Abstract
Authors
Holliday LS, McHugh KP, Zuo J, Aguirre JI, Neubert JK, Rody, Jr WJ
Objectives
Recent studies suggest that exosomes are involved in intercellular communication required for the maintenance of healthy bone. Exosomes are small (30-150 nm in diameter) extracellular vesicles that are formed in multivesicular bodies and are released from cells as the multivesicular bodies fuse with the plasma membrane. Regulatory exosomes have the capacity to exert profound control over target cells. They can stimulate plasma membrane receptors and are also internalized by the target cell delivering proteins, lipids, small molecules and functional RNAs from the cell of origin. We and others have recently reported on regulatory exosomes from osteoclasts and osteoblasts and these results will be highlighted.
Methods
Review
Results
Key candidate molecules identified in exosome-based regulation of bone remodeling include receptor activator of nuclear factor kappa B (RANK), RANK-ligand (RANKL), ephrinA2, semaphorin 4D, microRNA-146a and microRNA- 214-3p. Exosomes will likely prove to be crucial elements in the communication networks integrating bone cells (osteoclasts, osteoblasts, osteocytes) and linking bone to other tissue. Exosomes collected from bone cells grown in culture may prove useful to augment bone remodeling associated with orthodontic force application or required for the repair of craniofacial bone. Various technologies allow exosomes to be engineered to improve their targeting and efficacy for therapeutic purposes.
Conclusions
Exosomes have emerged as important elements of the machinery for intercellular communication between bone cells. They hold great promise as therapeutic targets, biomarkers and therapeutic agents for orthodontists.
Keywords: extracellular vesicle, microvesicle, osteoclast, osteoblast, RANK
Introduction
Exosomes are 30-150 nm in diameter vesicles that are released from multivesicular bodies by most or all eukaryotic cells (1). They were described in simultaneous publications by the Stahl and Johnstone laboratories in 1983 that showed reticulocytes jettisoned the transferrin receptor in vesicles that originated in multivesicular bodies (2;3). By 1996, evidence had emerged that exosomes could present antigen in the context of the major histocompatibility complex (4). It is now thought that exosomes can regulate target cells by stimulating cell surface receptors and by fusing with the target cell and releasing their contents into the membranes and cytosol (1). This allows exosomes to transfer active proteins, lipids, small molecules, and RNAs from their cell of origin to the target cell (5). Much recent work has focused on microRNAs in exosomes. These are small RNAs that bind to target mRNAs and prevent their translation (6). MicroRNAs in exosomes provide a means by which cells can directly control protein expression in target cells.
Exosomes in bone physiology
Physical characteristics and composition of exosomes from osteoclasts
To isolate exosomes from conditioned media of osteoclast cultures, we developed a method which utilized differential centrifugation in combination with ExoQuick (Systems Biosciences, Palo Alta, CA) (7). Two other groups independently reported studies of osteoclast exosomes. Li et al. (8) and Sun et al. (9) utilized differential centrifugation approaches to isolate exosomes. We characterized exosomes from osteoclasts and osteoclast precursors by measuring exosomes visualized by positive/negative stained electron microscopy (7). Exosomes from both sources had average diameters in the range of 40 nm. Li et al. did not provide physical characterization of osteoclast exosomes. Sun et al. made use of dynamic light scattering and identified an exosomal peak (9).
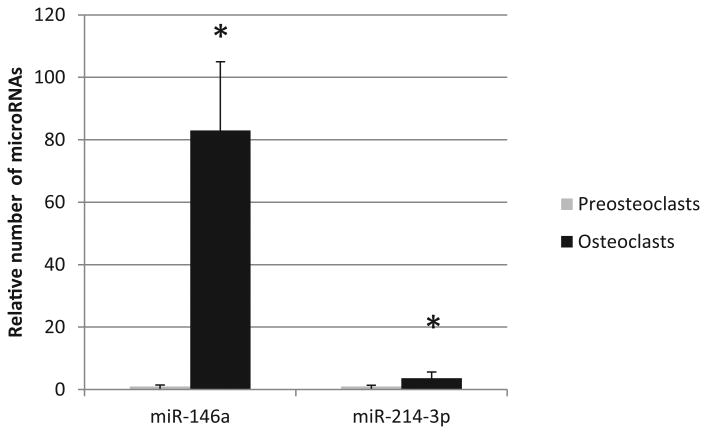
Proteins identified in osteoclast exosomes included the exosome markers; cell differentiation (CD)63 (7-9), epithelial cell adhesion molecule (EpCAM) (7), tumor susceptibility gene (TSG)101 (9), heat shock protein (HSP)70 (9), and β-actin (9). Our group found a subset of osteoclast exosomes that were enriched in receptor activator of nuclear factor kappa B (RANK) (7). Li et al. reported the presence of semaphorin 4D (8) and Sun et al. (9) detected exosomes enriched in ephrinA2 (9). Markers for contamination, transcription factor (TF) II B (6), lamin A/C (6), calnexin (7) and glycoprotein (GP)96 (7) were not detected in any of the exosome preparations. Both Li et al. and Sun et al. detected miR-214 as being enriched in osteoclast exosomes. Sun et al. identified several microRNAs that were enriched in osteoclast exosomes compared with exosomes from precursors, and others that were reduced. MicroRNA-214 was reported to be enriched 4-fold in osteoclasts (9). Our own results regarding microRNA-214 are similar to those of Sun et al., and we also have found that microRNA-146a is enriched over 80-fold in osteoclasts compared with precursors making it a candidate to be a regulatory molecule and biomarker (Figure 1).
Figure 1. MicroRNA-146a and microRNA-214-3p are enriched in exosomes derived from osteoclasts compared with precursors.
Total RNA was isolated from exosomes exactly as described in the protocol for the SeraMir Exosome RNA Purification Column Kit (System Biosciences). RNA was measured and assessed for quality using NanoDrop spectrophotometer. High-quality exosomal RNAs were analyzed by real-time quantitative PCR (qPCR). To determine mature miRNA expression, total RNAs were reverse-transcribed with High-Capacity cDNA Reverse Transcription Kit (Life Technologies) with a stem-loop RT primer designed based on the sequence of target genes (Applied Biosciences). Real-time PCR was performed using TaqMan® miRNA primers (Applied Biosciences) specific for miR-214-3p (002306) and miR-146a (000468) with a StepOnePlus Real-Time PCR System (Applied Biosystems). qRT-PCR were performed using an ABI Prism 7700 HT detection system (Applied Biosystems, Foster City, CA). Three biological replicates were used. In order to carry out absolute miRNA quantification, a serial dilution of a known concentration of appropriate miRNA mimics (Applied Biosciences) were used to plot standard curves and the number of miRNAs were determined as described. Data are presented as the relative number of microRNAs/unit of acetylcholinesterase activity (a quantitative marker of exosomes). The relative expression levels of the miRNAs were calculated using the 2−ΔΔCt method, values were analyzed by Student's t test with p< 0.05 considered significant. Asterisk indicates significantly different from precursor.
Regulatory activities of osteoclast exosomes
As an initial screen for regulatory activity, we tested exosomes isolated from osteoclasts or osteoclast precursors for their ability to regulate calcitriol-stimulated mouse marrow (C-SMM), which has been widely used as a surrogate for the local microenvironment of bone and the bone remodeling unit (BRU)(7). In C-SMM, both osteoclasts and osteoblasts differentiate over six days in a coordinated manner (10). Exosomes from osteoclast precursors stimulated increased numbers of osteoclasts in C-SMM, while the same number of exosomes from mature osteoclasts inhibited osteoclast formation. Neither type of exosome altered the differentiation of osteoclasts from precursors in pure hematopoietic cell cultures stimulated by recombinant RANKL and macrophage-colony stimulating factor. This suggested that osteoclast exosomes act in a paracrine manner, stimulating osteoblasts or other non-osteoclastic cells in C-SMM to produce factors that modulate osteoclast differentiation.
We identified RANK as a component of exosomes from mature osteoclasts, but not precursors. Initially, RANK was identified when we did mass spectroscopy on those concanavalin A-binding proteins, which had been separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and were found to be present in exosomes from osteoclasts, but not precursors. We then performed immunoblots using anti-RANK antibodies that showed RANK in exosomes from osteoclasts but not precursors (7). Finally, we executed quantitative immunogold labeling visualized by transmission electron microscopy to show that only a rare subset of exosomes from osteoclasts was rich in RANK (7).
MicroRNA-214 was identified as a candidate to be linked to bone remodeling by studies showing it regulates Osterix, and activating transcription factor (ATF)4, vital transcription factors in osteoblasts (11). Li, and colleagues found that microRNA-214 was present at higher levels in the serum of both elderly women with fractures and ovariectomized mice (8). A chemically engineered oligonucleotide that blocks other molecules from binding to a desired site on an mRNA (antagomir) directed against binding sites for microRNA-214 was delivered to osteoclasts and rescued the low bone formation phenotype in mice. In vitro, it was shown by using osteoblast-osteoclast co-cultures separated by transwell membranes (that blocked cells from passing but allowed exosomes through) that microRNA-214 was transferred from osteoclasts to osteoblasts in exosomes, where it inhibited osteoblast differentiation and mineralizing activity in vitro. The same mechanism was suggested for inhibition of bone formation in vivo (8). Li et al. also reported that semaphorin 4D was crucial for the binding of osteoclast-derived exosomes to osteoblasts.
Sun et al. also identified miR-214 as being mechanistically-involved in the regulation of osteoblasts by osteoclast-derived exosomes (9). They found miR-214 to be elevated in osteoclasts and osteoclast-exosomes compared with precursors and their exosomes. They identified ephrinA2 as a surface protein on osteoclast-derived exosomes that targeted osteoblasts based on ephrinA2 interacting with its receptor EphA2 on the surface of osteoblasts.
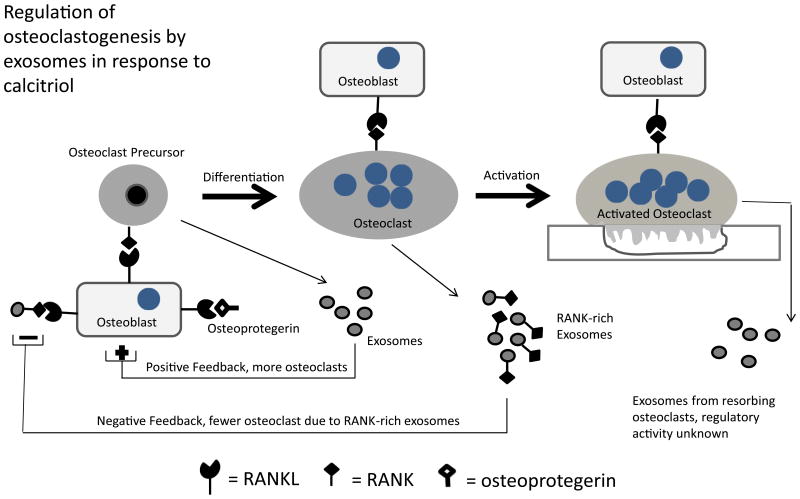
Unlike exosomes from osteoclasts, exosomes from osteoclast precursors stimulated osteoclast formation in C-SMM (7). This led to our proposal that exosomes might represent a means by which bone cells could sense the presence of osteoclasts and precursors, and based on that information, respond appropriately to calciotropic signals (Figure 2).
Figure 2. Regulation of osteoclastogenesis by exosomes released by osteoclasts.
In C-SMM, exosomes isolated from osteoclast precursors increased the number of osteoclasts produced. In contrast, RANK-rich exosomes from inactive osteoclasts inhibited osteoclast formation in the same culture. This suggests that exosomes released by osteoclasts and their precursors provide bone cells with information regarding how many osteoclasts and precursors are present, which the bone cells use to produce a response to the calciotropic hormone. Currently we do not know the regulatory activity of exosomes from resorbing osteoclasts.
Regulatory exosomes from osteoblasts
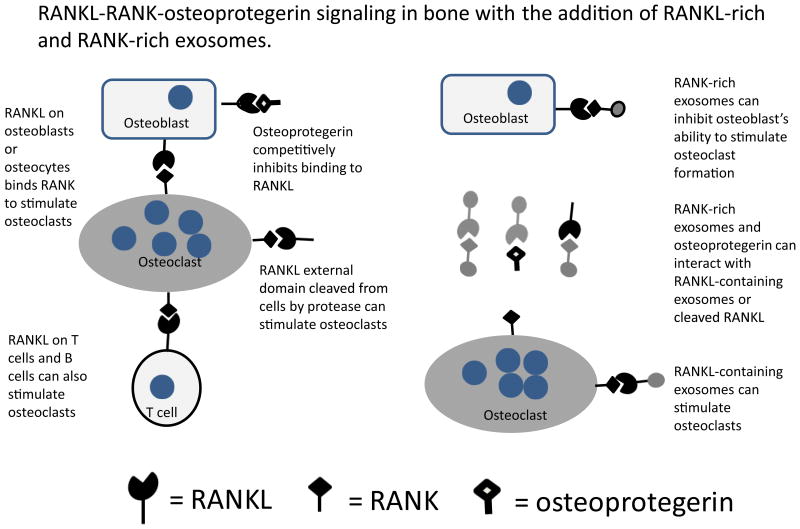
Microvesicles (probably exosomes) released by UAMS-32P cells, an osteoblastic cell line, carry Receptor Activator of Nuclear Factor Kappa B-ligand (RANKL), and these extracellular vesicles were sufficient to induce the differentiation of osteoclast-like cells from RAW 264.7 cells in culture (12). The finding that osteoblasts release extracellular vesicles containing RANKL, together with our data suggesting that osteoclasts release exosomes rich in RANK, suggest new branches to the RANKL-RANK-osteoprotegerin signaling network known to be at the heart of bone biology (Figure 3). RANKL-containing extracellular vesicles from osteoblasts may be able to stimulate osteoclast formation and activity without direct cell to cell contact. RANK-containing exosomes may be able to suppress RANKL in a manner similar to osteoprotegerin. In addition, the possibility exists that both RANKL and RANK-containing extracellular vesicles may be able to fuse with their target cells to release regulatory molecules, like miR-214 in the case of osteoclast exosomes, which may contribute to reprogramming the target cells.
Figure 3. Exosomes enriched in RANKL and RANK increase the potential regulatory complexity of the RANKL-RANK-osteoprotegerin regulatory system.
As shown in the schematic, new interactions between RANKL and RANK in exosomes produce new regulatory circuits and a means for extending the regulatory range of these molecules.
Exosomes and neuronal control of bone remodeling
It is known that bone is under neural control (13). The periosteum is densely innervated and marrow and mineralized bone also have numerous neurons (14). It is already known that exosomes are involved in the maintenance of the central nervous system and data suggest that calcitonin gene-related peptide released by nerve fibers can inhibit osteoclasts and stimulate mineralization by osteoblasts (15). Ephrins are well known for regulating the migration of axons and as promoters of angiogenesis (16) and have recently been shown to regulate bone remodeling (17). Recent data also indicate that the range of ephrins, which are membrane proteins, is extended by their incorporation into exosomes (18). This was reinforced by the identification of ephrinA2 in osteoclast-derived exosomes (9), which provides a direct link between a known controller of the development of the nervous system and a potential exosome-based regulator of bone remodeling.
Semaphorins have been shown to trigger axonal collapse and have been tied to the regulation of osteoblasts by osteoclasts. As in the case of ephrins, finding semaphorin 4D on exosomes released by osteoclasts (8) provides a potential mechanism for the coordinate regulation of bone remodeling with neural dynamics.
Engineering therapeutic exosomes
Exosomes can be engineered in various ways. These techniques for introducing proteins and RNAs into exosomes were recently reviewed (19). Protein cargoes can be introduced into exosomes by creating a fusion protein of the cargo-encoding gene to a gene encoding a protein known to be localized to exosomes. Another approach is based on the finding that oligomeric membrane-anchored proteins tend to be trafficked to exosomes. RNA can be added to isolated exosomes by electroporation. This strategy may be broadly applicable although there is evidence that certain sizes or conformations of RNA may be less amenable to electroporation (19). Another approach is to simply overexpress the cargo RNA in exosome producing cells. This method utilizes mass action to promote inclusion of the RNAs. Some microRNAs also have “zipcodes”, sequences in the 3′-untraslated region that target the RNA to exosomes (20).
Several companies have developed proprietary reagents that are advertised to transfect exosomes with RNAs. These include Exo-Fect (Systems Biosciences, Palo Alta, CA) and ExoFectin (101BIO, Palo Alta, CA). As the mechanisms involved in the targeting of native microRNAs to exosomes become clearer, the toolbox for exosome engineering should expand.
Exosomes for orthodontics
Exosomes are stable and can be frozen and stored for extensive periods (1). Unlike typical therapeutic agents, exosomes can, in principle, regulate the target cell at various levels and with great precision. A number of studies have indicated that much of the therapeutic benefit of mesenchymal stem cell therapy can be achieved by delivering exosomes derived from the stem cells grown in culture (21). Studies also indicate that exosomes injected into the blood stream tend to hone to their site of origin (22). Therefore, exosomes from osteoclasts and osteoblasts may have the capacity to be preferentially directed to the bone, or even to the alveolar bone surrounding teeth. This was directly supported by Li et al. who showed that osteoclast exosomes honed preferentially to bone after systemic injections (8).
It is possible that native exosomes collected from cell culture will be therapeutically beneficial. For example, a patient may donate peripheral monocytes, which can be differentiated into osteoclasts in vitro. The exosomes can then be collected and used for the treatment of bone related pathologies. This could represent a new and natural therapeutic approach that may avoid side effects like medicine –induced oral osteonecrosis of the jaw (MRONJ) (23).
Exosomes from osteoclast precursors stimulate osteoclastogenesis (7). This could be useful in increasing rates of orthodontic tooth movement. Evidence for the use of exosomes from other tissues to increase the speed and efficacy of healing has already appeared (21). Efforts are underway to test osteoclast-derived exosomes in a mouse model of orthodontic tooth movement (24).
Conclusions
Exosomes produced by bone cells represent a newly identified mechanism by which bone remodeling is regulated. These extracellular vesicles can be collected from cells obtained from the patient being treated and have the potential to regulate target cells very precisely and at multiple regulatory levels. They therefore offer the prospect of personalized and precise intervention to facilitate orthodontic tooth movement, and to treat various bone-associated pathologies.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Dental and Craniofacial Research R21 DE019862 (LSH).
Reference List
- 1.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–78. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 4.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 6.Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell. 2005;122:6–7. doi: 10.1016/j.cell.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 7.Huynh N, VonMoss L, Smith D, Rahman I, Felemban MF, Zuo J, et al. Characterization of Regulatory extracellular vesicles from osteoclasts. J Dent Res. 2016;95:673–679. doi: 10.1177/0022034516633189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D, Liu J, Guo B, Liang C, Dang L, Lu C, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7:10872. doi: 10.1038/ncomms10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun W, Zhao C, Li Y, Wang l, Nie G, Peng J, et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discovery. 2016;2:16015. doi: 10.1038/celldisc.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holliday LS, Dean AD, Lin RH, Greenwald JE, Gluck SL. Low NO concentrations inhibit osteoclast formation in mouse marrow cultures by cGMP-dependent mechanism. Am J Physiol. 1997;272:F283–F291. doi: 10.1152/ajprenal.1997.272.3.F283. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Guo B, Li Q, Peng J, Yang Z, Wang A, et al. miR-214 targets ATF4 to inhibit bone formation. Nat Med. 2013;19:93–100. doi: 10.1038/nm.3026. [DOI] [PubMed] [Google Scholar]
- 12.Deng L, Wang Y, Peng Y, Wu Y, Ding Y, Jiang Y, et al. Osteoblast-derived microvesicles: a novel mechanism for communication between osteoblasts and osteoclasts. Bone. 2015;79:37–42. doi: 10.1016/j.bone.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Neubert JK, Caudle RM, Dolce C, Toro EJ, Bokrand-Donatelli Y, Holliday LS. Neural Modulation of Orthodontic Tooth Movement. In: Naretto Silvano., Dr, editor. Principles in Contemporary Orthodontics. InTech; 2011. Available from: http://www.intechopen.com/books/principles-in-contemporary-orthodontics/neural-modulation-of-orthodontic-tooth-movement. [DOI] [Google Scholar]
- 14.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 15.Elefteriou F. Regulation of bone remodeling by the central and peripheral nervous system. Arch Biochem Biophys. 2008;473:231–236. doi: 10.1016/j.abb.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flanagan JG, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu.Rev. Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 17.Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep. 2014;3:481. doi: 10.1038/bonekey.2013.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong J, Korner R, Gaitanos L, Klein R. Exosomes mediate cell contact-independent ephrin-Eph signaling during axon guidance. J Cell Biol. 2016;214:35–44. doi: 10.1083/jcb.201601085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcus ME, Leonard JN. FedExosomes: Engineering Therapeutic Biological Nanoparticles that Truly Deliver. Pharmaceuticals (Basel) 2013;6:659–80. doi: 10.3390/ph6050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Strobel T, Erkan EP, et al. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol Ther Nucleic Acids. 2012;1:e10. doi: 10.1038/mtna.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82–88. doi: 10.1016/j.semcdb.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mager I, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. doi: 10.3402/jev.v4.26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fusco V, Santini D, Armento G, Tonini G, Campisi G. Osteonecrosis of jaw beyond antiresorptive (bone-targeted) agents: new horizons in oncology. Expert Opin Drug Saf. 2016;15:925–35. doi: 10.1080/14740338.2016.1177021. [DOI] [PubMed] [Google Scholar]
- 24.Toro EJ, Zuo J, Gutierrez A, La Rosa RL, Gawron AJ, Bradaschia-Correa V, et al. Bis-enoxacin inhibits bone resorption and orthodontic toothmovement. J Dent Res. 2013;92:925–31. doi: 10.1177/0022034513501876. [DOI] [PMC free article] [PubMed] [Google Scholar]