Abstract
Objectives
Craniofacial skeletal development requires deliberate coordination of two distinct mechanisms of endochondral and intramembranous ossification. Col2a1-expressing cells encompass growth-associated skeletal progenitors in endochondral bones of the limb. The objective of this study is to determine the contribution of Col2a1-expressing cells to the craniofacial skeletal cell lineages. We hypothesize that Col2a1-expressing progenitors significantly contribute to various modes of ossification associated with the craniofacial development.
Methods
Cellular fates of Col2a1-expressing cells were studied based on a cre-loxP system using a Col2a1-cre transgene and an R26R-tdTomato reporter allele. We analyzed three distinct locations of the craniofacial skeletal complex representing unique ossification mechanisms; the cranial base, the calvaria and the mandibular condyle.
Results
Col2a1-cre consistently marked a majority of skeletal cells in the cranial base. Interestingly, Col2a1-cre also marked a large number of osteoblasts and suture mesenchymal cells in the calvaria, in addition to chondrocytes in the underlying transient cartilage. In the mandibular condyle, Col2a1-cre marked chondrocytes and osteoblasts only during the growth phase.
Conclusions
Col2a1 is expressed by progenitors of the skeletal lineage in canonical endochondral bone formation occurring in the cranial base. In contrast, other ossification mechanisms of the craniofacial complex utilize Col2a1-expressing cells in a different manner, whereby Col2a1 may be expressed in more differentiated or transient cell types of the skeletal lineage.
Keywords: Type II collagen, Cell lineage tracing, Endochondral ossification, Intramembranous ossification, Mandibular condylar cartilage
Introduction
Craniofacial skeletal development requires deliberate coordination of two distinct mechanisms of endochondral and intramembranous ossification. The cranial base is initially formed by fusion of three primordial (1). The synchondroses, unique bidirectional growth plates, are formed between these ossification centers as spheno-ethmoidal (SES), intersphenoidal (ISS) and spheno-occipital synchondrosis (SOS), which play an important role in the growth of the cranial base. Recent studies suggest that chondrocytes within the growth plate serve as a major source of other skeletal lineage cells in growing bones (2,3). Our recent study revealed that Col2a1 promoter/enhancers are active in early growth-associated skeletal progenitors in the limb (4). The mandibular condylar cartilage of the skull is a secondary cartilage without growth plate structures. A recent study reports that direct transformation of chondrocytes into bone cells also occurs in these cartilages (5). Interestingly, subsets of osteoblast precursors in intramembranous ossification are known to express both ‘osteogenic’ and ‘chondrogenic’ markers; Col2a1 mRNA is transiently expressed by preosteoblasts and ‘chondrocyte-like osteoblasts’ during early development and adulthood (6,7). However, it is unclear how significant Col2a1-expressing cells contribute to the craniofacial skeletal cell lineages. Here, we hypothesize that Col2a1-expressing cells significantly contribute to various modes of ossification occurring in the craniofacial skeletal complex. The state-of-the-art in vivo cell fate-mapping technique that we utilize in this study has the possibility to reveal novel mechanisms for craniofacial skeletal development.
Materials and Methods
Mice
Col2a1-cre and Col1a1(2.3kb)-GFP have been described elsewhere (8,9). Rosa26-loxP-stop-loxP-tdTomato (R26R-tdTomato, Ai14, JAX007914 (10)) mice were acquired from the Jackson laboratory. All procedures were conducted in compliance with the Guideline for the Care and Use of Laboratory Animals approved by the University of Michigan.
Histology and Flow cytometry
These procedures were performed as previously described (4).
Results
Col2a1-expressing cells contribute to the majority of skeletal cells in the cranial base
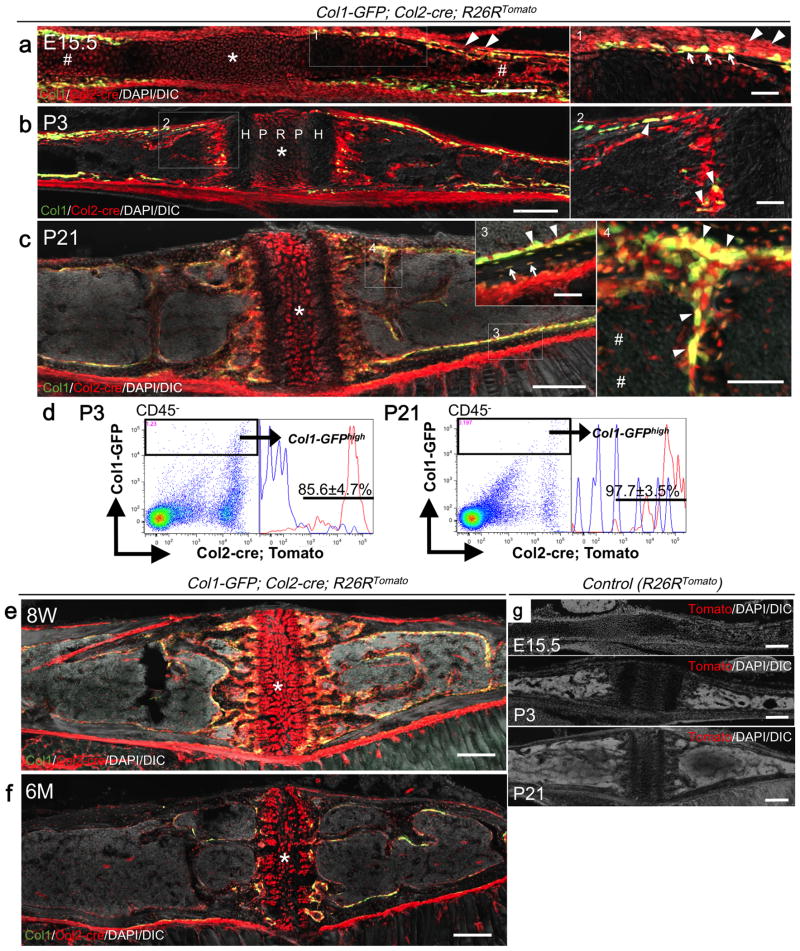
At E15.5, red cells occupied the synchondrosis (Fig.1a, asterisk: SOS), the ossification center (sharp) as well as the perichondrium (arrowheads). The vast majority of Col1a1-GFP+ osteoblasts appearing in the inner perichondrium were yellow (Fig.1a, right panel: arrows), therefore derived from Col2a1-cre+ cells. At P3, the synchondrosis generated a mirror-image growth plate composed of a common central resting zone (R) and proliferative (P) and hypertrophic (H) zones on both sides (Fig.1b). Col1a1-GFP+ osteoblasts on the trabecular surface and in the cortical bones were yellow, indicating that they were derived from Col2a1-cre+ cells (Fig.1b, right panels, arrowheads: osteoblasts). Although active cranial base growth had already ceased at P21, Col2a1-cre-derived red cells continued to contribute not only to chondrocytes and perichondrial cells in the synchondrosis, but also to osteoblasts, osteocytes and bone marrow stromal cells (Fig.1c, inset 3: arrows: osteocytes, right panel 4: arrowheads: osteoblasts, sharps: stromal cells). Flow cytometry analysis of dissociated cranial base cells revealed that Col2a1-cre-marked cells contributed to essentially all osteoblasts (Fig.1d, 85.6±4.7% and 97.7±3.5% of Col1a1-GFPhigh cells at P3 and P21, respectively). The synchondrosis was patent at 8 weeks old, and surprisingly, even at 6 months of age in these mice, associated with consistent robust generation of Col2al-cre-derived red cells as osteoblasts and bone marrow stromal cells (Fig.1e,f). No Tomato+ cells were observed in the absence of Col2a1-cre (Fig.1g). Therefore, these findings indicate that a large majority of chondrocytes, osteoblasts/cytes and bone marrow stromal cells in the cranial base are derived from Col2a1-expressing cells, underscoring the identical developmental mechanisms that support both the growth plate in the limb and the synchondrosis in the cranial base.
Fig.1.
Fate mapping of Col2a1-cre+ cells in the cranial base synchondrosis.
(a) Embryonic day 15.5 (E15.5). Asterisk: SOS. Sharp: the ossification center. Arrowheads: perichondrium.
(b) At postnatal day 3 (P3). Arrowheads: GFP/tdTomato+ osteoblasts.
(c) P21. Arrows: Tomato+ osteocytes. Arrowheads: GFP/tdTomato+ osteoblasts. Sharps: stromal cells.
(d) Flow cytometry analysis at P3 (left panels) and P21 (right panels). Right subpanels: histograms of the GFPhigh fraction developed for Tomato; blue lines: GFPhigh Tomatoneg control cells. X-axis: tdTomato, Y-axis: GFP. n=3 (P3), n=4 (P21). All data represented as mean ± S.D.
(e, f) 8 weeks (e) and 6 months (f) of age. Asterisk: SOS.
(g) Negative controls (R26RTomato) at E15.5, P3, P21.
Scale bars: 200μm in (main panels in a-c, e, and f), 100 μm in (right panels 1, 2 and 4 in a-c) and 50μm in (inset 3 in c)
Col2a1-expressing cells contribute to osteoblasts and suture mesenchymal cells in the calvaria
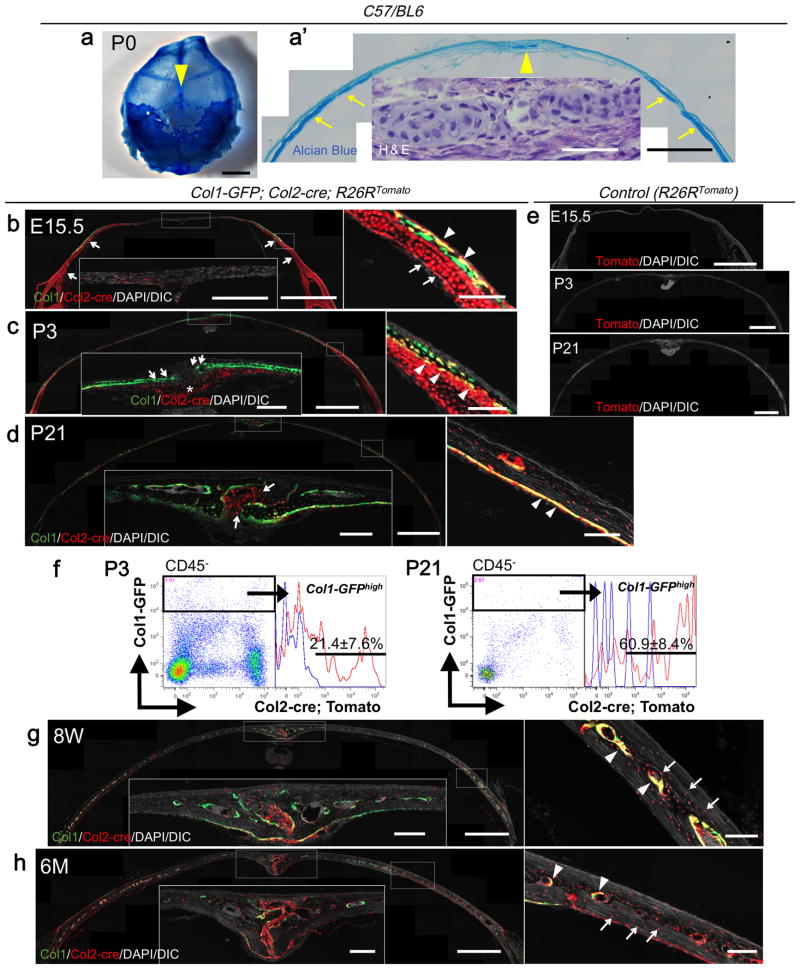
First, we verified the meshwork-like spotted cartilage in parietal bones near the midsagittal suture using whole-mount Alcian Blue staining and H&E staining at P0 in C57/BL6 mice (Fig.2a-a′, arrowheads: cartilage islands, arrows: underlying cartilage). Second, we took advantage of Col1a1-GFP; Col2a1-cre; R26R-tdTomato trigenic mice for more detailed fate mapping of Col2a1-expressing cells in the calvaria. At E15.5, a well-defined layer of cartilage underpinned the calvaria on the lateral portion of the parietal bones, in which chondrocytes and perichondrial cells were red, therefore marked by Col2a1-cre (Fig.2b, arrows: underlying cartilage). Many Col1-GFP+ calvarial osteoblasts were yellow, demonstrating that these cells were either derived from, or themselves were Col2a1-cre+ cells (Fig.2b, right panel, arrowheads: GFP/tdTomato+ osteoblasts). Few red cells were observed in the midsagittal suture at this stage. At P3, a Col2a1-cre-marked small piece of the underlying cartilage appeared directly underneath the midsagittal suture (Fig.2c, asterisk). On the lateral portion, many Col1a1-GFP+ calvarial osteoblasts located on the boundary between the calvarial bone and the underlying cartilage were yellow (Fig.2c, right panel, arrowheads). However, virtually all Col1a1-GFP+ osteoblasts near the suture were green, suggesting that they were not derived from Col2a1-cre+ cells (Fig.2c, arrows). Although the underlying cartilage had disappeared at P21, many Col1a1-GFP+ calvarial osteoblasts in the inner aspect of the calvaria and osteocytes were yellow and red, respectively (Fig.2d, right panel, arrowheads), thus marked by Col2a1-cre. In addition, Col2a1-cre-marked cells contributed to suture mesenchymal cells (Fig.2d, arrows), but not to Col1a1-GFP+ osteoblasts in proximity to the midsagittal suture. It could not be ascertained whether descendants of the underlying cartilage chondrocytes contributed to these suture mesenchymal cells, or Col2a1 promoter/enhancer activities occurred de novo among these cells at this stage. Flow cytometry analysis of digested calvarial cells revealed that 21.4±7.6% and 60.9±8.4% of Col1a1-GFPhigh osteoblasts were Tomato+ at P3 and P21, respectively (Fig.2f), indicating that the fraction of calvarial osteoblasts derived from Col2a1-expressing cells increased during active calvarial growth. This trend continued onto an adult and an aged stage at 8 weeks and 6 months of age (Fig.2g,h). Although osteoblasts on the bone surface were increasingly diminished in these later stages, osteoblasts on the endosteum and osteocytes were yellow and red, respectively (Fig.2g,h, right panels: arrowheads: osteoblasts, arrows: osteocytes). Abundant Col2a1-cre-marked suture mesenchymal cells continued to be observed, indicating persistent activities Col2a1 promoters/enhancers in the late stages. No Tomato+ cells were observed in the absence of Col2a1-cre (Fig.2e). Therefore, these findings indicate that a large number of osteoblasts, osteocytes and suture mesenchymal cells in the calvaria are either derived from Col2a1-expressing cells or themselves express Col2a1, revealing a significant contribution of Col2a1-expressing cells in the intramembranous skeletal lineage in the calvaria.
Fig.2.
Contribution of Col2a1-cre+ cells to osteoblasts and suture mesenchymal cells in the calvaria.
(a, a′) Whole-mount Alcian Blue (a) and H&E staining (a′) of P0 C57/BL6 mice. Arrowheads: cartilage islands. Arrows: underlying cartilage.
(b) E15.5. Arrows: underlying cartilage. Arrowheads: GFP/tdTomato+ osteoblasts.
(c) P3. Asterisk: underlying cartilage underneath the midsagittal suture. Arrows: GFP+/tdTomato- osteoblasts near the midsagittal suture. Arrowheads: GFP/tdTomato+ osteoblasts.
(d) P21. Arrows: suture mesenchymal cells derived from Col2a1-cre+ cells. Arrowheads: GFP/tdTomato+ osteoblasts in the inner aspect of the calvaria.
(e) Negative controls (R26RTomato) at E15.5, P3, P21.
(f) Flow cytometry analysis at P3 (left panels) and P21 (right panels). Right subpanels: histograms of the GFPhigh fraction developed for Tomato; blue lines: GFPhigh Tomatoneg control cells. X-axis: tdTomato, Y-axis: GFP. n=3 (P3), n=4 (P21). All data represented as mean ± S.D.
(g, h) 8 weeks (g) and 6 months (h) of age. Arrowheads: GFP/tdTomato+ osteoblasts on the endosteum. Arrows: tdTomato+ osteocytes.
Scale bars: 5mm in (a), 1mm in (a′,b-e, g and h) ,50μm in (inset in a′) ,200μm (insets in b-d, g and h) and 100μm in (right panels in b-d, g and h).
Transient contribution of Col2a1-expressing cells to the mandibular condyle
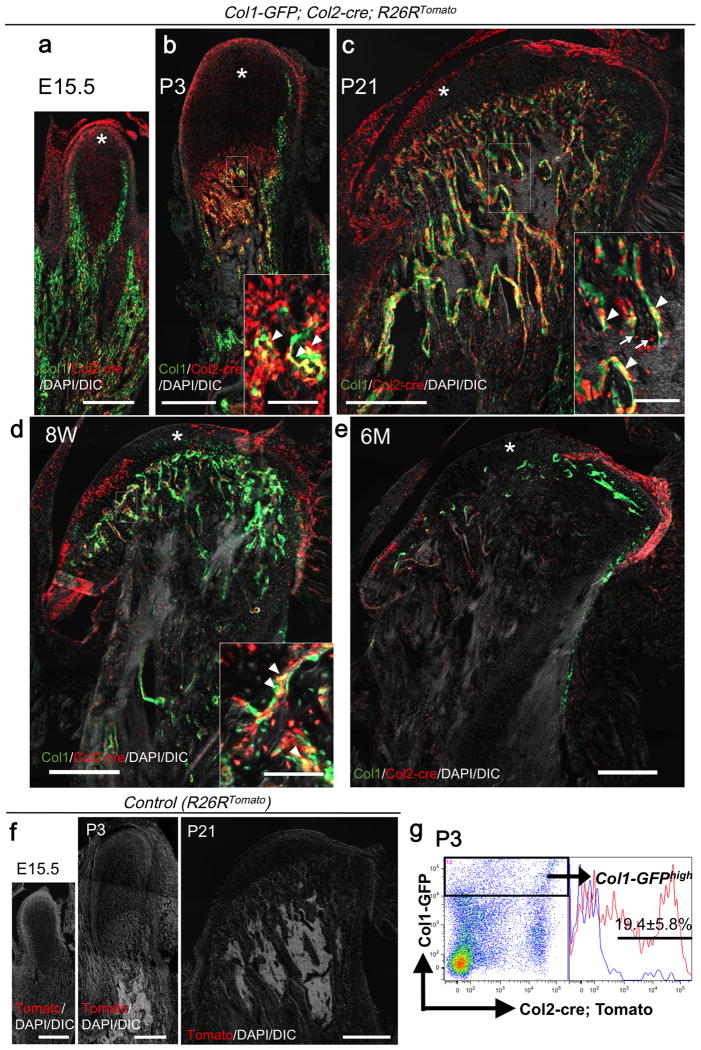
At E15.5, a number of Col2a1-cre-marked red cells were observed in the condyle anlage, distinct from sheet-like Col1a1-GFP+ osteoblasts of the mandible body (Fig.3a, asterisk: condylar cartilage). At P3, the condylar cartilage significantly increased in length, particularly in the proliferating and hypertrophic cell layers. These chondrocytes were red thus marked by Col2a1-cre (Fig.3b, asterisk). Abundant trabecular bone formation took place underneath the cartilage at this stage, where a majority of Col1a1-GFP+ trabecular osteoblasts were yellow thus derived from Col2a1-cre+ cells (Fig.3b, arrowheads: GFP/tdTomato+ osteoblasts). Flow cytometry analysis of digested whole mandible cells revealed that 19.4±5.8% of Col1a1-GFPhigh osteoblasts were Tomato+ at P3 (Fig.3g). The condylar cartilage reduced in its thickness but developed distinct structures at P21, whereas the domain for the subchondral trabecular bones expanded further. Most of condylar chondrocytes were red; in addition, and many Col1a1-GFP+ osteoblasts and osteocytes were yellow and red, respectively, thus derived from Col2al-cre+ cells (Fig.3c, arrowheads: osteoblasts, arrows: osteocytes). At 8 weeks of age, less red chondrocytes were noted in the condylar cartilage (Fig.3d, asterisk), and only a small fraction of Col1a1-GFP+ trabecular osteoblasts were marked by tdTomato (Fig.3d, arrowheads), indicating that contribution of Col2a1-cre+ cells to these osteoblasts diminished at this stage. At 6 months of age, Col2a1-marked cells largely disappeared from the condylar cartilage, with only a few yellow osteoblasts present in the trabecular bone (Fig.3e). No Tomato+ cells were observed in the absence of Col2a1-cre (Fig.3f). Therefore, these findings indicate that, while Col2a1-expressing cells make significant contribution to chondrocytes and subchondral osteoblasts during the early stages, these cells are replaced by other types of cells during the late stages. This underscores a transient contribution of Col2a1-expressing cells to the mandibular condylar cartilage and its subchondral bones.
Fig.3.
Transient contribution of Col2a1-cre+ cells to chondrocytes and subchondral osteoblasts in the mandibular condyle.
(a-e) E15.5 (a), P3 (b), P21 (c), 8 weeks (d) and 6 months (e) of age. Asterisk: condylar cartilage, arrowheads: GFP/tdTomato+ osteoblasts. Arrows: tdTomato+ osteocytes.
(f) Negative controls (R26RTomato) at E15.5, P3, P21.
Scale bars: 400μm in (a-f), 100 μm in (insets in b,c).
(g) Flow cytometry analysis at P3. Right subpanels: histograms of the GFPhigh fraction developed for Tomato; blue lines: GFPhigh Tomatoneg control cells. X-axis: tdTomato, Y-axis: GFP. n=3. All data represented as mean ± S.D.
Discussion
Two distinct types of skeletal progenitors, growth-associated skeletal progenitors and adult mesenchymal stromal progenitors, are present to promote growth, maintenance and repair of endochondral bones. The former cells reside within or in the proximity to the growth plate cartilage and can be marked by promoter/enhancers of chondrocyte-related genes such as Sox9 and Col2a1. We have previously reported that Col2a1-cre transgene can mark such early skeletal progenitors in the limb (4). The relationship of skeletal progenitors between endochondral and intramembranous bone formation is unknown. Here in this study, we first asked whether Col2a1-cre could mark similar early cells of the skeletal lineage in the cranial base where endochondral bone formation takes place. As expected, Col2a1-cre marked a great majority of skeletal cells, including chondrocytes, osteoblasts/cytes and bone marrow stromal cells. As Col2a1 is also reported to be present in intramembranous osteoblast precursors and/or preosteoblasts (6,7), we next asked whether Col2a1-cre could mark similar early cells in the calvaria where intramembranous bone formation takes place. Col2a1 mRNA is transiently expressed by preosteoblasts and ‘chondrocyte-like osteoblasts’ during early development of intramembranous bones (6), as well as by calvarial bone osteoblasts (7) . In addition, previous reports have demonstrated the existence of transient cartilages in the calvaria (11,12). We found that, during early stages, Col2a1-cre marked a number of osteoblasts/cytes especially in the lateral calvaria, where the underlying cartilage underpinned the bone structure during early stages (12). Subsequently, Col2a1-cre-marked osteoblasts/cytes increased during the postnatal growth phase. We could not determine whether their progenitor cells or osteoblasts themselves express Col2a1. More notably, Col2a1-cre did not mark osteoblasts/cytes near the midsagittal suture. Instead, a distinct group of suture mesenchymal cells were consistently marked by Col2a1-cre, also suggesting the possibility that suture mesenchymal cells might also express Col2a1. Third, we asked whether Col2a1-cre marks early skeletal progenitors in a secondary cartilage present in the mandibular condylar cartilage. The mandibular condyle cartilage is a secondary cartilage lacking growth plates (13), where direct transformation of chondrocytes into osteoblasts occurs (5). Development of the mandibular condyle starts at around E14.5 (14), when Sox9-positive mesenchymal cells condense on the posterior side of the mandible body (5). The proximal portion of the mandible has been suggested to contain osteo-chondroprogenitors (15). Secondary cartilages are derived from mesenchymal progenitor cells that express markers of early osteoblast differentiation, such as alkaline phosphatase (ALP) and runt-related transcription factor 2 (Runx2). This is different from the growth plate, where Runx2-expressing precursors are derived from Sox9-expressing progenitor cells (16). This reversal expression pattern of Runx2 and Sox9 is a unique feature of mandibular condylar cartilage formation; skeletal progenitor cells in the mandibular condylar cartilage are necessary to differentiate down an osteogenic lineage prior to chondrogenic differentiation (17). In contrast, some reports advocate that chondrocytes in the mandibular condylar cartilage can transform into osteoblasts (5); therefore the mechanism of development of mandibular condyle is complex. We found that, as expected, Col2a1-cre marked a majority of chondrocytes and osteoblasts/cytes during the early phase. However, Col2a1-cre only marked a small fraction of these cells in the later post-growth phase, suggesting that Col2a1-expressing cells only transiently contributed to the mandibular condylar formation. This discrepancy might be attributed to the unique complexity of the mandibular condylar cartilage. Our findings collectively suggest that, while Col2a1 is expressed by early progenitors of the skeletal lineage in ‘canonical’ endochondral bone formation occurring in the cranial base, other mechanisms of craniofacial bone formation utilizes Col2a1-expressing cells in a different manner. Deliberate considerations would be necessary in extrapolating cell lineage and molecular findings obtained from the limb to the craniofacial skeletal structures.
Conclusions
Col2a1 is expressed by early skeletal progenitors of the skeletal lineage in canonical endochondral bone formation occurring in the cranial base. In contrast, other ossification mechanisms of the craniofacial complex utilize Col2a1-expressing cells in a different manner, whereby Col2a1 may be expressed in more differentiated or transient cell types of the skeletal lineage.
Acknowledgments
The authors thank Henry Kronenberg (Massachusetts General Hospital) for providing Col1a1(2.3kb)-GFP and Col2a1-cre transgenic mice, as well as fruitful discussions leading up to this study. These authors acknowledge the support from National Institutes of Health Grant DE022564 to N.O. and University of Michigan MCubed 2.0 Grant to N.O. and W.O.
References
- 1.Thilander B, Ingervall B. The human spheno-occipital synchondrosis. II. A histological and microradiographic study of its growth. Acta Odontol Scand. 1973;31:323–34. doi: 10.3109/00016357309002520. [DOI] [PubMed] [Google Scholar]
- 2.Ono N, Kronenberg HM. Mesenchymal progenitor cells for the osteogenic lineage. Current molecular biology reports. 2015;1:95–100. doi: 10.1007/s40610-015-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ono N, Kronenberg HM. Bone repair and stem cells. Curr Opin Genet Dev. 2016;40:103–07. doi: 10.1016/j.gde.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ono N, Ono W, Nagasawa T, Kronenberg HM. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol. 2014;16:1157–67. doi: 10.1038/ncb3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jing Y, Zhou X, Han X, Jing J, von der Mark K, Wang J, et al. Chondrocytes Directly Transform into Bone Cells in Mandibular Condyle Growth. J Dent Res. 2015;94:1668–75. doi: 10.1177/0022034515598135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abzhanov A, Rodda SJ, McMahon AP, Tabin CJ. Regulation of skeletogenic differentiation in cranial dermal bone. Development. 2007;134:3133–44. doi: 10.1242/dev.002709. [DOI] [PubMed] [Google Scholar]
- 7.Szabova L, Yamada SS, Wimer H, Chrysovergis K, Ingvarsen S, Behrendt N, et al. MT1-MMP and type II collagen specify skeletal stem cells and their bone and cartilage progeny. J Bone Miner Res. 2009;24:1905–16. doi: 10.1359/JBMR.090510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ovchinnikov DA, Deng JM, Ogunrinu G, Behringer RR. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis (New York, NY : 2000) 2000;26:145–6. [PubMed] [Google Scholar]
- 9.Kalajzic Z, Liu P, Kalajzic I, Du Z, Braut A, Mina M, et al. Directing the expression of a green fluorescent protein transgene in differentiated osteoblasts: comparison between rat type I collagen and rat osteocalcin promoters. Bone. 2002;31:654–60. doi: 10.1016/s8756-3282(02)00912-2. [DOI] [PubMed] [Google Scholar]
- 10.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–40. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helms JA, Cordero D, Tapadia MD. New insights into craniofacial morphogenesis. Development. 2005;132:851–61. doi: 10.1242/dev.01705. [DOI] [PubMed] [Google Scholar]
- 12.Holmbeck K, Bianco P, Chrysovergis K, Yamada S, Birkedal-Hansen H. MT1-MMP-dependent, apoptotic remodeling of unmineralized cartilage: a critical process in skeletal growth. J Cell Biol. 2003;163:661–71. doi: 10.1083/jcb.200307061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen G, Darendeliler MA. The adaptive remodeling of condylar cartilage---a transition from chondrogenesis to osteogenesis. J Dent Res. 2005;84:691–9. doi: 10.1177/154405910508400802. [DOI] [PubMed] [Google Scholar]
- 14.Parada C, Chai Y. Mandible and Tongue Development. Curr Top Dev Biol. 2015;115:31–58. doi: 10.1016/bs.ctdb.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibata S, Suda N, Suzuki S, Fukuoka H, Yamashita Y. An in situ hybridization study of Runx2, Osterix, and Sox9 at the onset of condylar cartilage formation in fetal mouse mandible. J Anat. 2006;208:169–77. doi: 10.1111/j.1469-7580.2006.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci U S A. 2005;102:14665–70. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson J, O'Brien A, Chen J, Wadhwa S. Progenitor Cells of the Mandibular Condylar Cartilage. Current molecular biology reports. 2015;1:110–14. doi: 10.1007/s40610-015-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]