Abstract
Aims
Coexistence of lung cancer and granulomatous inflammation in the same patient confuses clinicians. We aimed to document the prevalence, clinicopathological features, treatment outcomes and prognosis in patients with coexisting granulomatous inflammation undergoing curative lung resection for lung cancer, in a tuberculosis (TB)-endemic country.
Methods
An observational cohort study of patients with lung cancer undergoing curative resection between 2012 and 2015 in a tertiary centre in Singapore.
Results
One hundred and twenty-seven patients underwent lung resection for cancer, out of which 19 (14.9%) had coexistent granulomatous inflammation in the resected specimen. Median age was 68 years and 58.2% were males. Overall median (range) survival was 451 (22–2452) days. Eighteen (14%) patients died at median duration of 271 days after surgery. The postsurgery median survival for those alive was 494 (29–2452) days in the whole group. Subgroup analysis did not reveal any differences in age, gender, location of cancer, radiological features, type of cancer, chemotherapy, history of TB or survival in patients with or without coexistent granulomatous inflammation.
Conclusions
Incidental detection of granulomatous inflammation in patients undergoing lung resection for cancer, even in a TB-endemic country, may not require any intervention. Such findings may be due to either mycobacterial infection in the past or ‘sarcoid reaction’ to cancer. Although all patients should have their resected specimen sent for acid-fast bacilli culture and followed up until the culture results are reported, the initiation of the management of such patients as per existing lung cancer management guidelines does not affect their outcome adversely.
Keywords: TUBERCULOSIS, LUNG CANCER, SURGERY, GRANULOMA
Introduction
Diagnosis of granulomatous inflammation is common in clinical practice.1 Granulomatous disorders comprise infections, vasculitis, immunological disorders, leucocyte oxidase defect, hypersensitivity, chemicals and neoplasia.2 Granulomatous inflammation seen in neoplastic diseases has been labelled as ‘sarcoid reaction’ or ‘sarcoid-like lymphadenopathy’.3–5 Sarcoid reaction is thought to be caused by immunological hypersensitivity to antigens derived from tumour cell leading to granuloma formation.6 Such sarcoid reaction occurs in 4.4% of carcinomas, 13.8% of patients with Hodgkin's disease, 7.3% of cases with non-Hodgkin's lymphoma, 50% of seminomas and 0.4% of sarcomas.7 8 It has also been observed in breast, gastric, colonic and laryngeal cancer along with head and neck cancer.9–11
However, literature reporting such non-specific granulomatous inflammation, namely sarcoid reaction in lung cancer, is limited.4 5 12–14 Most of the reports describe sarcoid-like lymphadenopathy in patients with lung cancer. The reported incidence of sarcoid-like lymphadenopathy ranges from 1.3% to 11%.4 5 12–14 Annual incidence of lung cancer and pulmonary tuberculosis (TB) in Singapore is similar, that is, 1300 and 1056, respectively.15–18 On the background of this near equal incidence, incidental finding of granulomatous inflammation in the mediastinal lymph nodes (LN) or resected lung parenchyma in patients with lung cancer confuses clinicians and creates unique challenges for their management.
First, trans-bronchial needle aspiration of mediastinal LN is often used to diagnose or stage lung cancer. If the diagnosis is solely based on LN aspiration, the histological findings of granulomatous inflammation in such LN specimens carry the risk of misdiagnosing lung cancer as TB. Second, frozen section done prior to lung resection showing granulomatous inflammation may lead to under-resection of the cancer, increasing the chances of recurrence. Third, detection of granulomatous inflammation together with cancer in the resected specimen may invoke the need for the treatment of TB, especially in a TB-endemic country. However, such treatment may be unnecessary as well as put patients at risk of side effects and drug interaction with chemotherapy. Fourth, the finding of granulomatous inflammation coexisting with the cancer in patients requiring chemotherapy raises concerns about the potential of exacerbating TB due to immune suppression associated with chemotherapy. Finally, appearance of enlarged LN on the surveillance CT or positron emission tomography scans after resection may be secondary to sarcoid reaction instead of recurrence of cancer, and administering chemotherapy without histological examination of these LN may be inappropriate.
We conducted this study to document the prevalence, clinicopathological features, treatment outcomes and prognosis in patients with coexisting granulomatous inflammation undergoing curative lung resection for lung cancer. A comparison with the lung cancer patients without granulomatous inflammation was also made from the same population.
Material and methods
The medical records of all patients undergoing curative resection of lung cancer in a tertiary hospital in Singapore between 2012 and 2015 were reviewed. Clinical data, including age, gender, symptoms at presentation, type of lung cancer, stage of lung cancer, history of TB, histological features of the resected specimen, mutation status, type of therapy and survival, were collected. Survival was calculated from date of surgery to date of death or date of last follow-up visit. Institutional review board approval was obtained for this study with the waiver of consent. All patients underwent thoracic surgery via conventional techniques. In patients requiring neoadjuvant or adjuvant chemotherapy, chemotherapy administered was platinum-based dual-agent combination therapy. Most patients received combination of carboplatin and paclitaxel. Among patients receiving epidermal growth factor receptortyrosine kinase inhibitors (TKIs), four types of TKIs were administered, namely erlotinib (Tarceva), gefitinib (Iressa), afatinib (giotrif), and crizoitinib (xalkori).
Data analysis
We used software (SPSS, V.17; SPSS, Chicago, Illinois, USA) for all statistical analyses. Where applicable, the results were compared using a Wilcoxon two-sample test or Fisher exact test. p Values were two sided and considered indicative of a significant difference if <0.05. Kaplan-Meier curve with log-rank test was used to analyse survival.
Results
One hundred and twenty-seven patients underwent resection of lung for lung cancer in a tertiary hospital in Singapore between 2012 and 2015. Median age was 68 (26–89) years and 58.2% were males. The most common cancer subtype was adenocarcinoma (64.5%). Eighteen (14.1%) patients died during the follow-up period. The median survival was 15 months and 1-year, 2-year and 3-year survival rates were 53.5%, 21.2% and 8.6%, respectively. Right and left upper lobes were the most commonly involved sites (table 1).
Table 1.
General characteristics and subgroup analysis of the patients with (n=19) and without (n=108) granulomatous inflammation on the specimen of resected lung
| Total | Only cancer | Coexisting granulomatous inflammation with lung cancer | p Value | |
|---|---|---|---|---|
| Number | 127 | 108 | 19 | |
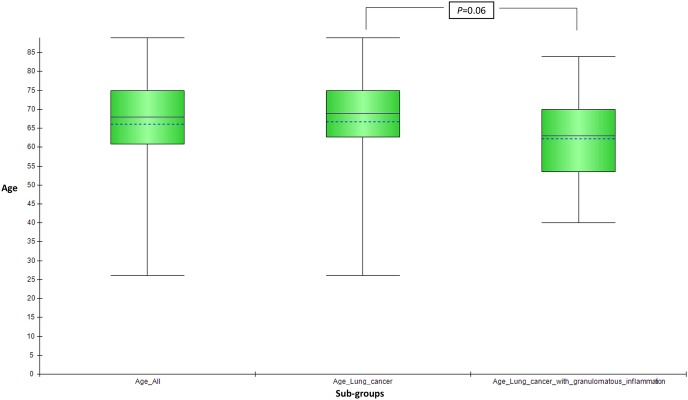
| Age | 68 (26–89) | 69 (26–89) | 63 (40–84) | 0.06 |
| Gender—males | 74 (58.2%) | 62 (57.4) | 12 (63.1) | 0.78 |
| Location of lesion | ||||
| Right upper lobe | 35 (27.5) | 28 (25.9) | 7 (36.8) | 0.40 |
| Right middle lobe | 13 (10.2) | 11 (10.1) | 2 (10.5) | 1.0 |
| Right lower lobe | 19 (14.9) | 17 (15.7) | 2 (10.5) | 0.73 |
| Left upper lobe | 23 (18.1) | 21 (19.4) | 2 (10.5) | 0.52 |
| Lingula | 2 (1.5) | 2 (1.8) | 0 | 1.0 |
| Left lower lobe | 20 (15.7) | 15 (13.8) | 5 (26.3) | 0.18 |
| Bilateral | 5 (3.9) | 3 (2.7) | 2 (10.5) | 0.16 |
| Type of surgery | ||||
| Wedge resection | 44 (34.6) | 36 (33.3) | 8 (42.1) | 0.42 |
| Lobectomy | 45 (35.4) | 36 (33.3) | 8 (42.1) | 0.42 |
| Others | 1 (0.07) | 36 (33.3) | 2 (10.5) | 0.16 |
| Histology type | ||||
| Adenocarcinoma | 82 (64.5) | 71 (66) | 11 (57.8) | 0.60 |
| Squamous cell carcinoma | 12 (9.4) | 10 (9) | – | 0.35 |
| Large-cell carcinoma | 2 (1.5) | 1 (1) | 1 (5.2) | 0.27 |
| Others | 27 (21.2) | 23 (21) | 4 (21) | 1.0 |
| Died | 18 (14.1) | 15 (14) | 3 (16) | 0.73 |
| History of tuberculosis | 4 (3.1) | 2 (1.8) | 2 (11) | 0.10 |
| History of non-tuberculous mycobacteria | 3 (2.3) | 1 (0.09) | 2 (11) | 0.05 |
| Chemotherapy | 30 (23.6) | 23 (21.2) | 7 (36.8) | 0.15 |
| Survival, median (range)—days | 451 (22–2452) | 449 (29–2452) | 478 (22–1569) | 0.56 |
| 1-year survival | 68 (53.5) | 58 (54) | 10 (53) | 1.0 |
| 2-year survival | 27 (21.2) | 20 (19) | 7 (37) | 0.12 |
| 3-year survival | 11 (8.6) | 7 (6) | 4 (21) | 0.06 |
| Tuberculosis treatment | 1 (0.07) | 0 | 1 (5.2) | 0.14 |
| Stage | ||||
| I and II | 97 (76.3) | 84 (77.7) | 13 (68.4) | 0.38 |
| IIIA | 30 (23.6) | 24 (22.2) | 6 (31.5) | 0.38 |
| Granulomatous inflammation in parenchyma | 19 (14.9) | – | 19 (100) | – |
| Granulomatous inflammation in parenchyma and lymph nodes | 5 (3.9) | – | 5 (26.3) | – |
Data presented as number (%) or median (range).
Nineteen out of 127 patients (14.9%) had histological features of granulomatous inflammation in the resected lung specimen with or without simultaneously involving the dissected LN (figure 1). Patients with coexisting granulomatous inflammation with lung cancer were younger than those with lung cancer alone, but without reaching statistical significance, p=0.06 (figure 2). The median survival in this group was 15.9 months with 1-year, 2-year and 3-year survival of 53%, 37% and 21%, respectively, with no difference in survival from patients without granulomatous inflammation, p=0.35 (figure 3). Three-year survival, however, showed a favourable trend in patients with coexisting granulomatous inflammation and lung cancer, although without reaching statistical significance, p=0.06. Ziehl-Neelsen stain for acid-fast bacillus (AFB) was negative in all 19 patients. Two patients in the coexistent granulomatous inflammation and lung cancer group and one patient in the lung cancer alone group had a history of non-tuberculous mycobacteria (NTM) based on the respiratory specimen (sputum). Similarly, two patients in the coexistent granulomatous inflammation and lung cancer group and two patients in the lung cancer alone group had a history of TB (table 1). The details of the stage of lung cancer, chemoradiotherapy (neoadjuvant or adjuvant) and AFB smear and culture results are summarised in table 2.
Figure 1.
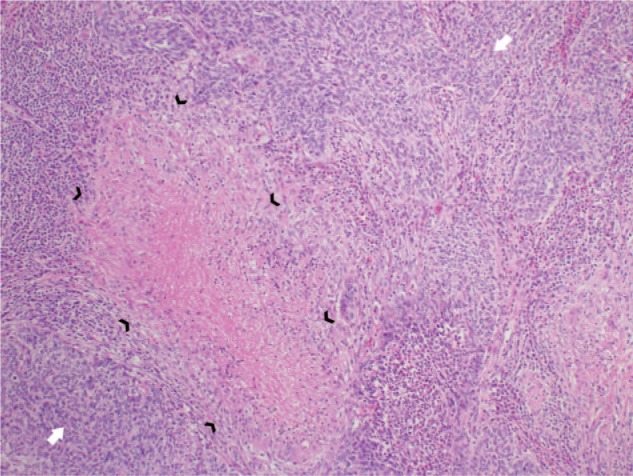
A representative case of coexisting granulomatous inflammation (black arrowheads) and squamous cell carcinoma (white arrows) in a lobectomy specimen in a 63-year-old male.
Figure 2.
Patients with coexisting granulomatous inflammation with lung cancer were younger than patients with lung cancer alone, without reaching statistical significance, p=0.06.
Figure 3.
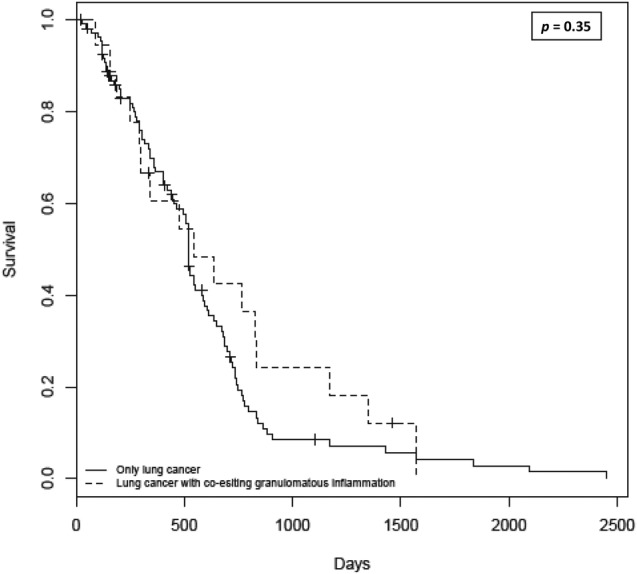
Kaplan-Meier curve showing similar survival in patients with coexisting granulomatous inflammation and lung cancer versus patients with lung cancer alone (log-rank test, p=0.35).
Table 2.
Summary of stage, chemotherapy and microbiological results
| Only cancer N=108 |
Coexisting granulomatous inflammation with lung cancer N=19 | |
|---|---|---|
| Stage I and II | 84 (77.7) | 13 (68.4) |
| T-1 | 60 (55.5) | 13 (68.4) |
| T-2 | 24 (22.2) | 6 (31.5) |
| N-0 | 10 (9.2) | 3 (15.7) |
| N-1 | 50 (46.2) | 10 (52.6) |
| N-2 | 24 (22.2) | 6 (31.5) |
| Chemotherapy | ||
| Neoadjuvant chemotherapy | 24 (22.2) | 6 (31.5) |
| Concurrent chemoradiotherapy | 27 (25) | 5 (26.3) |
| Epidermal growth factor receptor–tyrosine kinase inhibitor | 7 (6.4) | None |
| Acid-fast bacilli (AFB) culture | ||
| No. of patients in whom AFB cultures done | 27 (25) | 9 (47.3) |
| No. of patients in whom AFB culture was positive | None | 2 (non-tuberculous mycobacteria) |
| No. of patients in whom Ziehl-Neelsen stain done | 108 (100) | 19 (100) |
| No. of patients in whom Ziehl-Neelsen stain was positive for AFB | None | None |
Data presented as number (%).
Discussion
We illustrated that in a TB-endemic country, granulomatous inflammation was incidentally detected in the lung parenchyma with or without LN involvement in approximately 15% of patients undergoing lung resection for cancer. This granulomatous inflammation could be either secondary to TB, NTM or fungal infection in the past or it could be a ‘sarcoid reaction’ to cancer. Interval monitoring of these findings without any intervention did not have any adverse effect on the lung cancer management and outcomes. Empirical TB therapy may not be necessary, and adjuvant chemotherapy is tolerated safely in such patients.
Non-specific granulomatous inflammation called ‘sarcoid reaction’ has been observed in lung cancer previously. It has been observed both in the lung parenchyma and in the regional LN. The incidence varies from 1.3% to 11%.5 12–14 In the current study, the frequency of occurrence was 15%, which was higher than previously described.5 12–14 This may be due to inclusion of both parenchymal and LN lesions in the analysis as compared with previous studies, which have predominantly included the LN in their analysis.
Patients with coexisting granulomatous inflammation with lung cancer were slightly younger than those without coexisting granulomatous inflammation, although results did not reach statistical significance. This finding is similar to the findings of Kamiyoshihara et al13 who reported greater incidence of lung cancer with sarcoid reaction in younger patients. However, in another study by Tomimaru et al,14 no difference in the age was found between the patients with and without coexisting granulomatous lesions.
In terms of histological type of lung cancer, some authors have reported higher incidence of squamous cell carcinoma in patients with coexisting lesions and others have reported higher incidence of adenocarcinoma.12 However, in our study, no difference was found within the two groups of patients in terms of histology.
Granulomatous inflammation associated with malignancy does not carry prognostic value. In earlier studies by Kamiyoshihara et al13 and Tomimaru et al,14 no difference in survival was seen in patients with lung cancer with or without coexisting granulomatous inflammation. Similar to their findings, median survival was similar between the two groups in our cohort. However, the survival rate at 3 years after surgery was slightly higher in the patients with coexisting granulomatous inflammation in our cohort. This trend, interestingly, is in keeping with the existing literature on extrapulmonary malignancies where patients with coexisting granulomatous inflammation have been reported to have better prognosis in Hodgkin's disease and gastric cancer.19–22
Regarding the challenges posed by the coexistence of the granulomatous inflammation with lung cancer, if the diagnosis of lung cancer is solely based on LN aspiration, the histological findings of granulomatous inflammation in such LN specimens may misdiagnose cancer as TB with false-negative results. Six dissected LN stations in four patients (3.1%) revealed granulomatous inflammation in our cohort. If LN aspiration (either endobronchial ultrasound guided or blind) was the only technique employed to diagnose pulmonary mass in these patients, it would have led to misdiagnosis of cancer as TB or granulomatous inflammation of alternative aetiology.
In TB-endemic areas, detection of granulomatous inflammation together with cancer in the resected specimen may intuitively invoke the need for treatment of TB unnecessarily. Coincidentally, no patient received TB treatment after surgery in our cohort of patients with coexisting lesions. Despite this, no adverse effect was observed following chemotherapy, arguing against mycobacterial aetiology of this granulomatous inflammation. The decision of not to treat as TB was based on lung tumour discussion where waiting for the TB culture from resected specimen, or collecting fresh sample of sputum and sending it for TB culture was deemed prudent before starting TB treatment. However, no patient grew AFB on TB culture.
The finding of granulomatous inflammation coexisting with the cancer in patients requiring chemotherapy raises concerns regarding the potential of exacerbating TB due to immune suppression. Two patients showing granulomatous inflammation in the histological specimen in our cohort had stage-IIIA cancer requiring adjuvant chemotherapy. Six patients with coexisting granulomatous inflammation received chemotherapy without any evidence of exacerbation of TB in our cohort.
Eight of 19 patients had necrotising granulomatous inflammation, whereas six had non-necrotising granulomatous inflammation. In remaining five patients, the differentiation between necrotising and non-necrotising nature was not clear and these were reported as granulomatous inflammation. Out of two patients with the history of TB, one had necrotising type of granulomatous inflammation and the other had non-necrotising type of granulomatous inflammation. This is consistent with the existing literature where granulomas in TB have been reported to be caseating in 58.7%, non-caseating in 23.8% and atypical in 17.5% cases on autopsy studies.23 Out of two patients with history of NTM, both had necrotising type of granulomatous inflammation. Lack of difference in the number of patients with the history of TB or NTM in the group with or without coexistent granulomatous inflammation and lung cancer indicates lack of aetiological relationship between these two conditions and granulomatous changes found in patients with lung cancer.
Our study has following limitations. First, it is a single-centre retrospective study. Second, these findings may not be applicable to countries with much higher incidence of TB. Such countries may need to exclude TB infection before considering the diagnosis of ‘sarcoid reaction.’ Third, median follow-up period is short (1.5 years) in our cohort, and long-term follow-up duration demonstrating lack of emergence of TB is required to validate our results.
In conclusion, incidental detection of granulomatous inflammation in patients undergoing lung resection for cancer, even in a TB-endemic country may not require any intervention. Such findings may be due to either TB or NTM infection in the past or ‘sarcoid reaction’ to cancer. Although all patients should have their resected specimen sent for AFB culture and followed up until the culture results are reported, initiating the management of such patients as per existing lung cancer management guidelines does not affect their outcome adversely.
Take home messages.
Granulomatous inflammation can coexist with lung cancer in the lung parenchyma.
Such granulomatous inflammation could be due to a history of mycobacterial infection or sarcoid reaction to cancer and does not require treatment.
Chemotherapy is tolerated safely in such patients.
These patients may be managed as lung cancer patients without coexisting granulomatous inflammation.
Acknowledgments
Authors would like to thank Ms Ivy Yu Ling Ling for her valuable contribution in editing the figures.
Footnotes
Handling editor: Cheok Soon Lee
Contributors: AV conceived the study and participated in data collection, data interpretation and analysis and manuscript writing. RSD, CVC, ABA, DBAA, AC, AYHL, DYHT, KAC, GSK participated in the data collection and writing of the manuscript. JA participated in critical analysis and revising the manuscript.
Competing interests: None declared.
Ethics approval: NHG-DSRB.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Data were available to RSD and AV.
References
- 1.Bhatia A, Kumar Y, Kathpalia AS. Granulomatous inflammation in lymph nodes draining cancer: a coincidence or a significant association! Int J Med Med Sci 2009;1:13–16. [Google Scholar]
- 2.James DG. A clinicopathological classification of granulomatous disorders. Postgrad Med J 2000;76:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregori HB, Othersen HB, Moore MP. The significance of sarcoid-like lesions in association with malignant neoplasms. Am J Surg 1962;104:577–86. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy MP, Jimenez CA, Mhatre AD, et al. Clinical implications of granulomatous inflammation detected by endobronchial ultrasound transbronchial needle aspiration in patients with suspected cancer recurrence in the mediastinum. J Cardiothorac Surg 2008;3:8–13. 10.1186/1749-8090-3-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinfort DP, Irving LB. Sarcoid reactions in regional lymph nodes of patients with non-small cell lung cancer: incidence and implications for minimally invasive staging with endobronchial ultrasound. Lung Cancer 2009;66:305–8. 10.1016/j.lungcan.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 6.Sehgal S, Goyal P, Ghosh S, et al. Malignancy and granulomatosis: causality or coincidence? Narrative systematic review. Iran J Pathol 2014;9:237–44. [Google Scholar]
- 7.Khurana KK, Stanley MW, Powers CN, et al. Aspiration cytology of malignant neoplasms associated with granulomas and granuloma-like features: diagnostic dilemmas. Cancer 1998;84:84–91. [DOI] [PubMed] [Google Scholar]
- 8.Brincker H. Sarcoid reactions in malignant tumours. Cancer Treat Rev 1986;13:147–56. [DOI] [PubMed] [Google Scholar]
- 9.Ophir D, Nissim F, Marshak G. Granulomatous reaction in lymph nodes draining laryngeal carcinoma. Head Neck Surg 1986;8:214–17. [DOI] [PubMed] [Google Scholar]
- 10.Bigotti G, Coli A, Magistrelli P, et al. Gastric adenocarcinoma associated with granulomatous gastritis report and review of the literature. Tumori 2002;88:163–6. [DOI] [PubMed] [Google Scholar]
- 11.Marruchella A. Sarcoidosis or sarcoid reaction? Chest 2009;136:943–4. 10.1378/chest.09-0472 [DOI] [PubMed] [Google Scholar]
- 12.Laurberg P. Sarcoid reactions in pulmonary neoplasms. Scand J Respir Dis 1975;56:20–7. [PubMed] [Google Scholar]
- 13.Kamiyoshihara M, Hirai T, Kawashima O, et al. Sarcoid reactions in primary pulmonary carcinoma: report of seven cases. Oncol Rep 1998;5: 177–80. [PubMed] [Google Scholar]
- 14.Tomimaru Y, Higashiyama M, Okami J, et al. Surgical results of lung cancer with sarcoid reaction in regional lymph nodes. Jap J Clin Oncol 2007;37:90–5. [DOI] [PubMed] [Google Scholar]
- 15.Health Promotion Board, National registry of disease office. Interim Annual registry report Trends in cancer incidence in Singapore 2009–2013. http://www.nrdo.gov.sg (accessed 10 Apr 2016).
- 16.WHO global tuberculosis report. http://www.who.int/tb/publications/global_report/en/ (accessed 10 Apr 2016).
- 17.Countries with high Prevalence of Tuberculosis. https://shs.wustl.edu/MedicalAndHealthCare/Documents/Countries%20with%20a%20High%20Prevalence%20of%20Tuberculosis.pdf (accessed 10 Apr 2016).
- 18.The World Bank. http://data.worldbank.org/indicator/SH.TBS.INCD?page=3 (accessed 10 Apr 2016).
- 19.O'Connell MJ, Schimpff SC, Kirschner RH, et al. Epithelioid granulomas in Hodgkin disease. A favorable prognostic sign? JAMA 1975;233:886–9. [PubMed] [Google Scholar]
- 20.Sacks EL, Donaldson SS, Gordon J, et al. Epithelioid granulomas associated with Hodgkin's disease: clinical correlations in previously untreated patients. Cancer 1978;41:562–7. [DOI] [PubMed] [Google Scholar]
- 21.Takeuchi H, Suchi T, Suzuki R, et al. Histological study of immune parameters of regional lymph nodes of gastric cancer patients. Gan 1982;73:420–8. [PubMed] [Google Scholar]
- 22.Brincker H. Sarcoid reactions and sarcoidosis in Hodgkin's disease and other malignant lymphomata. Br J Cancer 1972;26:120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amarapurkar A, Agrawal V. Liver involvement in tuberculosis--an autopsy study. Trop Gastroenterol 2006;27:69–74. [PubMed] [Google Scholar]