Abstract
Objectives
A prospective parallel cohort trial was conducted to compare outcomes of patients treated with maxillary protraction vs. LeFort 1 maxillary advancement surgery.
Setting and Sample Population
The primary site for the clinical trial is Children’s Hospital Los Angeles; the satellite test site is Seattle Children’s Hospital. All patients have isolated cleft lip and palate and a skeletal Class III malocclusion.
Material & Methods
A total of 50 patients, ages 11–14 will be recruited for the maxillary protraction cohort. The maxillary surgery cohort consists of 50 patients, ages 16–21, who will undergo LeFort 1 maxillary advancement surgery. Patients with additional medical or cognitive handicaps were excluded from the study.
Results
Current recruitment of patients is on track to complete the study within the proposed recruitment period.
Conclusion
This observational trial is collecting information that will examine dental, skeletal, financial, and quality of life issues from both research cohorts.
Keywords: Class III malocclusion, Cleft lip and palate, jaw surgery, orthodontics
Introduction
Cleft lip and palate (CLP) is the most common facial birth defect occurring in 1/950 live births in the United States (1). Due to scarring from early surgeries to repair the lip and palate (2–4), 22–73% of patients with cleft lip and palate will develop a Class III malocclusion which requires orthognathic surgery at the end of pubertal growth (2). The standard of care is to surgically advance the maxillary bone and dentition in cleft patients with a Le Fort I (LF1) osteotomy after adolescent growth has completed (3). At the Children’s Hospital Los Angeles (CHLA), early adolescents–typically age 14 and younger–with cleft lip and palate-related Class III malocclusion are offered an alternative non-surgical approach to correct the malocclusion. At the age of 14 years and younger, typically the maxillary sutures have not fused and can be mobilized by alternating weekly expansion and constriction with a rapid palatal expander (RPE). This technique was originally described by Liou & Tsai in 2005 (7), but was modified due to problems of frequent breakage with the custom springs and open bite side effects (8–11). This modification uses standard Hyrax expanders, reverse pull face mask for night-time protraction and Class III elastics for 24 hour maintenance of the gains made by protraction. The patients are taught to “pull and hold.” The modified technique can be used to close anterior open bites in patients if the elastics pull downward toward the facemask. Although this technique is completely dependent on patient cooperation, our success rate with the non-surgical maxillary protraction procedure at CHLA over the past 10 years has been good. Currently, Seattle Children’s Hospital (SCH) does not offer non-surgical maxillary protraction procedure as standard of care for patients at this age; therefore, this method is considered experimental for SCH. The reported relapse rate for LF1 osteotomy in patients with cleft lip and palate range from 32%–80% with most teams reporting between 40–50%(4–6). In a pilot study done at CHLA, 30 patients underwent the maxillary protraction procedure, 24 were successfully treated to a positive incisor overjet, demonstrating an 80% success rate (12). Of these 24 successfully treated patients, none required additional surgery to correct residual problems. Of the original 30 patients, six patients failed to complete the protraction treatment because of poor cooperation.
Two cohorts of adolescents with cleft lip and palate and Class III malocclusion are being recruited to study the impact of LF1 maxillary advancement surgery vs. late maxillary protraction. Feasibility studies conducted by the principal investigator found that a randomized clinical trial (RCT) was not feasible or ethical for several reasons (13). Teenagers at ages 11–13 randomized to the surgical arm would need to wait 4–6 years for surgery; furthermore, orthognathic surgery requires informed consent. Most adolescents and parents wanted information to make an informed choice regarding whether or not to proceed with jaw surgery, a surgery that can alter facial appearance. Therefore, this study is not randomized but will follow the two cohorts receiving one of two therapies to determine the outcomes for each of the treatments.
The primary objective of this descriptive cohort study is to assess the correction of Class III malocclusion in both cohorts and to explore factors associated with successful outcome within each treatment. Occlusal correction is measured from study models using the Goslon Yardstick(20), a validated clinical tool that categorizes dental relationships. Cephalometric radiographs, study models and photographs will be used to analyze treatment relapse. Secondary outcomes are to assess the two treatment approaches based on facial aesthetics, quality of life, cost, periodontal health, stability of occlusal correction and treatment complications at 1 year after treatment.
Material & Methods
This descriptive prospective cohort study will enroll two groups of participants who chose their treatment to correct the Class III malocclusion associated with cleft lip and palate. These two cohorts are treated at different ages: early protraction treatment at ages 11–14, vs. late surgery treatment ages 16–21. This study is conducted at CHLA and SCH, two of the largest craniofacial centers in the United States. Both CHLA and SCH have orthodontic clinics dedicated to patients with cleft and craniofacial anomalies with full time craniofacial orthodontists. Both sites have craniofacial orthodontic fellowship programs. CHLA is the main site with a total recruitment goal of 100 patients, 50 patients per cohort. SCH will serve as a site to confirm the findings at CHLA with a lower number of recruited patients (Figure 1).
Figure 1.
Study Population at CHLA and SCH.
The protocol, informed consent form(s), and recruitment materials, were approved by both CHLA and SCH IRBs. Consent was obtained using a three-step process that gave the patients three opportunities to ask questions and decline participation. In the first step, the orthodontist explains the research study to the participant, the choices, risks and benefits and answers any questions that may arise. During the second step, the written consent is given to the patient to read but not sign before the next appointment. The patients are told that they do not have to participate to receive treatment and can participate fully or partially. During the third step, the participant is approached by the study coordinator to discuss the consent forms, and if willing to participate, signs the informed consent/assent document prior to any study-related assessments or procedures. The patients are informed that they may withdraw consent/assent at any time throughout the course of the study. A copy of the signed informed consent/assent document is given to participants for their records. The inclusion and exclusion criteria for the study population are shown in Table 1.
Table 1.
Inclusion and exclusion criteria for maxillary protraction and orthognathic surgery groups.
| Inclusion | Exclusion |
|---|---|
|
|
Intervention
In the maxillary protraction group, the procedure takes approximately 18–24 months with a total of 25–30 clinic visits. The first clinic visits, which take approximately 6 weeks, are devoted to fabricating and placing the RPE. Once placed, the patient is instructed to turn the device twice in the morning and twice in the evening for 1 week, using an external key to engage a turnbuckle on the RPE device. Following one week of expansion, the patient reverses direction and begins constriction. The patient is instructed to alternate expansion and constriction for 8 weeks. In the mandibular arch, only the canine teeth are bonded with brackets. An acrylic aligner with a canine bracket cutout is used to prevent retroclination of the lower incisors (Figure 2). After 8 weeks of sutural loosening, the patient begins the maxillary protraction procedure with intraoral Class III elastics in combination with nightly reverse pull facemask, while continuing the weekly altering of expansion and constriction. Class III elastics and the facemask are worn for approximately 5–6 months until the maximal correction of Class III. At this point, the RPE is removed with immediate replacement of the bands and brackets and placement of a 0.014 nickel titanium wire and Class III 2 oz. 5/16″ intra-oral elastics. Debanding usually occurs in 18–24 months from the beginning of treatment. Retention consists of vacuum-formed acrylic splints fabricated with Class III hooks in order to continue with light 5/16″ Class III elastics during retention. Maxillary protraction is attempted during early adolescence. If unsuccessful, then patients can still undergo orthognathic surgery when pubertal growth is complete.
Figure 2.
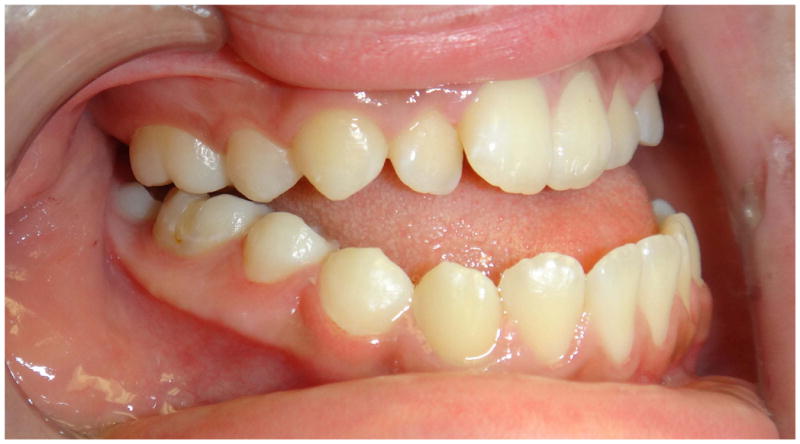

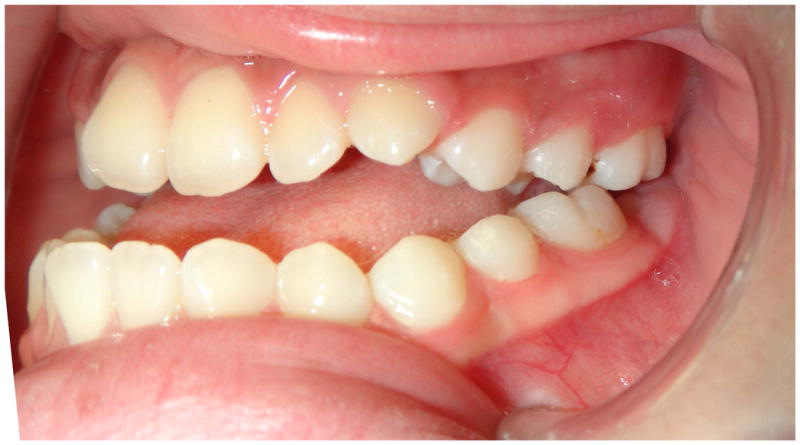



The protraction technique showing pre-treatment photographs and Cl III correction using a lower vacuform with Cl III elastics to canine brackets. Night time reverse pull facemask is also used.
In the orthognathic surgery cohort, edgewise appliances are placed on the upper and lower teeth to align crowded teeth, correct incisor angulation, and coordinate the upper and lower teeth so that the dentition will fit together when the maxilla is advanced to the appropriate position. During the pre-surgical orthodontic stage of treatment, there are periodic checks with progress models to confirm the surgical setup of the dentition. At the completion of pre-surgical orthodontics, the patient is enrolled in the study and pre-surgical T1 records are taken. After surgery and splint removal, orthodontic treatment is resumed to optimize the post-surgical occlusion. Retention consists of vacuum-formed acrylic retainers with hooks. Total treatment time for LF1 surgery group is 18 to 24 months of comprehensive care.
Prospective data will be collected prior to treatment (T1), at maximal correction (T2), at completion (T3), and at one year follow up (T4). Data sources will include clinical care records (charting, cephalometric and panoramic radiographs [LC/PAN], study models [SM], and photographs taken during treatment), data from procedures performed specifically for the study (LC/PAN, SM, and photographs taken at 1 year post-debanding or T4), and the study-specific assessments (adverse events, periodontal measurement, quality of life SF-12, Youth Quality of Life Instrument Facial Differences Module, Adult or Child Behavior Checklist [ABCL or CBCL], and health economic surveys). A summary of the clinical records and secondary variable data collection is shown in Table 2.
Table 2.
Summary of records taken during the study.
| T1 Pre-Treatment | T2 Maximal correction | T3 Completion of Treatment | T4 1-Year Follow Up | ||
|---|---|---|---|---|---|
| Clinical Records | Records (photos, LC/PAN, SM) | X | X | X | X |
| Periodontal measurements | X | X | |||
| Secondary Variables | Demographic survey | X | |||
| QOL SF-12 | X | X | X | X | |
| YQOL | X | X | X | X | |
| ABCL or CBCL | X | X | X | X | |
| Cost | X | ||||
The Goslon Yardstick is based on study model evaluations(20). Facial esthetics is scored using a visual analog scale for pre- and post-treatment photographs randomly presented to evaluators (15).
The cephalometric analyses are performed with imaging software and are based on the University of Southern California (USC) analysis (American Board of Orthodontics [ABO], Steiner, Tweed, soft tissue facial thirds, Wits, Harvold triangle) and the Arnett surgical analysis (hard and soft tissue analysis, facial angle and facial convexity) (9, 15). The final outcomes are compared using z-scores to record how many standards of deviation the treatment averages differ from the norm values (9).
Statistical analysis
Univariate descriptive patient characteristics of each group at baseline will be computed using N (%) for categorical outcomes and mean (95% confidence interval) or median for continuous outcomes that are normally or non-normally distributed, respectively. Bivariate comparisons between groups of potential covariates will be made. Missing data will be analyzed to determine whether the data is missing at random, missing completely at random, or not missing at random. For primary analyses, we will use the last-value carry forward and include any covariates related to missing data.
We are testing to determine whether the cohorts have similar outcomes. The primary outcome of improvement in the Goslon yardstick to scores of 1 or 2 from 3 to 5 at post-treatment (T3) will be made using logistic regression; the main factor will be treatment, with site, age, Goslon score at baseline, and cooperation as a priori covariates. The null hypothesis will be that there is at least 20% difference in improvement rate between groups, whereas the alternative hypothesis is that there is no difference between groups. The main hypothesis will be tested using an alpha level= 0.05.
Analysis of repeated measures outcomes will be made using repeated measures ANCOVA models facial aesthetic rating, quality of life, cephalometric measurements, and periodontal health between groups. Primary analyses will be performed using intent-to-treat, though secondary analyses will explore data providing for protraction group participants who switched groups.
A priori covariates were identified through meetings of the investigators and orthodontists of the Angle Society and American Cleft Palate Association. Covariate data (16) include age, sex, education level, ethnicity, number of missed appointments, number of appointments when the patient cannot demonstrate proper use of expanders or elastics, insurance type, clinic site, orthodontist and surgeon.
The primary outcome for each group will be the percentage of total patients in the group who have successful bite correction at end of treatment. Additionally, we will explore factors associated with success within each group using logistic regression with outcome (success/failure) to assess associations of potential predictors. Predictors of success will include demographic and medical characteristics of patients and self-reports about quality of life. We will also review clinical notes to explore other reported factors that may impact treatment success (e.g., parental involvement, adherence to treatment protocol).
At the end of the study, we will provide the clinician with information on success rate and profile the personal characteristics that are essential to success with each group. The data collected from 100 patients at CHLA will describe the skeletal and dental changes associated with each technique including treatment relapse, complications and quality of life scores before, during and after treatment. The photographic data will be used to test whether raters can tell a difference in final soft tissue outcomes between the two treatments. Cost data will be compared between the two groups.
Discussion
The current status of the trial is illustrated in Figure 3, which compares target vs. actual recruitment of the 100 patients for the primary trial at CHLA and the 10 protraction and 4 surgery patients at SCH. Figure 3 shows that 88% of the protraction patients have been recruited and 57% of the surgery patients have been recruited with 14 months left in the recruitment period. The study retention is currently 94%. Protraction patients were recruited ahead of schedule due to the longer overall treatment time. Rather than wait for the patients to come for their annual evaluation, all of the screened protraction patients were brought in during the summer in order to discuss the trial and to evaluate their eligibility for the trial. This move to bring in all of the potential study patients created a “surge” that helped to enroll patients in a manner that would allow them to complete the treatment within the study period.
Figure 3.
Study recruitment goal for protraction and surgery, expected and actual numbers.
The logistics of the trial required a full-time coordinator to screen patients and keep track of the data collection and patient appointments. A full-time dental assistant was added at the middle of the trial when the data collection, entry and storage was too much for the regular dental staff to conduct in a busy clinic. Additional research-only clinics were developed to collect the clinical data requiring long appointments and to keep the patients on track with their regular appointments during their treatment.
An unanticipated challenge to the study has been a paradigm shift in how surgeries are planned prior to surgery. The traditional model surgery approach is based on dental models and can take 3–6 hours to replicate the bite and perform the surgical movements for fabrication of surgical guides or splints (17). Virtual surgery requires less time as the details are worked out during an online discussion with technicians who can immediately perform the surgery on a computer screen(18). Once the plan is formulated, one or two jaw surgery splints can be 3-D printed with the press of a button; the surgeon does not have to fabricate the splint with acrylic. The extra cost of virtual surgery has been weighed against the savings in time and found to be cost-effective if the cost of the splints can be added to the hospital cost of the surgery paid by insurers (19). As the surgeons shift toward virtual surgery planning, there seems to be an inclination to do more two-jaw surgeries rather than one-jaw surgeries, lowering our total number of patients in the surgery cohort. Our strategy to make up the difference is to partner with local health maintenance organizations and county hospital facilities to increase the number of surgery patients in this study.
This methods paper is written as a reference for future analysis. It previews the type of design and data that are needed to conduct a comprehensive, prospective, observational, parallel cohort clinical trial.
Conclusion
Preliminary studies on late maxillary protraction support the use of this technique at ages 11–14 in patients with cleft lip and palate. This prospective cohort study of patients with cleft lip and palate undergoing maxillary protraction or LF1 maxillary advancement surgery will provide observational data on a large number of patients at CHLA and SCH. The primary outcome will be based on the Goslon yardstick; however, the collected data will evaluate quality of life, cost, orthodontic records, periodontal measurements and adverse events at four different time points.
Acknowledgments
This clinical trial is supported by NIDCR U01 DE022937-01A1.
References
- 1.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–16. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 2.Arosarena OA. Cleft lip and palate. Otolaryngol Clin N Am. 2007;40:27–60. doi: 10.1016/j.otc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Kaban LB, Troulis MJ. Sequential management of the child with cleft lip and palate. In: Kaban LB, Troulis MJ, editors. Pediatric Oral and Maxillofacial Surgery. 1. Philadelphia: Saunders; 2004. pp. 418–419. [Google Scholar]
- 4.Kramer FJ, Baethge C, Swennen G, Teltzrow T, Schulze A. Intra- and perioperative complications of the LeFort I osteotomy: a prospective evaluation of 1000 patients. J Craniofac Surg. 2004;15:971–977. doi: 10.1097/00001665-200411000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Robi MT, Farrell BB, Tucker MR. Complications in Orthognathic Surgery. A Report of 1000 Cases. Oral Maxillofac Surg Clin North Am. 2014;26:599–609. doi: 10.1016/j.coms.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Rachmiel A. Treatment of maxillary cleft palate: distraction osteogenesis versus orthognathic surgery-part one: maxillary distraction. J Oral and Maxillofac Surg. 2007;65:753–7. doi: 10.1016/j.joms.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Liou EJ, Tsai WC. A new protocol for maxillary protraction in cleft patients: repetitive weekly protocol of alternate rapid maxillary expansions and constrictions. Cleft Palate Craniofac J. 2005;42:121–7. doi: 10.1597/03-107.1. [DOI] [PubMed] [Google Scholar]
- 8.Liou EJ, Yen SL. Oral session presented at: Maxillary Protraction Workshop, 63rd Annual Meeting and Pre-Conference Symposium of the American Cleft Palate-Craniofacial Association; 2006 Apr 3–8; Vancouver, BC. [Google Scholar]
- 9.Borzabadi-Farahini A, Lance CJ, Yen SL. Late maxillary protraction in patients with unilateral cleft lip and palate: a retrospective study. Cleft Palate Craniofac J. 2014;51:e1–e10. doi: 10.1597/12-099. [DOI] [PubMed] [Google Scholar]
- 10.Morphis S, Yen SL. Unpublished thesis type. Los Angeles: University of Southern California; 2010. A Comparison of relapse and esthetic results with protraction vs. surgery in correcting Class III malocclusion in cleft lip and palate patients: lateral cephalometric analysis. [Google Scholar]
- 11.Alcazar AR. Unpublished thesis type. Los Angeles: University of Southern California; 2007. Modification of the Effective Maxilary Protraction Technique in the Maxillary Protraction Treatment of Cleft Patients. [Google Scholar]
- 12.Yen SL. Protocols for Late Maxillary Protraction in Cleft Lip and Palate Patients at Children’s Hospital Los Angeles. Semin Orthod. 2011;17:138–148. doi: 10.1053/j.sodo.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIlvaine E, Borzabadi-Farahini A, Lane CJ, Azen SP, Yen SL. Apriori feasibility testing of randomized clinical trial design in patients with cleft deformities and Class III malocclusion. Int J Pediatr Otorhinolarygol. 2014;78:725–30. doi: 10.1016/j.ijporl.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong FX, Heggie AA, Shand JM, Schneider PM. Skeletal stability of maxillary advancement with and without a mandibular reduction in the cleft lip and palate patient. Int J Oral Maxillofac Surg. 2016;45:1501–1507. doi: 10.1016/j.ijom.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Chung EH, Borzabadi-Farahini A, Yen CL. Clinicians and laypeople assessment of facial attractiveness in patients with cleft lip and palate treated with LeFort I surgery or late maxillary protraction. Int J Pediatr Otorhinolarygol. 2013;77:1446–50. doi: 10.1016/j.ijporl.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazhar K, Varma R, Choudhury F, Mckean-Cowdin R, Shtir CJ, Azen SP. Severity of diabetic retinopathy and health-related quality of life: The Los Angeles Latino Eye Study. Ophthalmology. 2011;118:649–655. doi: 10.1016/j.ophtha.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson KL, Bell WH, Goldsmith DH. Analytical model surgery. In: Bell WH, editor. Modern Practice in Orthognathic and Reconstructive Surgery. Vol. 1. Philadelphia: Saunders; 1992. pp. 155–216. [Google Scholar]
- 18.Bobek S, Farrell B, Choi C, Farrell B, Weimar K, Tucker M. Virtual surgical planning for orthognathic surgery using digital data transfer and an intraoral fiducial marker: the charlotte method. J Oral Maxillofac Surg. 2015;73:1143–58. doi: 10.1016/j.joms.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Resnick CN, Inverso G, Wrzosek M, Padwa BL, Kaban LB, Peacock ZS. Is there a cost difference between standard and virtual surgical planning for orthognathic surgery? J Oral Maxillofac Surg. 2016;74:1827–33. doi: 10.1016/j.joms.2016.03.035. [DOI] [PubMed] [Google Scholar]




