Abstract
Background
Psychiatric comorbidity is common among individuals with addictive disorders, with patients frequently suffering from anxiety disorders. While the genetic architecture of comorbid addictive and anxiety disorders remains unclear, elucidating the genes involved could provide important insights into the underlying etiology.
Methods
Here we examine a sample of 1284 Mexican-Americans from randomly selected extended pedigrees. Variance decomposition methods were used to examine the role of genetics in addiction phenotypes (lifetime history of alcohol dependence, drug dependence or chronic smoking) and various forms of clinically relevant anxiety. Genome-wide univariate and bivariate linkage scans were conducted to localize the chromosomal regions influencing these traits.
Results
Addiction phenotypes and anxiety were shown to be heritable and univariate genome-wide linkage scans revealed significant quantitative trait loci for drug dependence (14q13.2–q21.2, LOD = 3.322) and a broad anxiety phenotype (12q24.32–q24.33, LOD = 2.918). Significant positive genetic correlations were observed between anxiety and each of the addiction subtypes (ρg = 0.550–0.655) and further investigation with bivariate linkage analyses identified significant pleiotropic signals for alcohol dependence-anxiety (9q33.1–q33.2, LOD = 3.054) and drug dependence-anxiety (18p11.23–p11.22, LOD = 3.425).
Conclusions
This study confirms the shared genetic underpinnings of addiction and anxiety and identifies genomic loci involved in the etiology of these comorbid disorders. The linkage signal for anxiety on 12q24 spans the location of TMEM132D, an emerging gene of interest from previous GWAS of anxiety traits, whilst the bivariate linkage signal identified for anxiety-alcohol on 9q33 peak coincides with a region where rare CNVs have been associated with psychiatric disorders. Other signals identified implicate novel regions of the genome in addiction genetics.
Keywords: Addiction, Anxiety, Comorbidity, Pleiotropy
1. Introduction
Amongst individuals addicted to licit and illicit substances, the prevalence of comorbid psychiatric illnesses is high. In particular, epidemiological evidence for the pattern of comorbidity between addiction and anxiety is well established [1]; in addicted populations, there is a significant increase in the risk for anxiety disorders, even when covarying for demographic characteristics and other psychiatric diagnoses [2,3]. For example, among individuals with alcohol dependence the risk of panic disorder and posttraumatic stress disorder are doubled and that for generalized anxiety disorder is four times the population risk [4]. As the severity of substance misuse increases, so does the observed rate of anxiety comorbidity [2,5]. Psychiatric comorbidities are also associated with poorer outcomes among individuals with addictive disorders, including more severe symptomatology, greater social and functional impairments and increased suicide risk [6]. The lifetime prevalence of anxiety disorders and substance disorders are estimated at 18.1% and 14.6%, respectively [7], making the impact of this elevated risk substantial. However, the directionality of these effects is not clear. Increased comorbidity could be conceptualized as addiction increasing the likelihood of anxiety (due to drug use or withdrawal [8]), anxiety increasing the likelihood of addiction (to self-medicate symptoms [9]), or a third factor increasing the risk of both addiction and anxiety [5]. Disentangling these influences is highly complex, but as a potential starting point to unpick this relationship, twin and family studies focusing on alcohol disorders suggest that there may be a shared genetic etiology influencing liability to both addiction and anxiety [5,10,11].
Genetics play an important role in addiction disorders [12]. Heritability estimate for addiction to specific drugs are well established [13]. Furthermore, twin and family studies indicate that in addition to substance-specific genetic influences, there are common genetic factors acting across addiction phenotypes [14–16]. These findings suggest that genetic liability to addictive disorders arises from an increased propensity to addiction in general, combined with influences that are specific to that particular drug. Similarly, anxiety disorders are interlinked with one another, with high comorbidity rates (one study estimates 76% of anxiety cases have a experienced at least one other anxiety or depressive disorder [17]). We know that anxiety disorders are heritable [18,19], and as with addiction phenotypes, multivariate analyses exploring the genetic relationship between the different anxiety disorders have been undertaken. Results indicate an important role for common genetic influences acting across anxiety subtypes in addition to subtype-specific genetic influences [20–22]. Indeed, Tambs et al. [20] report that that heritability estimates for liability to any anxiety disorder (including PTSD and OCD) are higher than those for individual anxiety subtypes. Furthermore, the common genetic influences acting across anxiety subtypes substantially (but not completely) overlap with the genetic factors associated with neuroticism [23].
The high comorbidity between addictive and anxious phenotypes may reflect shared genetic influences. Genetics factors contribute substantially to the phenotypic correlations between alcohol consumption and anxiety or depression symptoms in twins [11]. Furthermore, a number of studies looking at patterns of transmission in families show shared familial risk factors for alcohol dependence and panic disorder [5,10], consistent with the hypothesis that addiction and anxiety are genetically correlated traits. Nevertheless, these studies have tended to focus on alcohol-related phenotypes; there is less work considering whether this genetic overlap generalizes across addiction subtypes.
To gain insight into the neurobiology underlying the overlap between addiction and anxiety, it is important to both understand the extent to which genetic liabilities are common among traits, and identify the genetic loci that contribute to this shared liability. Linkage analysis within family studies can allow us to do this by identifying chromosomal regions that segregate in families with disease. Furthermore, linkage methods capture a diverse range of types of genetic effects, including common and rare variants that are also important contributors to the risk for psychiatric disorders [24,25]. A number of univariate genome-wide linkage efforts have been published exploring anxiety disorders [26], and addiction [27–29]. While some genes implicated in this comorbidity have been linked separately to the risk for each disorder using candidate approaches, including neuropeptide Y [30,31] and GABA [32], we are unaware of any previous studies that have used linkage methods to investigate the comorbidity of these disorders on a genome-wide scale. Here, we employ bivariate linkage approaches looking at addiction and anxiety traits together, to identify chromosomal regions with pleiotropic influences on both traits. Bivariate linkage analysis provides greater statistical power to detect quantitative trait loci for correlated traits, as well as improved localization of the linkage signal [33], offering a powerful tool to identify genomic regions that jointly influence these comorbid disorders. We examine the genetic influences on addiction and anxiety phenotypes using a large randomly ascertained family sample and use both univariate and bivariate linkage analyses to identify chromosomal loci that underpin these phenotypes and their patterns of comorbidity.
2. Methods
2.1. Study sample
The sample consisted of 1284 Mexican-Americans from 75 extended pedigrees (containing 2–132 subjects, mean pedigree size = 16.36, SD = 19.41) and an additional 57 genetically unrelated spouses. The mean age of the sample was 46.08 years (SD = 14.89, range = 18–97 years), and 62.7% of the sample was female. Stated pedigree relationships were confirmed using PREST and available autosomal markers [34].
The sample is a subset of the San Antonio Family Study cohort of individuals randomly ascertained with the constraints that participants must live within the San Antonio region, be Mexican-American in ancestry, and part of a large family (at least 6 1st degree relatives). Full recruitment details are available elsewhere [35,36]. All subjects provided informed written consent (as approved by Institutional Review Boards at the university of Texas, health science center San Antonio and Yale university) and the cohort have been actively participating in research for over 18 years.
2.2. Phenotypic assessment
The semi-structured Mini International Psychiatric Interview Plus (MINI-Plus; [37]) was administered to all participants, with additional questions to establish lifetime history of psychiatric disorders, using DSM-IV criteria [38]. Lifetime history of chronic smoking was defined as ever having smoked cigarettes, cigars or a pipe every day for a month or more. Lifetime history of alcohol dependence was assessed, as was drug dependence (here defined as dependence on any drugs other than tobacco or alcohol; the Supplementary materials includes the prompts given). Anxiety cases were defined as those patients meeting criteria for any of the following; panic disorder, agoraphobia, social phobia, specific phobia, OCD, PTSD, or generalized anxiety disorder. Prevalence by subtype is included in the Supplementary materials. All interviewers administering the MINI-Plus had a doctorate or master’s degree in a mental health field or a bachelor’s degree with at least 2 years of relevant experience, and high levels of diagnostic reliability were reached for anxiety and addiction disorders (κ > = 0.90).
2.3. Genotyping
Genotyping for approximately one million SNPs was performed using Illumina HumanHap550v3, HumanExon510Sv1, Human1Mv1 and Human1M-Duov3 BeadChips, and following the Illumina Infinitum protocol. For quality control, SNPs and samples were removed if < 95% of genotypes were present or if the minor allele was present in less than 10 individuals. Allele frequencies were calculated using maximum likelihood methods to account for pedigree relationships [39] and SNPs were observed to be in Hardy Weinberg Equilibrium. SimWalk2 was used to check for Mendelian consistency and missing genotypes were imputed (utilizing the available pedigree information) within MERLIN [40].
For linkage analysis, a subset of 28,387 SNPs from the 1 M GWAS SNPs were selected using genotypes from 345 founders. SNPS were selected on each chromosome with a minimum spacing of 1 KB and a minor allele frequency > 5%. To limit linkage disequilibrium between SNPs, a limit of pairwise r2 < 0.0225 within a 100 kb sliding window was used. The selected subset of SNPs gave an average of 7–8 SNPs percentimorgan (cM). Using these 28,387 SNPs, multipoint identity-by-descent matrices were constructed at each centimorgan location, using a stochastic Markov Chain Monte Carlo procedure within LOKI [41].
2.4. Statistical analysis
SOLAR was used for all genetic analyses [42]. This software uses maximum likelihood decomposition methods to model the patterns of trait covariance between family members as a function of genetic relationships, and so estimate the genetic and environmental contributions to phenotypes within a family structure. All analyses included the covariates of age, age2, sex and their interactions.
To identify the relative contribution of additive genetic and environmental influences for addiction-related and anxiety traits, univariate variance decomposition was performed, yielding trait heritability estimates. For each trait showing significant heritability, we performed genome-wide multipoint linkage analysis, to identify chromosomal loci involved in the trait of interest. Based on a method derived by Feingold et al. [43], it was previously established in this sample that a LOD score greater than 2.9 indicates a genome-wide significant linkage peak, whilst a LOD score over 1.67 is indicative of a suggestive peak (likely to occur by chance less than once per genome-wide scan) [44]. These LOD scores are per genome-wide scan, whilst here, we examined four correlated phenotypes (alcohol dependence, drug dependence, smoking and anxiety). Therefore, using the method outlined by Cheverud [45] to assess the genetic correlations between traits, we established that the effective number of traits considered was 2.61.
We then explored the genetic relationships between addiction and anxiety. First, we examined the covariance between each of the three addiction-related traits and anxiety, to obtain bivariate genetic correlations. Then, for pairs of traits where significant genetic correlations were observed, bivariate linkage analysis was performed [33]. To enable comparison with the univariate results, bivariate LOD scores were converted to a one degree-of-freedom equivalent (based upon the P-value for the two degrees-of-freedom test of linkage to both traits versus linkage to neither). In order to assess whether bivariate linkage signals were truly bivariate in nature, nested models were compared to test whether the model could be explained in the absence of any shared genetic effect; that is where co-occurrence of linkage peaks in the two traits occurred by chance [46]. A confidence interval of maximum LOD score −1 was defined surrounding each significant linkage peak.
To follow up any significant linkage signals, we extracted SNPs that were located within each significant peak from the full 1 M GWAS SNPs. Using the measured genotype association (mga) method, where models include the fixed effect of the SNP and random effects of local and polygenic heritability, individual SNPs were associated traits of interest. An additive genetic model was assumed, and the first four principal components of the GWAS data were included as covariates to account for population and admixture substructure within pedigrees [47]. To test for significance, a likelihood ratio test was employed, comparing models where the genotype parameter is allowed to vary freely or is fixed to zero. Given linkage disequilibrium, the pairwise correlations between SNPs were used to calculate the effective number of independent tests within each region [48], for an appropriate multiple-testing correction.
3. Results
3.1. Univariate analyses
The prevalence and heritability of each trait is shown in Table 1. As expected, for addiction phenotypes, there are fewer female than male cases, but the majority of anxiety cases are female. When calculating the heritability of each trait, sex was a significant covariate for all phenotypes (details are given in the Supplementary materials).
Table 1.
Prevalence and heritability of addiction traits and anxiety.
| Trait | % cases (total n =1284) |
% female | Heritability
|
||
|---|---|---|---|---|---|
| h2 | P-value | SE | |||
| Alcohol dependence | 23.4 (n = 300) |
32.3 | 0.402 | 1.10E-05 | 0.110 |
| Drug dependence | 10.6 (n =136) |
27.9 | 0.609 | 1.87E-05 | 0.165 |
| Smoking | 33.9 (n = 435) |
45.3 | 0.554 | 4.79E-13 | 0.095 |
| Anxiety | 22.9 (n = 294) |
78.2 | 0.287 | 3.96E-04 | 0.102 |
Univariate genome-wide linkage scans were performed for each of the four phenotypes under consideration. Significant linkage peaks were observed for drug dependence on chromosome 14 at 41cM (LOD score = 3.322) and for anxiety on chromosome 12 at 164cM (LOD = 2.918). For alcohol dependence and smoking, genome-wide significance was not reached. The maximum linkage for alcohol dependence was on chromosome 16 at 54cM (LOD score = 2.752) and for smoking on chromosome 4 at 80cM (LOD score = 2.735). The maximum peak for every trait is shown in Fig. 1. The Supplementary materials includes details on the genes located within each of these peaks, as well as plots of genome-wide linkage results for all four traits. The linkage signal for anxiety on chromosome 12 encompasses TMEM132D, which has previously been associated with anxiety-related traits [49–53]. Each of the univariate linkage signals is specific to the trait of interest; LOD scores are below 0.7 for all other traits in each of the identified chromosomal loci.
Fig. 1.
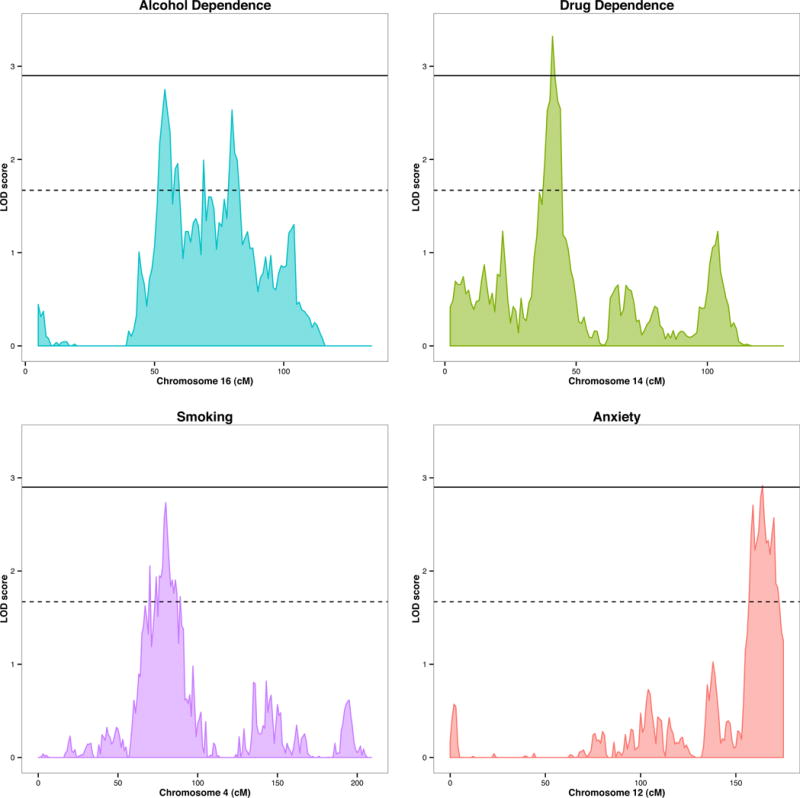
Signficant univariate linkage signals. Alcohol dependence (Chr 16LOD = 2.752 at 54cM); drug dependence (Chr 14LOD = 3.322 at 41cM); smoking (Chr 4 LOD = 2.735 at 80cM); anxiety (Chr 12 LOD = 2.918 at 164cM).
3.2. Bivariate analyses
The patterns of comorbidity between addiction subtypes and anxiety are shown in Table 2 (comorbidity rates amongst the addiction subtypes are in the Supplementary materials). Patterns of shared genetic underpinnings are evident (Table 3) with significant positive genetic correlations observed between anxiety and each of the addiction traits.
Table 2.
Addiction cases with comorbid anxiety.
| Percentage of cases with comorbid anxiety | |
|---|---|
| Alcohol dependence | 28.3% (n = 85) |
| Drug dependence | 38.2% (n = 52) |
| Smoking | 22.5% (n=98) |
Table 3.
Phenotypic and genetic correlations between addiction traits and anxiety.
| Phenotypic correlation with anxiety | Genetic correlation with anxiety | |
|---|---|---|
| Alcohol dependence | 0.281 (P=1.85E-06) |
0.655 (P=3.48E-03) |
| Drug dependence | 0.412 (P=2.75E-09) |
0.619 (P=7.82E-03) |
| Smoking | 0.112 (P=4.15E-02) |
0.550 (P=3.73E-03) |
Bivariate linkage scans were performed for anxiety with each of the addiction traits. Evidence of pleiotropic genomic regions were observed in two cases. For alcohol dependence and anxiety, a significant bivariate peak was observed on chromosome 9 (at 133cM, LOD = 3.054). At this location, the univariate linkage analyses gave a LOD = 2.562 for alcohol dependence and LOD = 1.673 for anxiety. Using nested models to compare coincident with pleiotropic models, this peak was observed to be pleiotropic (χ2[1] = 11.38, p = 3.71 × 10−4). A second significant bivariate linkage peak was observed for drug dependence and anxiety on chromosome 18 at 29 cm (LOD = 3.425, pleiotropy test χ2[l] = 16.02, p = 3.14 × 10−5). The univariate linkage signals at this locus were LOD = 1.098 for drug dependence and LOD = 1.887 for anxiety. No significant bivariate linkage was observed for anxiety and smoking. The significant bivariate linkage peaks are shown in Fig. 2. Genes located within these peaks and plots of the full genome-wide linkage results are in the Supplementary materials.
Fig. 2.
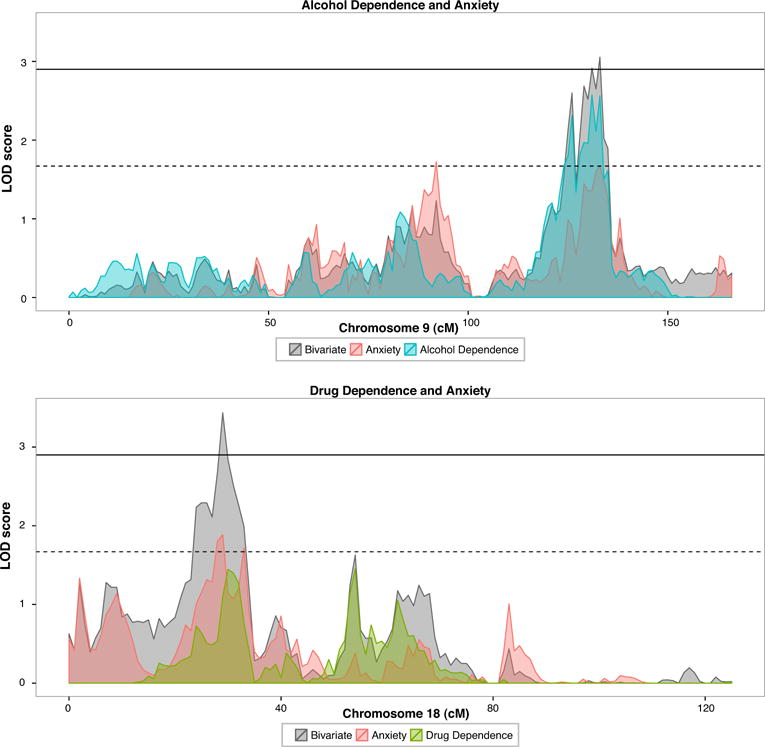
Significant bivariate linkage signals. Alcohol dependence and anxiety (Chr 9 at 133cM, bivariate LOD = 3.054); drug dependence and anxiety (Chr 18 at 29cM, bivariate LOD = 3.425). Bivariate linkage shown in grey, overlaid on univariate signals.
3.3. Association analysis of significant linkage peaks
We performed mga analysis using all of the available SNPs underneath each of the four significant linkage peaks identified. No SNPs reached peak-wide significance, for any of the four regions considered, although the most significant SNP for anxiety (rs10744424) is within TMEM132D. Details of the best performing SNPs for each region are given in Table 4.
Table 4.
Best performing snps in association analysis. No snps pass peak-wide significance.
| Drug dependence | Anxiety | Alcohol dependence/anxiety | Drug dependence/anxiety | |
|---|---|---|---|---|
| Chromosome | 14 | 12 | 9 | 18 |
| Size of linkage peak region (Mb) | 8.46 | 2.66 | 4.98 | 1.20 |
| No. of SNPs in peak | 2728 | 1443 | 1643 | 552 |
| Effective no. of SNPs in peak | 1364.97 | 952.619 | 898.131 | 337.16 |
| Peak-wide P-value threshold | 3.76E-05 | 5.38E-05 | 5.71E-05 | 1.52E-04 |
| Min P-value observed | 2.62E-04 | 7.80E-05 | 1.30E-04 | 2.27E-03 |
| Best SNP | rs17113722 | rs10744424 | rs10117151 | rs11661395 |
| Alleles | C/T | A/G | A/C | C/T |
| SNP MAF | 0.052 | 0.117 | 0.433 | 0.478 |
| Allele frequency in 1000 genomes MXL population | 0.070 | 0.133 | 0.469 | 0.523 |
| Allele frequency in 1000 genomes EUR population | 0.082 | 0.156 | 0.625 | 0.416 |
| Allele frequency in Alfred Pima Bajo population | 0.020 | 0.000 | 0.120 | Not available |
| Nearest gene | LRFN5 (nearest) | TMEM132D (intronic) | ASTN2 (nearest) | RAB12 (nearest) |
4. Discussion
4.1. Summary of findings
We have demonstrated that shared genetic influences play an important role in the high rates of comorbidity between anxiety and addiction (examining alcohol dependence, drug dependence and chronic smoking in turn). We observed genetic correlations between 0.550–0.655 within a Mexican-American extended pedigree sample. This is consistent with previous findings for anxiety and alcohol dependence [5,10,11] and our genetic correlation estimates are also similar to those observed when considering addiction in relation to major depression [54,55].
Having established a common genetic etiology, we used genome-wide linkage analyses to identify specific and pleiotropic quantitative trait loci for these phenotypes. Genome-wide significant univariate linkage signals were observed for drug dependence at 14q13.2–q21.2 and for anxiety at 12q24.32–q24.33. Genomewide significant bivariate linkage peaks were observed for alcohol dependence-anxiety at 9q33.1–q33.2 and for drug dependence-anxiety at 18p11.23–p11.22. For each of the phenotypes considered here, the identified quantitative trait loci were phenotype specific; LOD scores were low (< 0.7) in the univariate linkage scans of the other traits, despite the high genetic correlations among traits. It suggests that these loci are phenotype-specific, without pleiotropic effects on the other traits studied. However, given the limited power to detect loci of small effect, it is also possible that the loci were detected only through the phenotype on which they exert the largest effect and potential pleiotropic effects on the other phenotypes cannot be excluded. We used mga analyses to try to localize these linkage signals, using available SNP data. We were unable to identify any peak-wide significant SNP associations; this may be as we were only able to examine common SNPs or possibly due to sample size limitations.
4.2. Previously implicated genomic locations
The significant linkage signals identified in this analysis show some interesting convergence with previous findings in psychiatric genetics. First, in the univariate analysis of anxiety, we observed a significant linkage peak on chromosome 12q24.32–q24.33. While this peak only just reaches the threshold for genome-wide significance, it is of particular interest given that TMEM132D (transmembrane protein 132D) lies within this region. This gene was first implicated through GWAS investigation of panic disorder, with mouse model evidence supporting an involvement in anxious behavior [49]. Since then, both common and rare variants within the gene have been associated with anxiety disorders [50–52] and links have also been observed between variants in this gene, amygdala volume and anxiety traits in healthy controls [53]. Using association analysis in this region, the best performing SNP in our sample is rs10744424 (intronic within TMEM132D), although it does not reach significance when correcting for all SNPs available in the 2.66MB linkage region. Therefore, the identification here of a linkage peak for anxiety in the same genomic region as previous research is consistent with the role of the gene in liability to anxiety disorders, but as we are unable to more specifically localize the signal within this peak, our findings are not conclusive.
Second, we localize a bivariate linkage signal between alcohol dependence and anxiety at 9q33.1–q33.2. Previous work has linked rare CNVs within 9q33 to a number of traits, including neurodevelopmental delays and psychiatric disorders including anxiety [56]. There has also been some evidence of linkage in this region with addiction traits; in a study of alcohol-related traits a suggestive linkage peak was observed in the same location [57] and in a 2008 review of genome-wide linkage studies in smoking, this region had been implicated in four independent samples [58], although this signal was not observed in a later meta-analysis [27] and the signal has not been localized to a specific gene. Therefore, there is some tentative evidence that this genomic region may be linked to liability to a number of psychiatric traits.
The remaining linkage peaks (for drug dependence on chromosome 14 and for drug dependence-anxiety on chromosome 18) identified in this study appear to be in regions that have not previously been highlighted in addiction genetics. Generally, for illicit drugs, the genetic loci involved are not well established, which may in part be due to the difficulties in obtaining sufficiently large samples of drug dependent individuals for adequately powered genetic studies. Indeed, the drug dependent group contains individuals that are addicted to a range of different illicit drugs (such as cannabis, cocaine or tranquilizers). By grouping these individuals into a single category, we are able to increase statistical power to detect genetic effects that are common among these illicit drugs and detect both univariate and pleiotropic linkage signals, although drug-specific signals may be obscured.
It is of note that we did not observe genome-wide linkage signals within either univariate or bivariate models for smoking within this sample, using an “ever vs never” categorization to assess initiation of chronic smoking. This is despite significant trait heritability (h2 = 0.554) and genetic correlation with anxiety (ρg = 0.550). There has been greater success in the literature using smoking quantity or nicotine dependence phenotypes, which are genetically overlapping but not identical to smoking initiation [59]. However, these measures are unavailable in this sample. In a comprehensive meta-analysis of linkage studies looking at 3404 families, genome-wide significance was only observed with the phenotype of “maximum number of cigarettes per day”, on chromosome 20q13.12–13.32 [27]. Interestingly, in this metaanalysis, they failed to find evidence on linkage on chromosome 15q25, the location of the nicotinic acetylcholine receptor gene cluster which is a well-established hit for the phenotype of “cigarettes per day” from genome-wide association approaches [60].
4.3. Implications of findings
As a complex trait, addiction is likely to be highly polygenic, with both general and substance-specific components; the genetic loci identified to date only account for a small proportion of the variance in liability to addiction. Similarly, the genetic influences on anxiety traits remain poorly understood. By considering the relationships between addiction and anxiety, we aimed to reveal details of the genetic architecture underlying these comorbid traits. By demonstrating genetic correlations between addiction subtypes and anxiety, we have shown that shared genetic etiology plays an important role in the high rates of comorbidity, although this does not exclude the existence of causal pathways between the two types of disorder. It still remains that addiction could manifest as a result of anxiety symptoms, or indeed vice versa. We suggest that a single generality is unlikely, heterogeneity between individuals and a pattern of multiple interacting pathways fits the diverse clinical picture more accurately. However, to begin to understand the biological pathways responsible for the shared genetic etiology of these two disorders, we must identify the specific genes involved.
Leveraging the increased statistical power of bivariate linkage analyses to identify the regions of the genome involved in this shared genetic etiology [33], we have highlighted potential pleiotropic regions of the genome, implicating both previously identified and novel chromosomal loci that may contribute risk for each these diagnoses. These regions point to candidates for further research into these disorders, and offer a potential starting point to begin to unpick the underlying neurobiology for each diagnosis and understand how they might be interconnected.
4.4. Characteristics of the sample
Our study examines a Mexican-American sample. Within the USA, it has been shown that the prevalence of addiction and psychiatric disorders in amongst Hispanics is generally lower than amongst non-Hispanic Caucasians; this is also true for black and Asians minorities [7,61]. However, there is variation within ethnic minority subgroups, according to interacting factors including country of origin, level of discrimination and acculturation [62–65]. Relating the prevalence of disorder within the sample to US national averages, we find that addiction phenotypes are more common in this sample, however, rates of anxiety are comparable to those reported in large epidemiological surveys [7,61].
In defining anxiety in this sample, we grouped all anxiety diagnoses along with PTSD and OCD together into a single category. This approach of grouping rather than splitting was taken for two reasons; first due to low numbers of cases for some diagnoses. Second, whilst this grouping does not capture known anxiety disorder-specific genetic influences, previous work shows that when all anxiety disorders (including PTSD and OCD) are considered, a common pathway model best describes the relationship between disorders, with most of the genetic effects being shared across disorders [20]. Additional epidemiological [66] and twin [21] research supports this approach. Whilst we relied upon DSM-IV criteria [38] to classify alcohol dependence, substance dependence and anxiety disorders, we note that in DSM-V [67] (published after the collection of this data), OCD and PTSD have been moved from the Anxiety Disorders chapter (to adjacent chapters of Obsessive-Compulsive and Related Disorders and Trauma and Stressor-Related Disorders) and changes have been made to criteria defining substance use disorders.
It is known that patients recruited into a study from a treatment setting often differ from the general population of affected individuals, particularly in terms of symptom severity and patterns of comorbidity [68]. By using a randomly ascertained extended pedigree sample, we have side-stepped these potential biases. Additionally, the extended pedigree design that we employ here offers increased power to detect quantitative trait loci over smaller family units in most circumstances [69], and while family studies typically do not directly estimate the influence of environments shared by family members (as twin studies do), the inflation of heritability estimates by these shared environmental influences are much less likely in multigenerational families such as those included in this sample [70].
Given the contrasting prevalence by sex of addiction and anxiety, paired with overlapping genetic liability, the manifestation of symptoms associated with this shared vulnerability may differ by gender. It would be of interest to model genetic liability to these comorbidity disorders in a sex-specific manner. However, this sample is not well suited to tackle the issue, as sex-specific analyses would require breaking up the extended pedigree structure, resulting in a substantial loss of statistical power and further, only autosomal chromosomes are considered in this analysis.
5. Conclusions
In this study, we observe a number of significant quantitative trait loci that underlie the genetic overlap of the addiction subtypes of alcohol dependence, drug dependence and smoking with anxiety, within a randomly ascertained sample. The regions we identify show some convergence with previous literature, but also highlight a number of novel genomic loci involved in the comorbidity of these psychiatric traits.
Acknowledgments
Funding
Financial support for this study was provided by the National Institute of Mental Health grants MH078143 (PI: D.C. Glahn), MH078111 (PI: J. Blangero), and MH083824(PI: D.C. Glahn). SOLAR is supported by NIMH grant MH059490 (J. Blangero).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.eurpsy.2016.03.004.
Footnotes
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61(8):807–16. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 2.Compton WM, Thomas YF, Stinson FS, Grant BF, American medical association Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64(5):566–76. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- 3.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States. Arch Gen Psychiatry. 2007;64(7):830–42. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- 4.Klimkiewicz A, Klimkiewicz J, Jakubczyk A, Kieres-Salomonski I, Wojnar M. Comorbidity of alcohol dependence with other psychiatric disorders. Part I. Epidemiology of dual diagnosis Psychiatr Pol. 2015;49(2):265–75. doi: 10.12740/PP/25704. [DOI] [PubMed] [Google Scholar]
- 5.Merikangas KR, Stevens DE, Fenton B, Stolar M, O’Malley S, Woods SW, et al. Comorbidity and familial aggregation of alcoholism and anxiety disorders. Psychol Med. 1998;28(4):773–88. doi: 10.1017/s0033291798006941. [Cambridge university press] [DOI] [PubMed] [Google Scholar]
- 6.Jamal M, Willem Van der Does AJ, Cuijpers P, Penninx BWJH. Association of smoking and nicotine dependence with severity and course of symptoms in patients with depressive or anxiety disorder. Drug Alcohol Depend. 2012;126(1–2):138–46. doi: 10.1016/j.drugalcdep.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE, American medical association Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National comorbidity survey replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 8.Quitkin FM, Rifkin A, Kaplan J, Klein D, American medical association Phobic anxiety syndrome complicated by drug dependence and addiction. Arch Gen Psychiatry. 1972;27(2):159. doi: 10.1001/archpsyc.1972.01750260013002. [DOI] [PubMed] [Google Scholar]
- 9.George DT, Nutt DJ, Dwyer BA, Linnoila M. Alcoholism and panic disorder: is the comorbidity more than coincidence? Acta Psychiatr Scand. 1990;81(2):97–107. doi: 10.1111/j.1600-0447.1990.tb06460.x. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin RD, Lipsitz JD, Keyes K, Galea S, Fyer AJ. Family history of alcohol use disorders among adults with panic disorder in the community. J Psychiatr Res. 2011;45(8):1123–7. doi: 10.1016/j.jpsychires.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tambs K, Harris JR, Magnus P. Genetic and environmental contributions to the correlation between alcohol consumption and symptoms of anxiety and depression. Results from a bivariate analysis of Norwegian twin data. Behav Genet. 1997;27(3):241–50. doi: 10.1023/a:1025662114352. [DOI] [PubMed] [Google Scholar]
- 12.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6(7):521–32. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal A, Verweij KJH, Gillespie NA, Heath AC, Lessov-Schlaggar CN, Martin NG, et al. The genetics of addiction-a translational perspective. Transl Psychiatry. 2012;2:e140. doi: 10.1038/tp.2012.54. [Macmillan Publishers Limited] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kendler KS, Myers J, Prescott CA, American medical association Specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine, and nicotine dependence. Arch Gen Psychiatry. 2007;64(11):1313–20. doi: 10.1001/archpsyc.64.11.1313. [DOI] [PubMed] [Google Scholar]
- 15.Bierut LJ, Dinwiddie SH, Begleiter H, Crowe RR, Hesselbrock V, Nurnberger JI, et al. Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking. Arch Gen Psychiatry. 1998;55(11):982. doi: 10.1001/archpsyc.55.11.982. [DOI] [PubMed] [Google Scholar]
- 16.Palmer RHC, Button TM, Rhee SH, Corley RP, Young SE, Stallings MC, et al. Genetic etiology of the common liability to drug dependence: evidence of common and specific mechanisms for DSM-IV dependence symptoms. Drug Alcohol Depend. 2012;123(Suppl):S24–32. doi: 10.1016/j.drugalcdep.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110(4):585–99. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- 18.Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158(10):1568–78. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- 19.Smoller JW, Block SR, Young MM. Genetics of anxiety disorders: the complex road from DSM to DNA. Depress Anxiety. 2009;26(11):965–75. doi: 10.1002/da.20623. [DOI] [PubMed] [Google Scholar]
- 20.Tambs K, Czajkowsky N, Roysamb E, Neale MC, Reichborn-Kjennerud T, Aggen S, et al. Structure of genetic and environmental risk factors for dimensional representations of DSM-IV anxiety disorders. Br J Psychiatry. 2009;195(4):301–7. doi: 10.1192/bjp.bp.108.059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chantarujikapong SI, Scherrer JF, Xian H, Eisen SA, Lyons MJ, Goldberg J, et al. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and post-traumatic stress disorder in men. Psychiatry Res. 2001;103(2–3):133–45. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- 22.Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS, American medical association The structure ofgenetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62(2):182–9. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- 23.Hettema JM, Neale MC, Myers JM, Prescott CA, Kendler KS, American psychiatric association A population-based twin study of the relationship between neuroticism and internalizing disorders. Am J Psychiatry. 2006 doi: 10.1176/ajp.2006.163.5.857. [DOI] [PubMed] [Google Scholar]
- 24.Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper G, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320(5875):539–43. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 25.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–53. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smoller JW, Gardner-Schuster E, Misiaszek M. Genetics of anxiety: would the genome recognize the DSM? Depress Anxiety. 2008;25(4):368–77. doi: 10.1002/da.20492. [DOI] [PubMed] [Google Scholar]
- 27.Han S, Gelernter J, Luo X, Yang B. Meta-analysis of 15 genome-wide linkage scans of smoking behavior. Biol Psychiatry. 2010;67(1):12–9. doi: 10.1016/j.biopsych.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agrawal A, Hinrichs AL, Dunn G, Bertelsen S, Dick DM, Saccone SF, et al. Linkage scan for quantitative traits identifies new regions of interest for substance dependence in the collaborative study on the genetics of alcoholism (COGA) sample. Drug Alcohol Depend. 2008;93(1–2):12–20. doi: 10.1016/j.drugalcdep.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang BZ, Han S, Kranzler HR, Farrer LA, Elston RC, Gelernter J. Autosomal linkage scan for loci predisposing to comorbid dependence on multiple substances. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(4):361–9. doi: 10.1002/ajmg.b.32037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lappalainen J, Kranzler HR, Malison R, Price LH, Van Dyck C, Rosenheck RA, et al. A functional neuropeptide Y Leu7Pro polymorphism associated with alcohol dependence in a large population sample from the United States. Arch Gen Psychiatry. 2002;59(9):825. doi: 10.1001/archpsyc.59.9.825. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, et al. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452(7190):997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(6):599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Almasy L, Dyer TD, Blangero J. Bivariate quantitative trait linkage analysis: pleiotropy versus co-incident linkages. Genet Epidemiol. 1997;14(6):953–8. doi: 10.1002/(SICI)1098-2272(1997)14:6<953::AID-GEPI65>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 34.McPeek MS, Sun L. Statistical tests for detection of misspecified relationships by use of genome-screen data. Am J Hum Genet. 2000;66(3):1076–94. doi: 10.1086/302800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olvera RL, Bearden CE, Velligan DI, Almasy L, Carless MA, Curran JE, et al. Common genetic influences on depression, alcohol, and substance use disorders in Mexican-American families. Am J Med Genet B Neuropsychiatr Genet. 2011;156B(5):561–8. doi: 10.1002/ajmg.b.31196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKay DR, Knowles EEM, Winkler AAM, Sprooten E, Kochunov P, Olvera RL, et al. Influence of age, sex and genetic factors on the human brain. Brain Imaging Behav. 2014;8(2):143–52. doi: 10.1007/s11682-013-9277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-international neuropsychiatric interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 2):22–33. [quiz 34–57] [PubMed] [Google Scholar]
- 38.American psychiatric association. Diagnostic and statistical manual of mental disorders. 4th. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- 39.Boehnke M. Allele frequency estimation from data on relatives. Am J Hum Genet. 1991;48(1):22–5. [PMC free article] [PubMed] [Google Scholar]
- 40.Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin – rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet. 2002;30(1):97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- 41.Heath SC. Markov chain Monte Carlo segregation and linkage analysis for oligogenic models. Am J Hum Genet. 1997;61(3):748–60. doi: 10.1086/515506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feingold E, Brown PO, Siegmund D. Gaussian models for genetic linkage analysis using complete high-resolution maps of identity by descent. Am J Hum Genet. 1993;53(1):234–51. [PMC free article] [PubMed] [Google Scholar]
- 44.Knowles EEM, Carless MA, de Almeida MAA, Curran JE, McKay DR, Sprooten E, et al. Genome-wide significant localization for working and spatial memory: identifying genes for psychosis using models of cognition. Am J Med Genet B Neuropsychiatr Genet. 2014;165B(1):84–95. doi: 10.1002/ajmg.b.32211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheverud JM. A simple correction for multiple comparisons in interval mapping genome scans. Heredity (Edinb) 2001;87(1):52–8. doi: 10.1046/j.1365-2540.2001.00901.x. [DOI] [PubMed] [Google Scholar]
- 46.Williams JT, Begleiter H, Porjesz B, Edenberg HJ, Foroud T, Reich T, et al. Joint multipoint linkage analysis of multivariate qualitative and quantitative traits. II. Alcoholism and event-related potentials. Am J Hum Genet. 1999;65(4):1148–60. doi: 10.1086/302571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beuten J, Halder I, Fowler SP, Groing HHH, Duggirala R, Arya R, et al. Wide disparity in genetic admixture among Mexican Americans from San Antonio, TX. Ann Hum Genet. 2011;75(4):529–38. doi: 10.1111/j.1469-1809.2011.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moskvina V, Schmidt KM. On multiple-testing correction in genome-wide association studies. Genet Epidemiol. 2008;32(6):567–73. doi: 10.1002/gepi.20331. [DOI] [PubMed] [Google Scholar]
- 49.Erhardt A, Czibere L, Roeske D, Lucae S, Unschuld PG, Ripke S, et al. TMEM132D, a new candidate for anxiety phenotypes: evidence from human and mouse studies. Mol Psychiatry. 2011;16(6):647–63. doi: 10.1038/mp.2010.41. [DOI] [PubMed] [Google Scholar]
- 50.Erhardt A, Akula N, Schumacher J, Czamara D, Karbalai N, Muller-Myhsok B, et al. Replication and meta-analysis of TMEM132D gene variants in panic disorder. Transl Psychiatry. 2012;2:e156. doi: 10.1038/tp.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otowa T, Maher BS, Aggen SH, McClay JL, van den Oord EJ, Hettema JM. Genome-wide and gene-based association studies of anxiety disorders in European and African American samples. PLoS One. 2014;9(11):e112559. doi: 10.1371/journal.pone.0112559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quast C, Altmann A, Weber P, Arloth J, Bader D, Heck A, et al. Rare variants in TMEM132D in a case-control sample for panic disorder. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(8):896–907. doi: 10.1002/ajmg.b.32096. [DOI] [PubMed] [Google Scholar]
- 53.Haaker J, Lonsdorf TB, Raczka KA, Mechias M-L, Gartmann N, Kalisch R. Higher anxiety and larger amygdala volumes in carriers of a TMEM132D risk variant for panic disorder. Transl Psychiatry. 2014;4:e357. doi: 10.1038/tp.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kendler KS, American medical association Alcoholism and major depression in women. Arch Gen Psychiatry. 1993;50(9):690. doi: 10.1001/archpsyc.1993.01820210024003. [DOI] [PubMed] [Google Scholar]
- 55.Lyons M, Hitsman B, Xian H, Panizzon MS, Jerskey BA, Santangelo S, et al. A twin study of smoking, nicotine dependence, and major depression in men. Nicotine Tob Res. 2008;10(1):97–108. doi: 10.1080/14622200701705332. [DOI] [PubMed] [Google Scholar]
- 56.Lionel AC, Tammimies K, Vaags AK, Rosenfeld JA, Ahn JW, Merico D, et al. Disruption of the ASTN2/TRIM32 locus at 9q33.1 is a risk factor in males for autism spectrum disorders. ADHD and other neurodevelopmental phenotypes Hum Mol Genet. 2014;23(10):2752–68. doi: 10.1093/hmg/ddt669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuo P, Neale MC, Riley BP, Webb BT, Sullivan PF, Vittum J, et al. Identification of susceptibility loci for alcohol-related traits in the Irish affected sib pair study of alcohol dependence. Alcohol Clin Exp Res. 2006;30(11):1807–16. doi: 10.1111/j.1530-0277.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- 58.Li MD. Identifying susceptibility loci for nicotine dependence: 2008 update based on recent genome-wide linkage analyses. Hum Genet. 2008;123(2):119–31. doi: 10.1007/s00439-008-0473-0. [DOI] [PubMed] [Google Scholar]
- 59.Maes HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ, et al. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence. Psychol Med. 2004;34(7):1251–61. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- 60.The Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42(5):441–7. doi: 10.1038/ng.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hasin DS, Grant BF. The National epidemiologic survey on alcohol and related conditions (NESARC) Waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50(11):1609–40. doi: 10.1007/s00127-015-1088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salas-Wright CP, Clark TT, Vaughn MG, Cordova D. Profiles of acculturation among Hispanics in the United States: links with discrimination and substance use. Soc Psychiatry Psychiatr Epidemiol. 2015;50(1):39–49. doi: 10.1007/s00127-014-0889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Otiniano Verissimo AD, Grella CE, Amaro H, Gee GC. Discrimination and substance use disorders among Latinos: the role of gender, nativity, and ethnicity. Am J Public Health. 2014;104(8):1421–8. doi: 10.2105/AJPH.2014.302011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramisetty-Mikler S, Caetano R, Rodriguez LA. The HispanicAmericans Baseline Alcohol Survey (HABLAS): alcohol consumption and sociodemographic predictors across Hispanic national groups. J Subst Use. 2010;15(6):402–16. doi: 10.3109/14659891003706357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alegría M, Canino G, Shrout PE, Woo M, Duan N, Vila D, et al. Prevalence of mental illness in immigrant and non-immigrant U.S. Latino groups. Am J Psychiatry. 2008;165(3):359–69. doi: 10.1176/appi.ajp.2007.07040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Michael T, Zetsche U, Margraf J. Epidemiology of anxiety disorders. Psychiatry. 2007;6(4):136–42. [Google Scholar]
- 67.American psychiatric association. DSM-V. Am J Psychiatry. 2013;20:31–32. [Google Scholar]
- 68.Galbaud du Fort G, Newman SC, Bland RC. Psychiatric comorbidity and treatment seeking. Sources of selection bias in the study of clinical populations. J Nerv Ment Dis. 1993;181(8):467–74. [PubMed] [Google Scholar]
- 69.Wijsman E, Amos CI. Genetic analysis of simulated oligogenic traits in nuclear and extended pedigrees: Summary of GAW10 contributions. Genet Epidemiol. 1997;14(6):719–35. doi: 10.1002/(SICI)1098-2272(1997)14:6<719::AID-GEPI28>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 70.Gur RE, Nimgaonkar VL, Almasy L, Calkins ME, Ragland JD, Pogue-Geile MF, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164(5):813–9. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]


