Abstract
Purpose
To evaluate technologies and techniques available for the diagnosis of ocular surface tumors.
Methods
A review of the literature from 1947 to 2017, through the PubMed Database, was conducted in order to evaluate current diagnostic methods for ocular surface tumors.
Results
Ocular surface squamous neoplasia, conjunctival melanoma, and conjunctival lymphoma are the three most common ocular surface malignancies. Technologies available to assist with diagnosis of these conditions, in addition to full thickness biopsy, include vital dyes, aspiration and impression cytology, in vivo confocal microscopy, ultrasound biomicroscopy, genetic testing, and anterior segment optical coherence tomography.
Conclusions
Histology remains the gold standard for diagnosis for all 3 of these malignancies. However, multiple diagnostic techniques are available to assist in making preliminary and early diagnoses, in differentiating between similar-appearing lesions, and in some cases, avoiding biopsy prior to initiating treatment. As imaging and technology continue to evolve, these adjunctive techniques will likely continue to play a greater role in clinical practice.
Keywords: Ocular surface squamous neoplasia, conjunctival melanoma, conjunctival lymphoma, impression cytology, confocal microscopy, optical coherence tomography, OSSN, conjunctival intraepithelial neoplasia, CIN, OCT
Ocular surface tumors represent a range of conditions, from benign to malignant lesions. They originate from a variety of cell types, forming epithelial, melanocytic, lymphoid, leukemic, fibrous, lipomatous, and other lesions. The most common malignant tumors of the cornea and conjunctiva are ocular surface squamous neoplasia (OSSN), conjunctival melanoma (CM), and conjunctival lymphoma (CL)1. Each of these tumors arises from a similar-appearing pre-malignant lesion or can resemble a pre-malignant lesion. Differentiating between benign and malignant lesions, as well as between these three malignant conditions, can sometimes present a clinical challenge. However, accurate diagnosis is paramount because of the differences in treatment of these lesions. In this review, we will highlight the various modalities available for the diagnosis of conjunctival tumors and in particular for these three most common malignant tumors.
I. Ocular Surface Squamous Neoplasia
OSSN is an umbrella term encompassing a spectrum of disorders ranging from dysplasia to invasive squamous cell carcinoma, and is the most common malignant tumor of the ocular surface2. Risk factors include ultraviolet light exposure, human immunodeficiency virus and other forms of immunosuppression, age, and male sex3–7. OSSN generally presents as unilateral disease, but may be bilateral in immunosuppressed patients. Patients are often asymptomatic, but the most common presenting symptoms are redness, tearing, grittiness, or pain7.
OSSN can present on the cornea or conjunctiva in a variety of ways, including as a gelatinous mass, an elevated growth with overlying leukoplakia, a nodule, or when on the cornea, as a flat opalescent layer. The diagnosis can often be made clinically, based on a classic appearance. At times, however, the lesion can be subtle, and difficult to determine clinically. In addition, there is a wide breadth of lesions, both benign and malignant, that can masquerade as OSSN. These include pterygia, amelanotic melanoma, corneal pannus, corneal nodular degeneration, pyogenic granuloma, sebaceous cell carcinoma, and others8,9. In order to differentiate between these growths, other diagnostic modalities besides clinical exam are sometimes required.
The gold standard for the diagnosis of OSSN involves excisional biopsy, with histology of the specimen. The advantage of excisional biopsy is that it allows one to obtain a histologic diagnosis for the entirety of the specimen. However, for large and diffuse lesions without clear margins, even intended excisional biopsy may not remove the entire lesion. Also, for large or recurrent lesions, excisional biopsies lead to increased risks of limbal stem cell deficiency, symblepharon, and scarring10,11. Lastly, as medical options for treatment of OSSN increase in number and become more utilized, non-invasive methods of diagnosis become more important12.
A. Vital Dyes
In addition to clinical exam alone, vital dye staining can assist in making the diagnosis of OSSN both inexpensively and quickly; these vital dyes include rose bengal, methylene blue, and toluidine blue. Rose bengal is a iodinated derivative of fluorescein that stains degenerating, devitalized, and dead epithelial cells in a bright pink color. Due to its ability to stain unhealthy and metabolically deranged cells, this dye highlights epithelial neoplasms on the eye13 (Figure 1). However, because it stains any devitalized tissue, it is not specific for OSSN and will also stain exposed epithelium along an elevated pterygium or unhealthy epithelium in dry eye syndrome. Methylene blue is an acidophilic dye that penetrates cells and has a selective affinity for nucleic acids, thus staining cells with a high metabolic rate. This dye is useful for detecting malignant lesions, as surface neoplasias pick up the stain at a high rate. Like rose bengal, however, methylene blue is not specific for OSSN and benign lesions can also show positive dye uptake14. Toluidine blue, another acidophilic dye, also stains cells with a high mitotic rate, as well as accumulating between cells and therefore staining tissues with poor cell-to-cell adhesion. Similar to methylene blue, the test is sensitive, meaning that OSSN lesions have a high rate of staining; however, benign lesions may also take up dye15. The staining of benign lesions with these acidophilic dyes is likely because of a similar causal pathway between OSSN and benign lesions such as pterygia and actinic keratosis. Vital dyes are therefore helpful adjuncts to clinical examination but their staining is not specific to malignant squamous lesions. Rose bengal can also cause some significant burning at the time of instillation, less so for the other agents.
Figure 1.
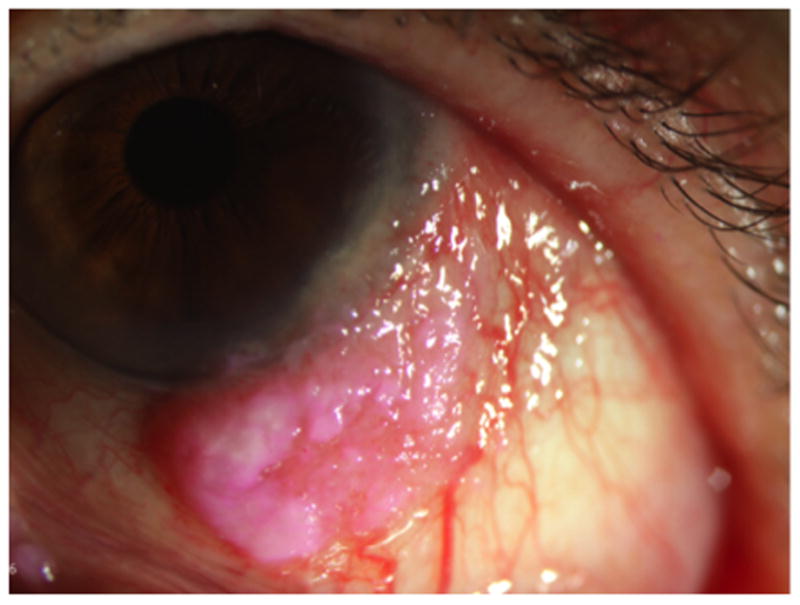
An 81 year old female with OSSN of the left eye. Rose bengal highlights devitalized and metabolically deranged epithelial cells, thus highlighting her limbal tumor.
B. Cytology
Another adjunctive diagnostic method involves cytology, either by aspiration or impression. In aspiration cytology (AC), cells are aspirated from the conjunctival surface with a fluid-filled syringe; a syringe without a needle is used rather than aspiration of cells from within the tumor.16 A small study of AC for OSSN has shown good histological correlation between this technique and subsequent biopsy16. However, limitations include lack of adequate sampling, both in terms of cell number and sampling of only superficial cells, thereby not enabling a differentiation between invasive and non-invasive disease. The technique also requires experience, both of the practioner performing the aspiration cytology and of the cytologist reading the specimen. Due to these limitations, AC has limited applicability16.
Impression cytology (IC) uses a technique of collecting superficial cells from the surface of the eye with filter paper; this paper is attached to a collecting device. The device is applied to the surface of the eye to adhere cells to the paper. Cells are then fixed, stained, mounted, and evaluated for atypia, based on features such as enlarged nuclei, irregular nuclear outline, coarse chromatin and presence of prominent nucleoli, and hyperchromasia17. Studies of IC, like AC, show overall good correlation, from 80 to 84%, with histology specimens from biopsy17,18. Similar to AC and unlike biopsy, IC has the advantage of being minimally invasive and preserving limbal stem cells. It is quick and can be performed even on children in clinic. However, similar limitations to AC are present, including the technique being limited to superficial sampling of cells and the requirement of an experienced cytologist. Additionally, IC has decreased sensitivity when used for keratotic lesions, as the abundance of surface keratin can decrease the sampling of actual cells. Consecutive repeated applications of filter paper, in order to access the deeper epithelium and remove any superficial keratin, have been shown to increase sensitivity. However, invasive disease can still not be definitively identified19.
C. In Vivo Confocal Microscopy
In vivo confocal microscopy (IVCM) is a non-invasive technique that allows en face images of the ocular surface. It utilizes a point or slit light source and a point or slit detector with the same focal point; the source and detector use conjugate pinholes to increase optical resolution by decreasing interference from light contamination20. Impressive resolution, down to the cellular level, is obtained, although only one plane can be imaged during any time point. The technique is safe and non-invasive and can be performed in clinic so the information is immediately available. However, although it is non-invasive, there are both contact and non-contact forms; the contact technique does have risks of corneal abrasion and infection.
Studies of IVCM for OSSN have had mixed conclusions. Some have shown several characteristic features of the tumor, including hyperreflective pleomorphic cells of varying shapes and sizes, large nuclei, bright dots within middle layer cells corresponding to prominent nucleoli, a basal cell layer with poorly defined cell borders and bright nuclei, and the apparent absence of subbasal nerves due to the high reflectivity of the OSSN cells causing difficulty in imaging (not due to actual degenerated nerves)21–23. In small studies of IVCM, it has been shown that the technology has good correlation to histology; however, as these were all correlation studies, the sensitivity and positive predictive value of this technique is not yet known21–23. One of these studies did show that IVCM is useful for predicting the grade of dysplasia and the extent of epithelial involvement as well as stromal invasion23.
In contrast to these smaller studies, in the largest study to date of IVCM for OSSN did not find IVCM to be as helpful; this study included 120 patients, 52 of which underwent excisional biopsy with histopathology. A significant overlap was identified between IVCM findings for benign and malignant lesions, including hyperreflective and pleomorphic cells and a “starry night” appearance of the basal cell layer in both groups24. In addition, some OSSN lesions showed no suspicious IVCM characteristics at all. While IVCM may be a useful adjuvant to histology, these findings indicate that it cannot replace biopsy. Limitations include the requirement for operator expertise in capturing and the interpretation of images, and decreased visualization in very thick/keratinized lesions that degrade the optical properties of the normally transparent conjunctival and corneal epithelium22,23.
D. Anterior Segment Optical Coherence Tomography
The advent of optical coherence tomography (OCT) has allowed clinicians to obtain an in vivo, cross-sectional, high-resolution image of ocular tissue. The OCT, which was initially limited to the posterior segment, was first used for anterior segment imaging in 199425. It has recently been shown to be very helpful for ocular surface lesions as well9,26–32.
OCT uses a Michelson interferometer, which splits a beam of light into a reference beam that is reflected from a mirror, and a measurement beam, which is reflected from the variably reflective tissue layers of the eye. These lights are then combined upon reflection to create an interference pattern, which is detected. Multiple interference patterns are created over the structure being imaged, and used to create a series of A-scans that are put together into a composite, cross-sectional image of the tissue.
With the original time-domain OCT (TD-OCT) technology, the images are created as the reference mirror moves in a linear fashion. In contrast, with the newer spectral domain OCT (SD-OCT), the detector arm of the interferometer uses a spectrometer instead of a single detector, allowing simultaneous detection of a broad range of depths; the entire A-scan is then produced using Fourier transformation of spectrometer analysis. As scanning can be performed much faster using SD-OCT, resolution is also better33. Compared to 18 micron resolution with TD-OCT, resolution with SD-OCT is 3 microns with custom-built ultra-high resolution devices30 and 5 microns with commercially-available high-resolution machines.
While an initial study using TD-OCT for imaging conjunctival tumors found that TD-OCT and UBM had a similar ability to image surface tumors26 the improved resolution of SD-OCT improves the diagnostic ability of the OCT devices. Classic characteristics of anterior segment OCT (AS-OCT) for OSSN, using SD-OCT, include an abrupt transition between the normal and abnormal epithelium, as well as epithelial thickening and epithelial hyper-reflectivity in the area of the tumor28,31 (Figure 2). SD-OCT for the anterior segment is useful in differentiating between OSSN and benign conditions such as pterygium, scarring, Salzmann’s nodular degeneration, and limbal stem cell deficiency as well as between OSSN and other malignant conditions such as melanoma9,30,31.
Figure 2. A 63 year old male with OSSN of the left eye.
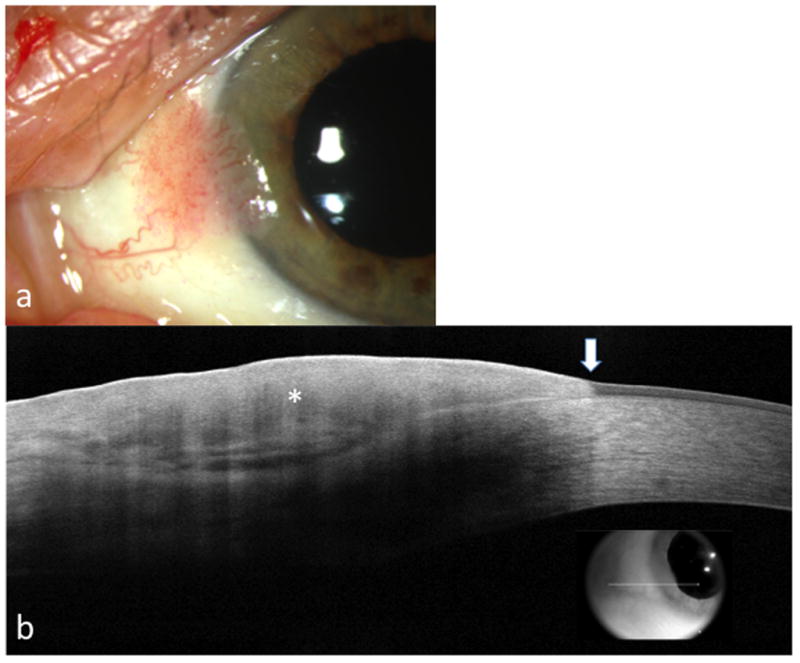
a. Slit lamp photograph of a sessile conjunctival lesion extending to the limbus.
b. AS-OCT reveals an abrupt transition (arrow) between normal and thickened hyperreflective (asterisk) epithelium
Advantages of OCT compared with other imaging for the anterior segment include that is that it is not only non-invasive but also non-contact. Imaging can be performed in clinic, so results are immediately available. And, unlike confocal microscopy, which requires an experienced user for interpretation, AS-OCT can be quickly taught to even novice users with moderate sensitivity but high specificity (unpublished data). However, limitations of this technology include difficulty in imaging the posterior portion of thick, keratinized, and pigmented lesions due to shadowing of the image30. With thick tumors, imaging can be done at the edges to minimize shadowing, but it remains difficult to image the entire posterior border. There is also limited knowledge regarding the ability of the technique to detect invasion into the stroma.
II. Conjunctival Melanoma
CM is a rare but potentially life-threatening tumor of the ocular surface. It comprises 2% of all ocular malignancies, but similar to cutaneous melanoma, the incidence of CM is rising34. CM is most common in non-Hispanic white populations, but it can occur in any group, including blacks, Hispanics, Asians, and Native Americans35. CM generally arises from primary acquired melanosis (PAM), but can also arise from conjunctival nevi or de novo36. The clinical presentation of CM is typically a pigmented and nodular lesion on the bulbar conjunctiva with surrounding feeder vessels. However, CM can also occur on the palpebral conjunctiva or caruncle, and may be minimally elevated37,38. Additionally, these tumors may be amelanotic or minimally pigmented in up to one-fifth of cases, causing diagnostic difficulty and often, diagnostic delay36. Risk of metastatic disease with CM varies with origin, and at ten years is 25–49%, while 10-year tumor related mortality is 9–35%36. Risk factors for both metastatic disease and mortality include increased tumor thickness, local recurrence, and de novo tumor origin.36,39–41
As with OSSN, the gold standard for diagnosis of CM is histology, after excision with cryotherapy is performed for complete removal of the lesion. Excisional surgery allows for removal of the entire lesion, so that all areas can be examined for invasive disease and so that margins can be evaluated for atypical cells. A total excision, although important for all tumors, has even greater implications for conjunctival melanoma, as incomplete excision leads to elevated risk of local recurrence, metastatic disease, and tumor-related death42,43. Diagnostic imaging is therefore especially important in CM, to avoid an incisional biopsy and to proceed directly to full excisional procedures. The diagnosis of CM depends on differentiation not only between various malignant tumors but also between CM and its precursors. Diagnostics for CM precursors will therefore also be discussed in this section.
A. Impression Cytology
The first study of IC for lesions of melanocytic origin was published in 1992 and evaluated IC for PAM, nevi, and melanoma44. Cytologic features of malignancy in melanocytic lesions include an increased nucleus to cytoplasmic ratio, enlarged nucleoli, irregular nuclear chromatin pattern, anisokaryosis, and mitoses; a high relative proportion of atypical cells with these features is consistent with a diagnosis of malignant melanoma. Correlation between impression cytology and histology for diagnosis in that study was 73%; errors included the diagnosis of melanoma as PAM in one eye44. A second study with IC using similar grading criteria found a correlation with histology of 88%, with errors again including melanoma being diagnosed by IC as a pre-malignant condition rather than melanoma45.
IC, as discussed previously, has advantages of being painless and minimally invasive. Additionally, it takes advantage of the ascent of atypical melanocytes to the epithelial surface that it is indicative of malignancy44. A recent case report of an unusual amelanotic lesion on the cornea, in which IC provided the first indication of melanoma, demonstrates the utility of IC in challenging diagnostic cases and its use as an adjuvant to histologic diagnosis46. However, with the risk of local and distant metastasis increasing as the tumor grows and thickens, the false negatives seen in both studies do raise concern. Also, as IC is limited to the superficial cells, it is not able to detect the atypia in early PAM that begins at the basal epithelium. IC is also limited by tumor location, as sampling is difficult for tumors in the fornices, palpebral conjunctiva, and caruncle44,45.
II. In Vivo Confocal Microscopy
IVCM characteristics for PAM, nevi, and melanoma were developed by Messmer et al.47 For conjunctival nevi, IVCM demonstrates nests or collections of medium-sized, uniform stromal cells as well as pseudocyst-like structures. For PAM with atypia, hyper-reflective cells and large dendritic cells are seen throughout the epithelium, while in PAM without atypia the hyper-reflective cells remain mostly in the basal epithelium and the dentritic cells are small. Conjunctival melanoma has the characteristic feature of large cells with prominent nuclei/nucleoli. Subepithelial highly reflective cells are also directly visible, confirming invasion. In vivo confocal microscopy for the diagnosis of CM has a sensitivity of 89% and a specificity of 100%47. While this indicates that IVCM cannot replace histology as the gold standard of diagnosis, it does suggest that the need for excisional biopsy can be guided by IVCM findings. Similar results have also been found using a handheld dermatologic confocal device in place of the floor-mounted ophthalmic devices, which allows for easier alignment with the region of interest, including more difficult to image locations such as the caruncle48.
III. Anterior Segment Optical Coherence Tomography
AS-OCT can be helpful in differentiating between various tumors of melanocytic origin. As first noted by Shields et al., AS-OCT of conjunctival nevi demonstrates intrinsic cysts that infer a benign lesion49. Using high-resolution AS-OCT, intrinsic cysts may be seen even when not visible by clinic exam; in addition, with high-resolution OCT, nevi demonstrate hyperreflective basal epithelial layers30,31. For AS-OCT of primary acquired melanosis, multiple studies have demonstrated a uniform hyper-reflective band along the basal epithelium, with normal overlying epithelium and an absence of cysts. However, AS-OCT, even at ultra-high resolution, is unable to differentiate between PAM with and without atypia, as these devices are unable to evaluate cellular details and there is no difference between hyper-reflective band thickness between lesions with and without atypia30–32. For CM, both pigmented and amelanotic, AS-OCT reveals a hyper-reflective epithelium of variable thickening overlying a hyper-reflective sub-epithelial mass, and an absence of cysts30,31.
AS-OCT, in the diagnosis of CM, is principally helpful in differentiating it from other types of tumors. The distinct differences between AS-OCT for CM and OSSN allow differentiation of these tumors, even in difficult cases such as amelanotic melanomas (Figure 3) or pigmented OSSN9. The technology does have limitations in the diagnosis of pigmented lesions, however. Optical shadowing is a concern for AS-OCT for all pigmented lesions, as it prevents calculations of depth. Additionally, unlike cytology and IVCM, atypia cannot be identified at a cellular level.
Figure 3. A 56 year old male with amelanotic melanoma of the left eye.
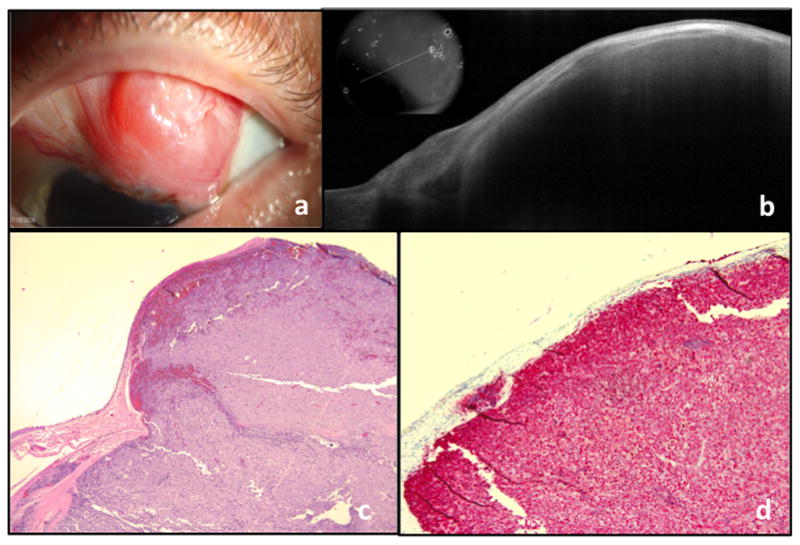
a. Slit lamp photograph of a conjunctival lesion, extending to the limbus. The mass is predominately amelanotic with the exception of small area of pigment at the limbus.
b. AS-OCT showing a thin epithelium overlying a large and elevated subepithelial mass with shadowing of the underlying tissue. The OCT is consistent with the appearance of a conjunctival melanoma.
c. Atypical basophilic cells with prominent nucleoli present within the substantia propria (Original magnification x40)
d. Melan-A with red chromogen highlights the tumor cells within the substantia propria (Original magnification x100), consistent with conjunctival melanoma
IV. Ultrasound Biomicroscopy (UBM)
UBM uses sound waves to analyze structures; the frequency of waves used is higher than classic ocular ultrasound, which increases resolution but decreases penetration into tissue. The resolution of UBM is 42 microns and penetration is 4–5mm50. UBM is able to penetrate opaque tumors, allowing the posterior margin of CMs, even heavily pigmented melanomas, to be visualized. This ability improves detection of tumor invasion26. A small study of patients with CM also showed good correlation between tumor thickness on UBM and histology, which is useful for prognostication51. However, while UBM is able to delineate tumor margins and extent, the resolution of internal tumor details is limited. Other disadvantages of UBM are that it requires contact with the ocular surface and is generally only available in large centers.
V. Genetic markers
The genetics of melanoma, while not strictly used for diagnosis, are important in the diagnostic process because of their potential ability to guide therapy. The mitogen-activated protein kinase (MAPK) pathway is one of the main regulatory pathways involved in CM development, particularly through mutations in BRAF, NRAS, and KIT52–54. BRAF mutations, seen in up to 50% of CM, are common in cutaneous melanoma but rare in mucosal and uveal melanoma43,52,55. BRAF mutations appear to be early events in the development of CM, as they are seen in conjunctival nevi as well43,55. CM with BRAF mutations tends to occur in younger patients and be associated with CM of nevus origin43. Mutations in NRAS, which comprise up to 18% of mutations in CM, do occur in cutaneous melanoma also, but are more common in mucosal melanoma; NRAS mutations are very rare, however, in uveal melanomas. KIT mutations, which comprise the least common CM mutation in the MAPK pathway, occur in both cutaneous and mucosal melanomas.
For cutaneous melanoma, targeted therapies to BRAF (BRAF-inhibitors) have extended life expectancy in patients with those mutations56. For CM, while no intralesional or topical therapy exists at this time targeted to genetic mutations, systemic targeted medications can be useful in patients with metastatic disease57. Additionally, understanding these genetic mutations opens the door in the future for new and better local treatments.
III. Conjunctival lymphoma
Lymphomas are a group of neoplasms derived from clonal proliferations of lymphocytes. Approximately one-quarter of ocular adnexal lymphomas are confined to the conjunctiva58. Ninety-eight percent of conjunctival lymphomas (CL) are derived from B-cell lineage, and most of these are derived from four major subtypes: 1) extranodular marginal zone lymphoma, 2) follicular lymphoma, 3) diffuse large B-cell lymphoma, and 4) mantle cell lymphoma. CL tends to present in an elderly population, but can occur at any age. It generally presents as a pink, or salmon-colored lesion, which is asymptomatic59. It also has a follicular clinical form, which may be subtle.60
CLs, despite being malignant tumors, often present without classically malignant features of feeder vessels or rapid growth. No clear clinical difference can be identified between CL and reactive lymphoid hyperplasia, the latter of which is benign. In general, malignant lesions, when compared to benign lesions, tend to occur in older patients, in the fornix, and tend to be larger and bilateral61; however, none of these characteristics definitively characterize a mass as malignant. As mentioned above, CL can present as a chronic follicular conjunctivitis, an entity that has many etiologies, most of which are also benign60. For a lesion suspicious for CL, the gold standard of diagnosis is a conjunctival biopsy, which is then sent for both histology and flow cytometry and gene rearrangement. The mainstay of treatment of CL involves orbital radiation if the disease is local or systemic chemotherapy if the disease is metastatic62. Rarely, excision with cryotherapy or intralesional chemotherapy or immunotherapy is utilized63–65. Approximately 17% of CLs with unilateral involvement and 47% of CLs with bilateral involvement manifest systemic disease, either at the time of diagnosis or that develops thereafter63.
A. In Vivo Confocal Microscopy
Limited work using IVCM has been performed for lymphoid lesions. The first demonstration, in 2008, of IVCM for CL showed small, hyper-reflective, tightly-packed cells in nests or cyst-like spaces66. Cellular atypia, to differentiate these cells from those seen in inflammatory conditions, however, cannot be identified48. IVCM may therefore be helpful in differentiating CL from other malignant tumors, but given its similarity in appearance to inflammatory lesions, cannot supplant histology for a definitive diagnosis.
B. Anterior Segment Ocular Coherence Tomography
AS-OCT for CL is characterized by a homogenous, hypo-reflective mass, with shadowing of the underlying tissue in thick lesions (Figure 4). On UHR-OCT, the dark mass appears to be made up of small, hyper-reflective stippled dots, while on lower resolution OCT, these dots may not be visualized30,31. Furthermore, on HR-OCT, a hyper-reflective band of tissue can be seen overlying the tumor31. This likely represents conjunctival tissue displaced upward by the subconjunctival infiltrate. No study of AS-OCT to date, however, has demonstrated a comparison between conjunctival lymphoma and benign reactive hyperplasia, so it is unclear whether AS-OCT can be used for differentiation between CL and its benign masquerading counterparts.
Figure 4. A 57 year old male with conjunctival lymphoma of the left eye.
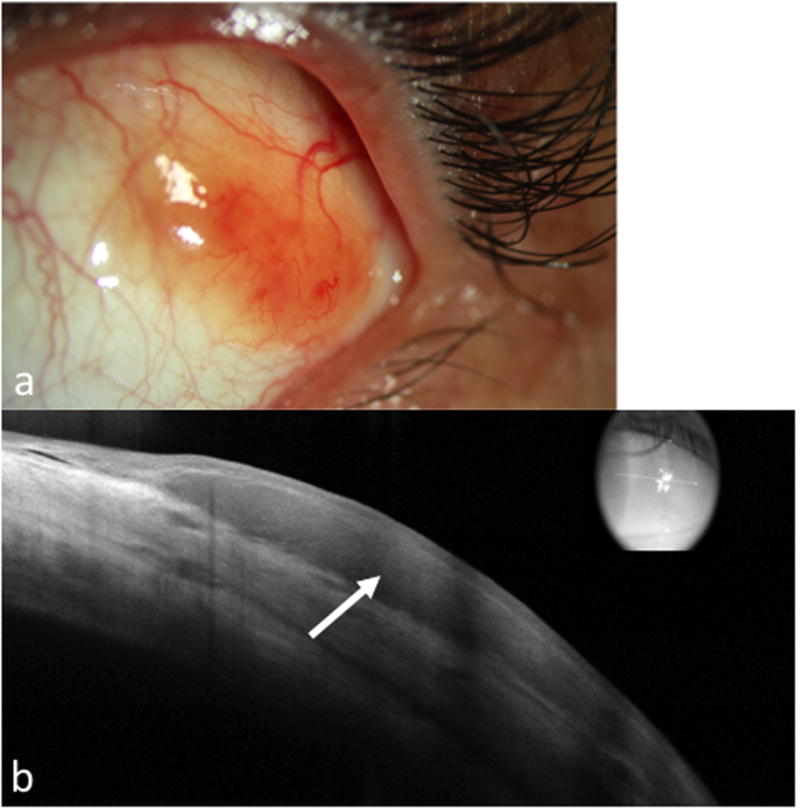
a. Slit lamp photograph of the left eye showing a nasal, fleshy, salmon patch lesion.
b. AS-OCT shows a dark, monomorphic, hyporeflective subepithelial lesion (arrow) with normal overlying epithelium.
IV. Summary
Ocular surface squamous neoplasia, conjunctival melanoma, and conjunctival lymphoma are the most common malignant tumors of the ocular surface. Each has a classic clinical appearance, but may also present in a subtle or atypical fashion, thus requiring more than slit lamp examination for diagnosis. The gold standard for diagnosis of each of these lesions remains histology. However, numerous modalities including cytology, IVCM, AS-OCT, and UBM can assist with prompt diagnosis. Understanding the genetics of these tumors is also important, as it improves the development of targeted therapies. As technology continues to improve, we will likely see a host of new diagnostic modalities in the future and an expansion of their use for ocular surface pathologies.
Acknowledgments
Financial support:
NIH Center Core Grant P30EY014801, RPB Unrestricted Award and Career Development Awards, Department of Defense (DOD- Grant#W81XWH-09-1-0675), The Ronald and Alicia Lepke Grant, The Lee and Claire Hager Grant, The Jimmy and Gaye Bryan Grant, The H. Scott Huizenga Grant, The Robert Baer Family Grant, The Gordon Charitable Foundation, The Richard Azar Family Grant, and The Mark Feldberg Family Grant (Bascom Palmer Eye Institute institutional grants). NIH Grant P30EY10572, departmental funding from Research to Prevent Blindness (Casey Eye Institute institutional grants).
References
- 1.Shields CL, Demirci H, Karatza E, Shields JA. Clinical survey of 1643 melanocytic and nonmelanocytic conjunctival tumors. Ophthalmology. 2004;111(9):1747–1754. doi: 10.1016/j.ophtha.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Grossniklaus HE, Green WR, Luckenbach M, Chan CC. Conjunctival lesions in adults. A clinical and histopathologic review. Cornea. 1987;6(2):78–116. doi: 10.1097/00003226-198706020-00002. [DOI] [PubMed] [Google Scholar]
- 3.Lee GA, Williams G, Hirst LW, Green AC. Risk factors in the development of ocular surface epithelial dysplasia. Ophthalmology. 1994;101(2):360–364. doi: 10.1016/s0161-6420(94)31328-5. [DOI] [PubMed] [Google Scholar]
- 4.Waddell K, Kwehangana J, Johnston WT, Lucas S, Newton R. A case-control study of ocular surface squamous neoplasia (OSSN) in Uganda. International journal of cancer. 2010;127(2):427–432. doi: 10.1002/ijc.25040. [DOI] [PubMed] [Google Scholar]
- 5.Guech-Ongey M, Engels EA, Goedert JJ, Biggar RJ, Mbulaiteye SM. Elevated risk for squamous cell carcinoma of the conjunctiva among adults with AIDS in the United States. International journal of cancer. 2008;122(11):2590–2593. doi: 10.1002/ijc.23384. [DOI] [PubMed] [Google Scholar]
- 6.Furahini G, Lewallen S. Epidemiology and management of ocular surface squamous neoplasia in Tanzania. Ophthalmic epidemiology. 2010;17(3):171–176. doi: 10.3109/09286581003731544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee GA, Hirst LW. Retrospective study of ocular surface squamous neoplasia. Australian and New Zealand journal of ophthalmology. 1997;25(4):269–276. doi: 10.1111/j.1442-9071.1997.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 8.Hirst LW, Axelsen RA, Schwab I. Pterygium and associated ocular surface squamous neoplasia. Arch Ophthalmol. 2009;127(1):31–32. doi: 10.1001/archophthalmol.2008.531. [DOI] [PubMed] [Google Scholar]
- 9.Thomas BJ, Galor A, Nanji AA, et al. Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. Ocul Surf. 2014;12(1):46–58. doi: 10.1016/j.jtos.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palamar M, Kaya E, Egrilmez S, Akalin T, Yagci A. Amniotic membrane transplantation in surgical management of ocular surface squamous neoplasias: long-term results. Eye (London, England) 2014;28(9):1131–1135. doi: 10.1038/eye.2014.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka TS, Demirci H. Cryopreserved Ultra-Thick Human Amniotic Membrane for Conjunctival Surface Reconstruction After Excision of Conjunctival Tumors. Cornea. 2016;35(4):445–450. doi: 10.1097/ICO.0000000000000737. [DOI] [PubMed] [Google Scholar]
- 12.Adler E, Turner JR, Stone DU. Ocular surface squamous neoplasia: a survey of changes in the standard of care from 2003 to 2012. Cornea. 2013;32(12):1558–1561. doi: 10.1097/ICO.0b013e3182a6ea6c. [DOI] [PubMed] [Google Scholar]
- 13.Wilson FM., 2nd Rose bengal staining of epibulbar squamous neoplasms. Ophthalmic surgery. 1976;7(2):21–23. [PubMed] [Google Scholar]
- 14.Steffen J, Rice J, Lecuona K, Carrara H. Identification of ocular surface squamous neoplasia by in vivo staining with methylene blue. Br J Ophthalmol. 2014;98(1):13–15. doi: 10.1136/bjophthalmol-2013-303956. [DOI] [PubMed] [Google Scholar]
- 15.Gichuhi S, Macharia E, Kabiru J, et al. Toluidine Blue 0.05% Vital Staining for the Diagnosis of Ocular Surface Squamous Neoplasia in Kenya. JAMA Ophthalmol. 2015;133(11):1314–1321. doi: 10.1001/jamaophthalmol.2015.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grossniklaus HE, Stulting RD, Gansler T, Aaberg TM., Jr Aspiration cytology of the conjunctival surface. Acta cytologica. 2003;47(2):239–246. doi: 10.1159/000326510. [DOI] [PubMed] [Google Scholar]
- 17.Tole DM, McKelvie PA, Daniell M. Reliability of impression cytology for the diagnosis of ocular surface squamous neoplasia employing the Biopore membrane. Br J Ophthalmol. 2001;85(2):154–158. doi: 10.1136/bjo.85.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tananuvat N, Lertprasertsuk N, Mahanupap P, Noppanakeepong P. Role of impression cytology in diagnosis of ocular surface neoplasia. Cornea. 2008;27(3):269–274. doi: 10.1097/ICO.0b013e31815b9402. [DOI] [PubMed] [Google Scholar]
- 19.Kheirkhah A, Mahbod M, Farzbod F, Zavareh MK, Behrouz MJ, Hashemi H. Repeated applications of impression cytology to increase sensitivity for diagnosis of conjunctival intraepithelial neoplasia. Br J Ophthalmol. 2012;96(2):229–233. doi: 10.1136/bjo.2010.201103. [DOI] [PubMed] [Google Scholar]
- 20.Chiou AG, Kaufman SC, Kaufman HE, Beuerman RW. Clinical corneal confocal microscopy. Surv Ophthalmol. 2006;51(5):482–500. doi: 10.1016/j.survophthal.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Alomar TS, Nubile M, Lowe J, Dua HS. Corneal intraepithelial neoplasia: in vivo confocal microscopic study with histopathologic correlation. Am J Ophthalmol. 2011;151(2):238–247. doi: 10.1016/j.ajo.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 22.Parrozzani R, Lazzarini D, Dario A, Midena E. In vivo confocal microscopy of ocular surface squamous neoplasia. Eye (London, England) 2011;25(4):455–460. doi: 10.1038/eye.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y, Zhou Z, Xu Y, et al. The clinical value of in vivo confocal microscopy for diagnosis of ocular surface squamous neoplasia. Eye (London, England) 2012;26(6):781–787. doi: 10.1038/eye.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguena MB, van den Tweel JG, Makupa W, et al. Diagnosing ocular surface squamous neoplasia in East Africa: case-control study of clinical and in vivo confocal microscopy assessment. Ophthalmology. 2014;121(2):484–491. doi: 10.1016/j.ophtha.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izatt JA, Hee MR, Swanson EA, et al. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol. 1994;112(12):1584–1589. doi: 10.1001/archopht.1994.01090240090031. [DOI] [PubMed] [Google Scholar]
- 26.Bianciotto C, Shields CL, Guzman JM, et al. Assessment of anterior segment tumors with ultrasound biomicroscopy versus anterior segment optical coherence tomography in 200 cases. Ophthalmology. 2011;118(7):1297–1302. doi: 10.1016/j.ophtha.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Vajzovic LM, Karp CL, Haft P, et al. Ultra high-resolution anterior segment optical coherence tomography in the evaluation of anterior corneal dystrophies and degenerations. Ophthalmology. 2011;118(7):1291–1296. doi: 10.1016/j.ophtha.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Shousha MA, Karp CL, Perez VL, et al. Diagnosis and management of conjunctival and corneal intraepithelial neoplasia using ultra high-resolution optical coherence tomography. Ophthalmology. 2011;118(8):1531–1537. doi: 10.1016/j.ophtha.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 29.Kieval JZ, Karp CL, Abou Shousha M, et al. Ultra-high resolution optical coherence tomography for differentiation of ocular surface squamous neoplasia and pterygia. Ophthalmology. 2012;119(3):481–486. doi: 10.1016/j.ophtha.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 30.Shousha MA, Karp CL, Canto AP, et al. Diagnosis of ocular surface lesions using ultra-high-resolution optical coherence tomography. Ophthalmology. 2013;120(5):883–891. doi: 10.1016/j.ophtha.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nanji AA, Sayyad FE, Galor A, Dubovy S, Karp CL. High-Resolution Optical Coherence Tomography as an Adjunctive Tool in the Diagnosis of Corneal and Conjunctival Pathology. Ocul Surf. 2015;13(3):226–235. doi: 10.1016/j.jtos.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alzahrani YA, Kumar S, Abdul Aziz H, Plesec T, Singh AD. Primary Acquired Melanosis: Clinical, Histopathologic and Optical Coherence Tomographic Correlation. Ocular oncology and pathology. 2016;2(3):123–127. doi: 10.1159/000440960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramos JL, Li Y, Huang D. Clinical and research applications of anterior segment optical coherence tomography - a review. Clinical & experimental ophthalmology. 2009;37(1):81–89. doi: 10.1111/j.1442-9071.2008.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu GP, Hu DN, McCormick S, Finger PT. Conjunctival melanoma: is it increasing in the United States? Am J Ophthalmol. 2003;135(6):800–806. doi: 10.1016/s0002-9394(02)02288-2. [DOI] [PubMed] [Google Scholar]
- 35.Hu DN, Yu G, McCormick SA, Finger PT. Population-based incidence of conjunctival melanoma in various races and ethnic groups and comparison with other melanomas. Am J Ophthalmol. 2008;145(3):418–423. doi: 10.1016/j.ajo.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Shields CL, Markowitz JS, Belinsky I, et al. Conjunctival melanoma: outcomes based on tumor origin in 382 consecutive cases. Ophthalmology. 2011;118(2):389–395. e381–382. doi: 10.1016/j.ophtha.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 37.Jakobiec FA, Folberg R, Iwamoto T. Clinicopathologic characteristics of premalignant and malignant melanocytic lesions of the conjunctiva. Ophthalmology. 1989;96(2):147–166. doi: 10.1016/s0161-6420(89)32920-4. [DOI] [PubMed] [Google Scholar]
- 38.Paridaens AD, Minassian DC, McCartney AC, Hungerford JL. Prognostic factors in primary malignant melanoma of the conjunctiva: a clinicopathological study of 256 cases. Br J Ophthalmol. 1994;78(4):252–259. doi: 10.1136/bjo.78.4.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Missotten GS, Keijser S, De Keizer RJ, De Wolff-Rouendaal D. Conjunctival melanoma in the Netherlands: a nationwide study. Invest Ophthalmol Vis Sci. 2005;46(1):75–82. doi: 10.1167/iovs.04-0344. [DOI] [PubMed] [Google Scholar]
- 40.Tuomaala S, Eskelin S, Tarkkanen A, Kivela T. Population-based assessment of clinical characteristics predicting outcome of conjunctival melanoma in whites. Invest Ophthalmol Vis Sci. 2002;43(11):3399–3408. [PubMed] [Google Scholar]
- 41.Esmaeli B, Wang X, Youssef A, Gershenwald JE. Patterns of regional and distant metastasis in patients with conjunctival melanoma: experience at a cancer center over four decades. Ophthalmology. 2001;108(11):2101–2105. doi: 10.1016/s0161-6420(01)00782-5. [DOI] [PubMed] [Google Scholar]
- 42.Anastassiou G, Heiligenhaus A, Bechrakis N, Bader E, Bornfeld N, Steuhl KP. Prognostic value of clinical and histopathological parameters in conjunctival melanomas: a retrospective study. Br J Ophthalmol. 2002;86(2):163–167. doi: 10.1136/bjo.86.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larsen AC. Conjunctival malignant melanoma in Denmark: epidemiology, treatment and prognosis with special emphasis on tumorigenesis and genetic profile. Acta Ophthalmol. 2016;94:1–27. doi: 10.1111/aos.13100. Thesis 1. [DOI] [PubMed] [Google Scholar]
- 44.Paridaens AD, McCartney AC, Curling OM, Lyons CJ, Hungerford JL. Impression cytology of conjunctival melanosis and melanoma. Br J Ophthalmol. 1992;76(4):198–201. doi: 10.1136/bjo.76.4.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keijser S, Missotten GS, De Wolff-Rouendaal D, et al. Impression cytology of melanocytic conjunctival tumours using the Biopore membrane. Eur J Ophthalmol. 2007;17(4):501–506. doi: 10.1177/112067210701700404. [DOI] [PubMed] [Google Scholar]
- 46.de Barros JN, Motono M, Costa FD, Cunha MC, Chojniak MM. Amelanotic corneally displaced malignant conjunctival melanoma: a case report evaluated with impression cytology. Arq Bras Oftalmol. 2014;77(1):57–59. doi: 10.5935/0004-2749.20140015. [DOI] [PubMed] [Google Scholar]
- 47.Messmer EM, Mackert MJ, Zapp DM, Kampik A. In vivo confocal microscopy of pigmented conjunctival tumors. Graefes Arch Clin Exp Ophthalmol. 2006;244(11):1437–1445. doi: 10.1007/s00417-006-0284-8. [DOI] [PubMed] [Google Scholar]
- 48.Cinotti E, Perrot JL, Labeille B, et al. Handheld reflectance confocal microscopy for the diagnosis of conjunctival tumors. Am J Ophthalmol. 2015;159(2):324–333.e321. doi: 10.1016/j.ajo.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 49.Shields CL, Belinsky I, Romanelli-Gobbi M, et al. Anterior segment optical coherence tomography of conjunctival nevus. Ophthalmology. 2011;118(5):915–919. doi: 10.1016/j.ophtha.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Garcia JP, Jr, Rosen RB. Anterior segment imaging: optical coherence tomography versus ultrasound biomicroscopy. Ophthalmic Surg Lasers Imaging. 2008;39(6):476–484. doi: 10.3928/15428877-20081101-02. [DOI] [PubMed] [Google Scholar]
- 51.Ho VH, Prager TC, Diwan H, Prieto V, Esmaeli B. Ultrasound biomicroscopy for estimation of tumor thickness for conjunctival melanoma. Journal of clinical ultrasound : JCU. 2007;35(9):533–537. doi: 10.1002/jcu.20343. [DOI] [PubMed] [Google Scholar]
- 52.Spendlove HE, Damato BE, Humphreys J, Barker KT, Hiscott PS, Houlston RS. BRAF mutations are detectable in conjunctival but not uveal melanomas. Melanoma research. 2004;14(6):449–452. doi: 10.1097/00008390-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Griewank KG, Westekemper H, Murali R, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(12):3143–3152. doi: 10.1158/1078-0432.CCR-13-0163. [DOI] [PubMed] [Google Scholar]
- 54.Beadling C, Jacobson-Dunlop E, Hodi FS, et al. KIT gene mutations and copy number in melanoma subtypes. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(21):6821–6828. doi: 10.1158/1078-0432.CCR-08-0575. [DOI] [PubMed] [Google Scholar]
- 55.Goldenberg-Cohen N, Cohen Y, Rosenbaum E, et al. T1799A BRAF mutations in conjunctival melanocytic lesions. Invest Ophthalmol Vis Sci. 2005;46(9):3027–3030. doi: 10.1167/iovs.04-1449. [DOI] [PubMed] [Google Scholar]
- 56.Dossett LA, Kudchadkar RR, Zager JS. BRAF and MEK inhibition in melanoma. Expert opinion on drug safety. 2015;14(4):559–570. doi: 10.1517/14740338.2015.1011618. [DOI] [PubMed] [Google Scholar]
- 57.Dagi Glass LR, Lawrence DP, Jakobiec FA, Freitag SK. Conjunctival Melanoma Responsive to Combined Systemic BRAF/MEK Inhibitors. Ophthalmic plastic and reconstructive surgery. 2016 doi: 10.1097/IOP.0000000000000833. [DOI] [PubMed] [Google Scholar]
- 58.Sjo LD. Ophthalmic lymphoma: epidemiology and pathogenesis. Acta Ophthalmol. 2009;87:1–20. doi: 10.1111/j.1755-3768.2008.01478.x. Thesis 1. [DOI] [PubMed] [Google Scholar]
- 59.Kirkegaard MM, Coupland SE, Prause JU, Heegaard S. Malignant lymphoma of the conjunctiva. Surv Ophthalmol. 2015;60(5):444–458. doi: 10.1016/j.survophthal.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Duncan J, Chen Y, Fuchs D, Cantu C, Wang M. Follicular Lymphoma Presenting Solely as Chronic Follicular Conjunctivitis. Cornea. 2016;35(3):395–398. doi: 10.1097/ICO.0000000000000696. [DOI] [PubMed] [Google Scholar]
- 61.Shields CL, Alset AE, Boal NS, et al. Conjunctival Tumors in 5002 Cases. Comparative Analysis of Benign Versus Malignant Counterparts. The 2016 James D. Allen Lecture. Am J Ophthalmol. 2017;173:106–133. doi: 10.1016/j.ajo.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 62.Kirkegaard MM, Rasmussen PK, Coupland SE, et al. Conjunctival Lymphoma--An International Multicenter Retrospective Study. JAMA Ophthalmol. 2016;134(4):406–414. doi: 10.1001/jamaophthalmol.2015.6122. [DOI] [PubMed] [Google Scholar]
- 63.Shields CL, Shields JA, Carvalho C, Rundle P, Smith AF. Conjunctival lymphoid tumors: clinical analysis of 117 cases and relationship to systemic lymphoma. Ophthalmology. 2001;108(5):979–984. doi: 10.1016/s0161-6420(01)00547-4. [DOI] [PubMed] [Google Scholar]
- 64.Ferreri AJ, Govi S, Colucci A, Crocchiolo R, Modorati G. Intralesional rituximab: a new therapeutic approach for patients with conjunctival lymphomas. Ophthalmology. 2011;118(1):24–28. doi: 10.1016/j.ophtha.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 65.Blasi MA, Tiberti AC, Valente P, et al. Intralesional interferon-alpha for conjunctival mucosa-associated lymphoid tissue lymphoma: long-term results. Ophthalmology. 2012;119(3):494–500. doi: 10.1016/j.ophtha.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 66.Pichierri P, Martone G, Loffredo A, Traversi C, Polito E. In vivo confocal microscopy in a patient with conjunctival lymphoma. Clinical & experimental ophthalmology. 2008;36(1):67–69. doi: 10.1111/j.1442-9071.2007.01654.x. [DOI] [PubMed] [Google Scholar]


