloS one. 2014;9(5):e94640. doi: 10.1371/journal.pone.0094640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bogg T, Fukunaga R, Finn PR, Brown JW. Cognitive control links alcohol use, trait disinhibition, and reduced cognitive capacity: Evidence for medial prefrontal cortex dysregulation during reward-seeking behavior. Drug and Alcohol Dependence. 2012;122(1–2):112–8. doi: 10.1016/j.drugalcdep.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mongrain V, Carrier J, Dumont M. Circadian and homeostatic sleep regulation in morningness-eveningness. Journal of Sleep Research. 2006;15(2):162–6. doi: 10.1111/j.1365-2869.2006.00532.x. [DOI] [PubMed] [Google Scholar]
- 63.Mullin BC, Phillips ML, Siegle GJ, Buysse DJ, Forbes EE, Franzen PL. Sleep deprivation amplifies striatal activation to monetary reward. Psychological Medicine. 2013;43(10):2215–25. doi: 10.1017/S0033291712002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30(5):603–9. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- 65.Bennett CM, Miller MB. How reliable are the results from functional magnetic resonance imaging? Annals of the New York Academy of Sciences. 2010;1191(1):133–55. doi: 10.1111/j.1749-6632.2010.05446.x. [DOI] [PubMed] [Google Scholar]
- 66.Plichta MM, Schwarz AJ, Grimm O, Morgen K, Mier D, Haddad L, et al. Test-retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. NeuroImage. 2012;60(3):1746–58. doi: 10.1016/j.neuroimage.2012.01.129. [DOI] [PubMed] [Google Scholar]
- 67.Van Leijenhorst L, Westenberg PM, Crone EA. A developmental study of risky decisions on the cake gambling task: age and gender analyses of probability estimation and reward evaluation. Developmental neuropsychology. 2008;33(2):179–96. doi: 10.1080/87565640701884287. [DOI] [PubMed] [Google Scholar]
- 68.Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. Journal of Biological Rhythms. 2003;18(1):80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- 69.Harford TC, Hy Yi, Faden VB, Chen CM. The dimensionality of DSM‐IV alcohol use disorders among adolescent and adult drinkers and symptom patterns by age, gender, and race/ethnicity. Alcoholism: Clinical and Experimental Research. 2009;33(5):868–78. doi: 10.1111/j.1530-0277.2009.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Macoveanu J, Fisher PM, Madsen MK, Mc Mahon B, Knudsen GM, Siebner HR. Bright-light intervention induces a dose-dependent increase in striatal response to risk in healthy volunteers. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.06.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


