Abstract
Background
Immunotherapies directed against methamphetamine (MA) abuse have shown success in rodent models, however only a limited number of studies have investigated active vaccination in female mice and none in female rats. It is critical to determine if potential immunotherapeutic strategies generalize across sex, particularly for drugs that may produce significant sex-differences on behavioral or physiological endpoints.
Methods
Female Wistar rats were initially vaccinated with keyhole-limpet hemocyanin (KLH) or an anti-methamphetamine-KLH conjugate (MH6-KLH) three times over five weeks and implanted with radiotelemetry devices to assess locomotor activity and body temperature responses to MA. Rats were first exposed to MA via vapor inhalation (100mg/mL in propylene glycol) and then by injection (0.25–1.0 mg/kg, i.p.) and vapor after a final vaccine boost.
Results
The MH6-KLH vaccine generated an increase in antibody titers across the initial 6-week, 3 immunization protocol and a restoration of titer after a week 14 booster. Locomotor stimulation induced by 0.25 mg/kg MA, i.p, in the KLH group was prevented in the MH6-KLH group. MH6-KLH animals also exhibited an attenuated locomotor stimulation produced by 0.5 mg/kg MA, i.p. No group differences in locomotion induced by vapor inhalation of MA were observed and body temperature was not differentially affected by MA across the groups, most likely because vapor inhalation of MA that produced similar locomotor stimulation resulted in ~10-fold higher plasma MA levels.
Conclusions
This study confirms the efficacy of the MH6-KLH vaccine in attenuating the effects of MA in female rats.
Keywords: methamphetamine, vaccine, rat, female, vapor inhalation
1. INTRODUCTION
D-methamphetamine (MA) addiction is a public health problem; however, there are currently no medications approved for the treatment of addiction and abuse disorders involving MA. Immunopharmacotherapy, specifically through active immunization, has been utilized as a method for the sequestration of drug molecules from the brain and for the reduction of drug effects. By eliciting high-affinity drug-specific antibodies, anti-drug vaccines have been developed as potential therapeutics (see (Kosten and Domingo, 2013; Ohia-Nwoko et al., 2016; Skolnick, 2015; Zalewska-Kaszubska, 2015) for review), for psychomotor stimulants such as MA and cocaine (Kosten et al., 2014) and even recently emerged designer cathinones, such as alpha-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone (Nguyen et al., 2016c). Multiple reports followed a seminal demonstration that an anti-cocaine vaccine produced an attenuation of cocaine-stimulated locomotor increases in male Wistar rats (Carrera et al., 1995). There have been at least four programs investigating active anti-MA vaccines that have provided evidence of in vivo efficacy of anti-MA vaccine in animal models (Byrnes-Blake et al., 2001; Duryee et al., 2009; Miller et al., 2015; Miller et al., 2013b; Ruedi-Bettschen et al., 2013; Shen et al., 2013). Specifically these studies have shown that vaccination is effective against MA-induced suppression of food-maintained responding in male Sprague-Dawley rats (Ruedi-Bettschen et al., 2013), produces initially increased self-administration of MA followed by extinction in lever-trained male rats (Duryee et al., 2009; Miller et al., 2015) and delays the acquisition of self-administration in behaviorally naïve male rats (Miller et al., 2015). Evidence for efficacy against locomotor stimulant effects of MA is mixed, with vaccines attenuating effects of MA in wheel activity and spontaneous locomotion in rats (Miller et al., 2013b) and mice (Shen et al., 2013) at some doses and a failure to protect against locomotor activating effects of 3 mg/kg MA, i.p., in male rats (Byrnes-Blake et al., 2001) reported.
Determining the behavioral or physiological targets that are most likely to be affected by anti-MA vaccination in laboratory models would enhance the prospects for successful clinical trials. Sex differences are one potential critical issue which may affect vaccine effectiveness, yet these have not been well delineated in anti-drug vaccine development efforts. Women may be at differential risk for drug dependence, for example, methamphetamine dependence starts earlier in women (Rawson et al., 2005), MDMA dependence occurs more frequently in women (Bruno et al., 2009; Uosukainen et al., 2015) and discontinuation of cocaine is harder (DeVito et al., 2014). More generally, the escalation of substance use and resistance to treatment is higher in women (Westermeyer and Boedicker, 2000). Alternately, cocaine dependent women may respond favorable to an experimental drug where men do not (Fox et al., 2014). These observations reinforce the recent United States National Institutes of Health policy position identifying a need for additional sex-difference comparisons across biomedical domains (Clayton and Collins, 2014) and show that study of potential immunotherapies in female animal models are needed. Previous investigations of the effects of anti-MA vaccination found attenuation of locomotor stimulation and place conditioning in female BALB/c mice (Haile et al., 2015; Shen et al., 2013) but there have been no investigations of anti-MA vaccination in female rats.
This study was therefore undertaken to determine if the efficacy of the MH6-KLH vaccine (Miller et al., 2015; Miller et al., 2013b) extends to female rats. Experiments were conducted to determine potential protective effects against methamphetamine-induced stimulation of spontaneous locomotor activity after intraperitoneal injection. The vapor inhalation model was selected in addition to parenteral injection because humans who are dependent on methamphetamine use inhalation more than other routes of administration (Das-Douglas et al., 2008; Heinzerling et al., 2010; Wood et al., 2008) and this route is not commonly tested in nonhuman animal models.
2. METHODS
2.1 Subjects
Female (N=32) Wistar rats (Charles River, New York) were housed in humidity and temperature-controlled (23±1 °C) vivaria on 12:12 hour light:dark cycles. Rats entered the laboratory at 10 weeks of age. Vaccinated animals were 11 weeks of age on Week 0 of this study and the animals used for pharmacokinetics were 13 weeks of age at the start of those studies. Animals had ad libitum access to food and water in their home cages. All procedures were conducted under protocols approved by the Institutional Care and Use Committees of The Scripps Research Institute and in a manner consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Radiotransmitter Implantation
Rats were anesthetized with an isoflurane/oxygen vapor mixture (isoflurane 5% induction, 1–3% maintenance), and sterile radiotelemetry transmitters (Data Sciences International; TA-F40) were implanted in the abdominal cavity through an incision along the abdominal midline posterior to the xyphoid space as previously reported (Aarde et al., 2015; Miller et al., 2013a; Wright et al., 2012). Absorbable sutures were used to close the abdominal muscle incision and the skin incision was closed with the tissue adhesive. A minimum of 7 days was allowed for surgical recovery prior to starting experiments. For the first three days of the recovery period, an antibiotic Cefazolin (Hikma Farmaceutica, Portugal; 0.4 mg/kg, i.m. in sterile water Day 1, s.c. Day 2–3) and an analgesic flunixin (FlunixiJect, Bimeda USA, Oakbrook Terrace, IL; 2.5 mg/kg, s.c. in saline) were administered daily.
2.3 Drugs and Hapten
The D-methamphetamine HCl (MA) was obtained from the Drug Supply Program of the U.S. National Institute on Drug Abuse. MA was delivered in propylene glycol (PG) vehicle (at a concentration of 100 mg/mL) using e-cigarette-type cartridges for vapor inhalation sessions conducted in an exposure chamber as described below (Section 2.6). Four 10-s vapor puffs were delivered with 2-s intervals every 5 minutes that resulted in use of approximately 0.125 mL per 40 min exposure session. The chamber exhaust was vacuum controlled to 1 L air per minute during puffs to ensure that vapor entered the chamber on each device-triggering event. MA administered via intraperitoneal injection (0.25, 0.5, 1.0 mg/kg) was dissolved in physiological saline using an injection volume of 1 mL/kg. Dosing is expressed as the salt in all cases. MA doses were tested in each rat in a randomized order within the first vapor study and the i.p. study. The second vapor study, conducted a week after the i.p. study, began with all rats first exposed to 40 min of MA (100 mg/mL) inhalation. Next, the 20 minute PG and MA (100 mg/mL) studies were conducted in a balanced order within the groups. There was a minimum 3–4 day interval between test days for all MA studies.
Methamphetamine hapten (MH6) was coupled with a keyhole limpet hemocyanin (KLH) carrier protein and administered (100 micrograms per immunization) in formulation with the Sigma Adjuvant System® as previously reported (Miller et al., 2013b).
2.4 Vaccination Procedure
Rats (N=8 per group) were vaccinated during weeks 0, 2, 5 and 14. For vaccination, MH6-KLH or KLH was added to adjuvant to create a 0.5 mL vaccine for each rat, which was administered across three sites (0.2 mL s.c. in the nape; 0.2 mL s.c. in the hind quadriceps; 0.1 mL i.p.).
2.5 Immunologic Assays
For characterization of anti-MA antibody titers, rats were anesthetized with an isoflurane/oxygen vapor mixture (isoflurane 5% induction, 1–3% maintenance), and blood was collected from the jugular vein during weeks 1–6, 14, 20 and 30. Antibody titer was defined by the dilution required to achieve a 50% signal using enzyme-linked immunosorbent assay (ELISA) with a Biomek 4000 liquid handling robot. 96-well assay plates were coated with 25 μg/well MH6-BSA conjugate and blocked with skim milk. Twelve 1:1 rat plasma dilutions were added to the plate starting at 1:200 and allowed to incubate for 2 h. Following a wash step, goat anti-rat HRP IgG (SouthernBiotech) at 1:10,000 dilution was incubated in the plates for 18 h at 4 °C. After a second wash step the plates were developed using a 3,3′-5,5′-tetramethylbenzidine (TMB) substrate kit (Thermo Pierce) and 2 M H2SO4 as a stopping solution. The well absorbance values were read at 450 nm and normalized to the highest value for each sample in GraphPad Prism version 6, followed by curve fitting with log(inhibitor) vs. normalized response – variable slope to find the midpoint titer. One individual sample was unavailable in Week 6 and one animal was lost to the study prior to the Week 14, 20 and 30 time-points; the values were replaced with the group average for the time point for statistical analysis.
2.6 Inhalation Apparatus
Sealed exposure chambers were modified from the 259mm X 234mm X 209mm Allentown rat cage to regulate airflow and the delivery of vaporized drug to rats using e-cigarette type devices as has been previously described (Nguyen et al., 2016a; Nguyen et al., 2016b). A custom e-cigarette cartridge-triggering unit (Model SSV-1; La Jolla Alcohol Research, Inc; La Jolla CA) was controlled by MedPC IV software (MedAssociates, St Albans, VT) to deliver vapor puffs as scheduled. The chamber air was vacuum controlled by a chamber exhaust valve (i.e., a “pull” system) to flow room ambient air through an intake valve at 1 L per minute. This functioned to ensure that vapor filled the chamber on each device triggering event, i.e., the vapor stream was integrated with the ambient air stream once triggered.
2.7 Radiotelemetry Measures of Locomotor Activity and Body Temperature
Locomotor activity and temperature data were collected while animals were housed in clean standard plastic home-cages (thin layer of bedding) in a dark testing room (dim red-light illumination), separate from the vivarium, during the (vivarium) dark cycle. Radiotelemetry transmissions were collected via receiver plates (Data Sciences International; RPC-1) placed under the cages as described in prior investigations (Aarde et al., 2013; Miller et al., 2013a; Taffe et al., 2015; Wright et al., 2012).
Sessions started with a 30-minute interval in the recording cage to determine a pre-treatment baseline of activity and temperature. For the inhalation exposure, rats were placed in the chamber, in pairs, for 40 min and then returned to their individual recording cages. Intraperitoneal injection sessions were conducted in the recording chamber only, with the drug administered after the baseline interval. The three telemetry samples taken prior to moving the rat to the inhalation chamber or prior to injection were used as the pre-treatment baseline for data analysis. Primary analysis focused on the average activity rate and body temperature in 30 min intervals as derived from the primary 5 min sampling bins. Inhalation data are timed to the initiation of vape session thus the “60 min” time bin reflects the average of 3–4 samples collected after return to the recording chamber following the 40 min vapor exposure. All subsequent time bins reflect the average of 6 five-min samples. Any missing body temperature data (e.g., due to radio interference or animal’s location within the chamber at the time of sampling) was interpolated across preceding and succeeding recorded values. One MH6-KLH animal was excluded from the first vapor challenges (N=7) and two from the i.p. and final vapor challenges (N=5) due to transmitter failure. Two KLH animals were excluded from the final 20 minute vapor inhalation challenge due to transmitter failure.
2.8 Evaluation of Plasma Methamphetamine Content
Female Wistar rats (N = 16) received MA via vapor inhalation (12.5 or 100 mg/mL) or i.p. injection (0.25 or 1.0 mg/kg) and blood was obtained at 60 or 120 min by acute venipuncture under gas inhalation anesthesia. One month elapsed between the vapor and i.p. collections. Groups of N=4 were studied at each dose and time point within route of administration and each animal participated in one inhalation and one i.p. treatment condition. The blood samples were stored on ice, then centrifuged at 10–13,000 rpm for ten minutes. The plasma was collected and stored at −20 °C until analysis by LCMS.
(±)-Methamphetamine-d5 (d5-MA) in a 1 mg/mL solution of methanol was purchased from Cerilliant (Cerilliant Corp., Round Rock, TX). On the day of plasma analysis, 20 μL of plasma sample was added to a 40 μL aliquot of acetonitrile, containing 1 μg/mL concentration of d5-MA as an internal standard. The samples were vortexed and then centrifuged at 14,000 rpm for ten minutes. A 40 μL aliquot of supernatant was removed for LCMS analysis. A 5 μL aliquot of each sample was injected into an LCMS system and passed through an Agilent Zorbax 300SB-C8 5 urn column. The samples were run using Solvent A: 0.1 % formic acid and Solvent B: 0.1% formic acid in acetonitrile as the mobile phase solvents on a gradient method (0–4 min, 10–100% Solvent B, 4–9 min, 100% Solvent B) with a flow rate of 0.5 mL/min, followed by a 4 min wash phase at 5% Solvent B.
Peaks of deuterated and non-deuterated masses were extracted using the Extract Ion function on Agilent LC/MSD ChemStation and the area under the peaks were integrated. Non-deuterated peaks were normalized against their d5-MA peaks and quantified for their plasma concentration. A standard curve for MA with d5-MA was generated using blank rat plasma that was spiked with known concentrations of MA (0–5 μg/mL) and diluted with a 40 μL aliquot 1 μg/mL d5-MA in acetonitrile, and treated the same as described above for plasma samples. Three of the four 0.25 mg/kg i.p. samples (t = 2 h), as well as one 0.25 mg/kg i.p. sample at t = 1 h, were below the limit of detection for the method. These data were not excluded from analysis because inclusion of the data did not significantly influence the trends observed across routes of administration, dosing, or time after exposure.
2.9 Data Analysis
Antibody titer data were analyzed with repeated-measure one-way Analysis of Variance (rmANOVA) with post-hoc multiple comparisons analysis using the Tukey procedure. The locomotor activity and body temperature data were analyzed with rmANOVA with an initial omnibus 2-way with Group/Dose as a between-subjects factor and Time Post-initiation (vapor) or Post-injection (i.p.) as the within-subjects factor. Significant ANOVA findings in the i.p. study were followed with 2-way ANOVA within-group with Dose and Time Post-injection as repeated-measures factors. Follow-up analysis of the average activity rate for each hour after i.p. injection included Dose as a repeated-measures factor and Group/Hour as a between-subjects factor. Significant effects on the ANOVAs were further examined with Tukey (within group) or Sidak’s (between group) post-hoc analysis. The criterion for significant results was P < 0.05 and all analyses were conducted using Prism 6 or 7 for Windows (v. 6.02 or v. 7.00; GraphPad Software, Inc, San Diego CA).
3. Results
3.1 Titers
The antibody titer for the female group (N=8) inoculated with the MH6-KLH vaccine were analyzed across the 9 blood samples obtained across the experiment (Figure 1) and the ANOVA confirmed a significant effect of time [F (2.172, 15.21) = 3.76; P<0.05]. The Tukey post-hoc test confirmed that antibody titer was significantly higher in Weeks 4 and 20 relative to Week 1, higher in Weeks 20 and 30 compared with Week 14 and lower in Week 30 compared with Week 20.
Figure 1.
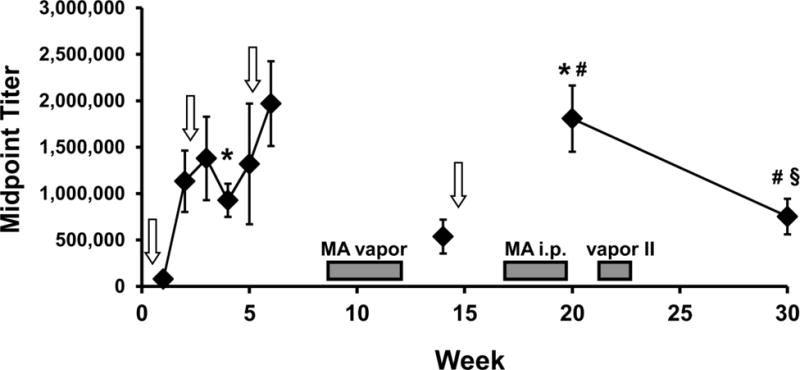
Mean (N=8; ±SEM) antibody titer for female Wistar rats treated with the MH6-KLH vaccine (as indicated by vertical arrows). The timing of methamphetamine (MA) challenge experiments are indicated in shaded bars. A significant difference from week 1 is indicated by #, from week 14 by # and from week 20 by §.
3.2 Locomotor Activity and Body Temperature- Vapor
Exposure to vaporized MA (100 mg/ml) increased locomotor activity in MH6-KLH and KLH vaccinated rats (Figure 2). The ANOVA confirmed main effects of group [F (3, 26) = 10.56; P = 0.0001], of time [F (7, 182) = 44.08; P < 0.0001] and of the interaction of group with time [F (21, 182) = 10.55; P < 0.0001]. The post-hoc test confirmed that activity differed significantly from the baseline activity rate following MA inhalation in MH6-KLH (60–210 minutes after vapor initiation) and KLH (60–180 minutes) groups and 180 minutes after PG inhalation in the MH6-KLH rats. Activity also differed between vehicle and MA inhalation conditions for the MH6-KLH (60–210 minutes after vapor initiation) and KLH (60–120 minutes after vapor initiation) groups. The post-hoc test further confirmed that activity differed between the MH6-KLH and KLH vaccine groups following MA exposure at 210 min after the start of inhalation.
Figure 2.
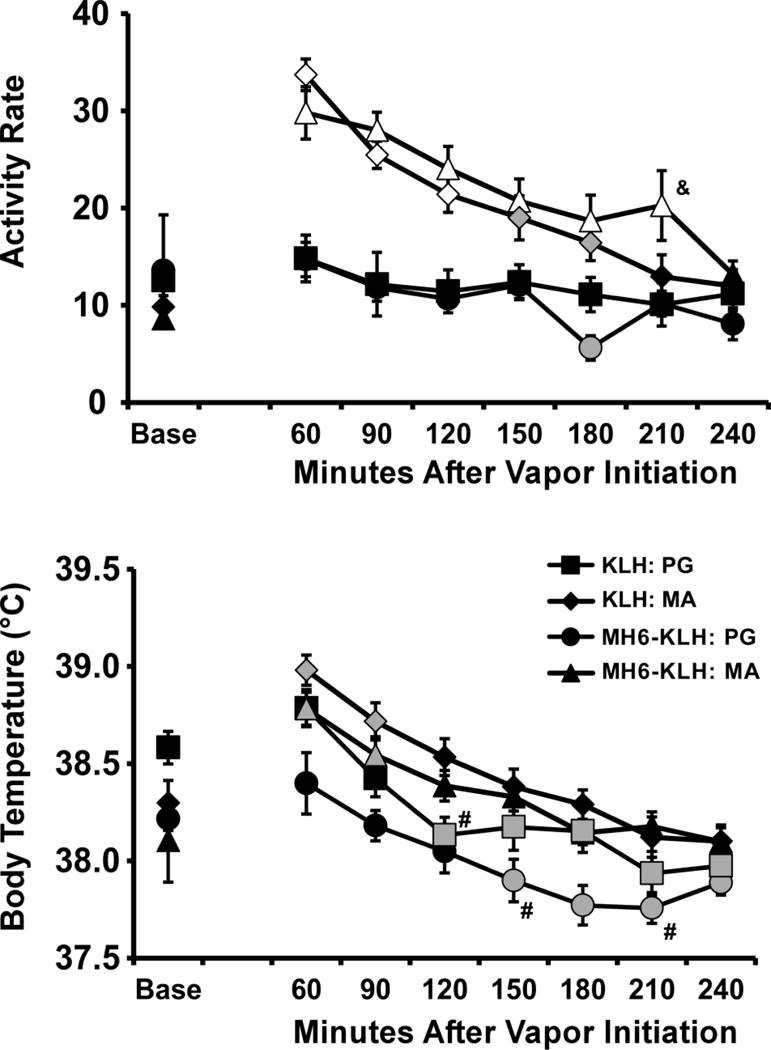
Mean (±SEM) activity rates and body temperature for MH6-KLH (N=7) and KLH (N=8) female rats after inhalation of PG or d-methamphetamine (100 mg/mL in PG) vapor. A significant difference from both the baseline and PG vehicle is indicated by the open symbols and from the baseline by the gray shaded symbols. A significant difference between groups is indicated by & and between PG and MA inhalation within-group by #. Base= pre-inhalation baseline.
The body temperature was increased by inhalation of MA (100 mg/ml) and decreased by PG vehicle (Figure 2). The ANOVA confirmed main effects of group [F (3, 26) =4.30; P < 0.05], of time [F (7, 182) = 57.04; P < 0.0001] and of the interaction of group with time [F (21, 182) = 3.56; P < 0.0001]. The post-hoc test confirmed body temperature following inhalation of MA (100 mg/ml) was significantly increased compared with baseline in both KLH (60–90 min) and MH6-KLH (60–90 min) rats and temperature was significantly decreased relative to baseline following inhalation of PG vehicle in KLH (120–240 min) and MH6-KLH (150–240 min) rats. In addition, the post-hoc test confirmed that temperature was higher in the MA versus the PG inhalation condition for MH6-KLH (150, 210 minutes) and KLH (120 minutes) groups.
3.3 Locomotor Activity and Body Temperature- i.p. injection
The injection of MA increased locomotor activity in both groups (Figure 3) as was confirmed by the omnibus ANOVA in which activity rate was significantly affected by the Group/Dose factor [F (7, 44) = 4.41; P<0.001], by time post-injection [F (8, 352) = 58.83; P<0.0001] and by the interaction of factors [F (56, 352) = 4.32; P<0.0001]. MA significantly increased activity rate in KLH rats (Figure 3) as was confirmed by significant main effects of dose [F (3, 21) = 18.33; P < 0.0001], of time [F (8, 56) =64.86; P < 0.0001] and of the interaction of dose with time [F (24, 168) = 5.444; P < 0.0001] in the within-group ANOVA. The Tukey post-hoc test confirmed that the activity rate was significantly increased compared to PG vehicle following 0.25 mg/kg, 0.5 mg/kg (30–150 minutes after injection) and 1.0 mg/kg (30–210 minutes). The activity rate was also increased in MH6-KLH rats following intraperitoneal injection (Figure 3) as was confirmed by the follow-up within-subjects ANOVA finding of significant main effects of dose [F (3, 12) = 13.09; P = 0.0004], of time [F (8, 23) =16.43; P < 0.0001] and of the interaction of dose with time [F (24, 96) = 3.103; P < 0.0001] on activity. The Tukey post-hoc test further confirmed that the activity rate of MH6-KLH rats was significantly increased compared to PG vehicle only after the 0.5 mg/kg (30–60 minutes) and 1.0 mg/kg (30–240 minutes) doses, but not after the 0.25mg/kg dose.
Figure 3.
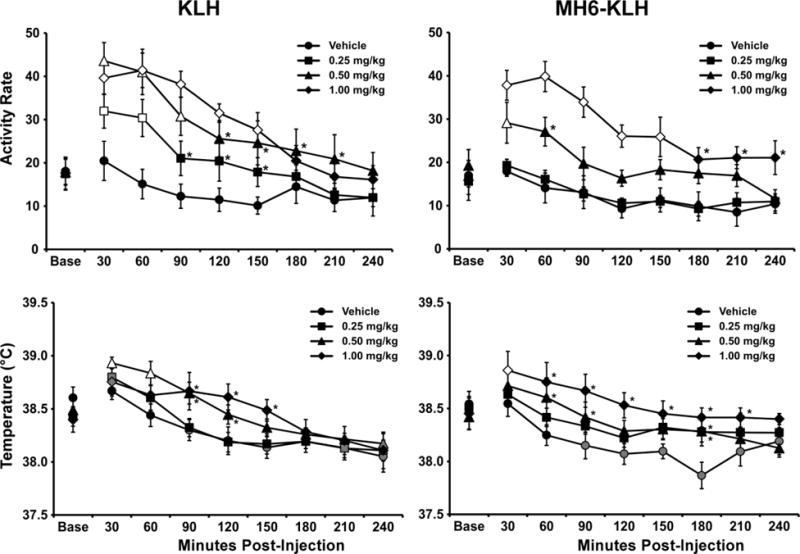
Mean (±SEM) activity rates for KLH (N=8) and MH6-KLH (N=5) female rats after administration of the Vehicle or d-methamphetamine (0.25, 0.5, 1.0 mg/kg, i.p.). Significant differences from the baseline and vehicle within group are indicated by the open symbols, from the baseline (only) by the gray shaded symbols and from the vehicle by *.
The summary analysis of the activity in the first two hours after injection (Figure 4) confirmed significant main effects of MA dose [F (3, 66) = 47.73; P<0.0001] and of the Group/Hour factor [F (3, 22) = 4.89; P<0.01]. The post-hoc analysis confirmed that the KLH group’s activity was elevated relative to the vehicle (0.25–1.0 mg/kg) and 0.25 mg/kg (0.5–1.0 mg/kg) conditions the first hour. The MH6-KLH group’s activity was only increased relative to the vehicle after the 0.5–1.0 doses and relative to the 0.25 mg/kg condition after the 1.0 mg/kg dose in the first hour. Activity rates in the second hour were elevated in the KLH group after 1.0 mg/kg (vs Vehicle or 0.25 mg/kg) and 0.5 mg/kg (vs Vehicle) and in the MH6-KLH group after 1.0 mg/kg (vs Vehicle, 0.25 or 0.5 mg/kg).
Figure 4.
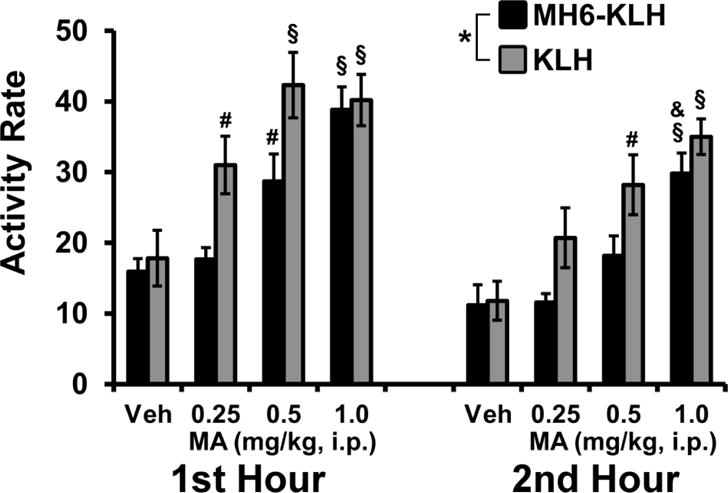
Mean (±SEM) activity rates for KLH (N=8) and MH6-KLH (N=5) female rats after administration of the Vehicle (Veh) or d-methamphetamine (MA; 0.25, 0.5, 1.0 mg/kg, i.p.) are directly compared for the first two hours. Significant differences from the Vehicle and 0.25 mg/kg within Group and Hour are indicated by §, from Vehicle (only) by # and from the 0.5 mg/kg condition by &.
The body temperature of the rats was also altered by injection of MA (Figure 3). The omnibus ANOVA confirmed significant effects of the time post-injection [F (8, 352) = 47.21; P<0.0001] and by the interaction of factors [F (56, 352) = 1.96; P<0.0005], but not of the Group/Dose factor. Follow-up ANOVA confirmed significant effects of time post-injection and the interaction in both KLH [F (8, 56) = 21.78; P<0.0001, F (24, 168) = 2.48; P<0.0005] and MH6-KLH groups [F (8, 32) = 18.6; P<0.0001, F (24, 96) = 2.22; P<0.005]. The post-hoc analysis of the temperature confirmed that the KLH group’s temperature was elevated relative to the baseline after the 0.25 mg/kg (30 minutes post-injection), 0.5 mg/kg (30–60 minutes) and 1.0 mg/kg (30 minutes) MA doses but not after vehicle. Similarly, body temperature was elevated relative to the vehicle condition in the KLH animals following injection of 0.5 mg/kg (30–120 minutes) and 1.0 mg/kg (90–150 minutes) MA, i.p. The post-hoc analysis of the MH6-KLH group confirmed that body temperature was elevated relative to the baseline 30 min after 1.0 mg/kg MA and relative to the vehicle condition after the 0.25 mg/kg (180 minutes), 0.5 mg/kg (60–90, 180 minutes) and 1.0 mg/kg (30–210 minutes) MA doses.
3.4 Locomotor Activity and Body Temperature- Vapor II
Exposure to vaporized MA (100 mg/mL) for 40 minutes decreased locomotor activity, while a 20-minute exposure increased locomotor activity, in MH6-KLH and KLH vaccinated rats (Figure 5). The analysis of the 40-minute MA inhalation confirmed main effects of group [F (1, 11) = 6.50; P<0.05] and of time [F (7, 77) = 3.68; P<0.005], but not of the interaction of factors, on activity. The post-hoc test confirmed activity was significantly different from the 60-minute time point for the 120–180 minute time points within the KLH group. No individual time point differences were confirmed within the MH6-KLH group or between groups. Analysis of the 20-minute study confirmed main effects of group/dose [F (3, 18) = 6.03; P<0.01], of time [F (8, 144) = 17.67; P<0.0001] and of the interaction of group with time [F (24, 144) = 4.27; P<0.0001]. The post-hoc test confirmed that activity differed significantly between PG and MA inhalation within MH6-KLH (30–180 minutes after vapor initiation) and KLH (30–120 minutes) groups. No significant individual time point differences were confirmed between groups following either PG or MA inhalation.
Figure 5.
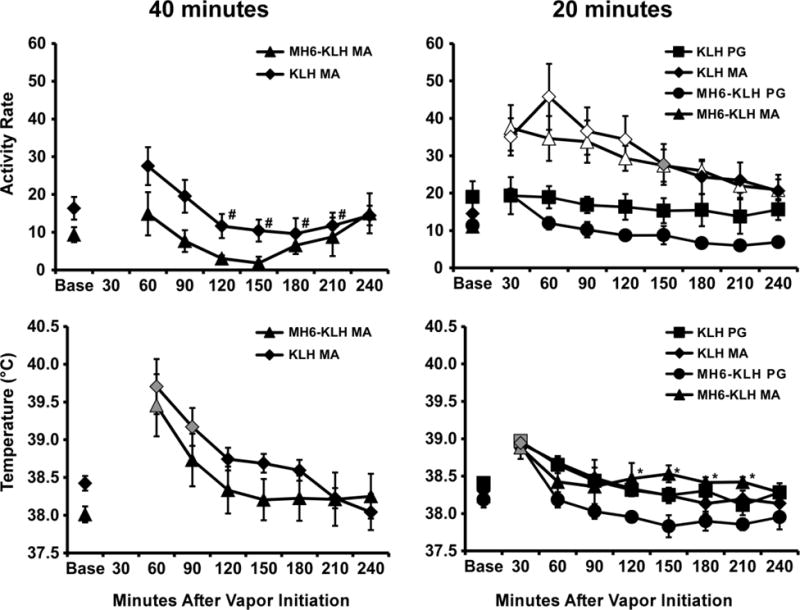
Mean activity rates and body temperature for MH6-KLH (N=5) and KLH (N=6–8) female rats after inhalation of PG (20 minutes) or d-methamphetamine (100 mg/mL in PG; 40 or 20 minutes) vapor. Bars indicate SEM. A significant difference from both the baseline and PG vehicle within group is indicated by the open symbols and from the baseline (only) by the gray shaded symbols. A significant difference from the PG within group is indicated by * and a significant difference from the 60 minute time point within-group by #. Base= pre-inhalation baseline.
Body temperature was also significantly altered by the inhalation of MA (Figure 5). The 40 min inhalation condition significantly increased body temperature [main effect of time; F (7, 77) = 15.03; P<0.0001] in both groups with the post-hoc test confirming significant differences from baseline for KLH (60–90 minutes post-initiation) and MH6-KLH (60 minutes) groups. The analysis of the 20 minute inhalation study confirmed significant main effects of Time Post-Initiation [F (8, 144) = 27.99; P<0.0001] and the interaction of Time Post-Initiation with the Group/Dose factor [F (24, 144) = 1.71; P<0.05]. The Tukey post-hoc test confirmed that temperature was increased 30 minutes after the initiation of vapor in both PG and MA conditions for both groups. In addition, temperature was higher in the MH6-KLH group following MA inhalation compared with the PG inhalation condition (120–210 minutes after vapor initiation).
3.5 Plasma Methamphetamine after Vapor Inhalation or Intraperitoneal Injection
A standard curve was generated using the ratio of peak area of drug analyte to the internal standard (d5-MA) versus concentration (Figure 6A). Linearity of the curve was confirmed by a correlation coefficient greater than 0.990. MA plasma concentrations were determined using the six point standard curve. This analysis found that the mean plasma MA levels observed depended on dose, route of administration and time since administration (Figure 6B,C). Notably, the concentrations of MA in plasma were an order of magnitude greater after inhalation exposure (100 mg/mL) compared with i.p. injection (1.0 mg/kg). In all treatment conditions there was a substantial decrease in MA plasma concentration from the first to the second hour ranging from a 50% reduction in the 100 mg/mL inhalation condition to a 65% reduction in the 0.25 mg/kg, i.p., condition. The three-way ANOVA confirmed main effects of Route of Administration [F (1, 1) = 18.74; P<0.0005], of Dose [F (1, 1) = 7.84; P<0.01] and of the interaction of Route with Dose [F (1, 1) = 6.06; P<0.05]. Follow-up one-way ANOVA within route of administration confirmed main effects of Dose for vapor inhalation [F (1, 12) = 6.96; P<0.05] and i.p. injection [F (1, 12) = 5.06; P<0.05] and a significant effect of Time Post-Injection for i.p. injection [F (1, 12) = 5.81; P<0.05].
Figure 6.
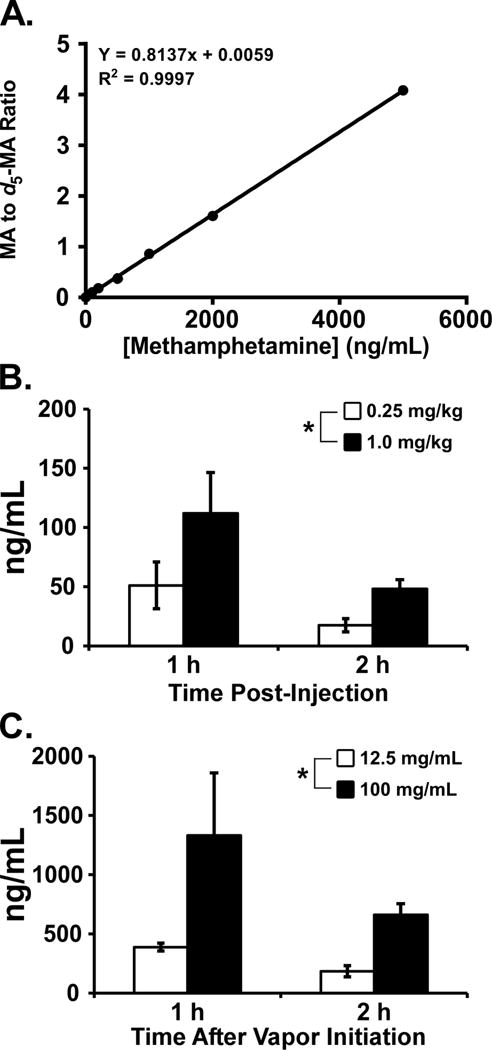
A) Standard curve generated for MA using blank plasma samples and known concentration of drug (0 – 500 ng/mL). Mean (N=4; ±SEM) Concentrations of MA in plasma 1 and 2 hours after B) i.p. injection and C) vapor inhalation. Plasma concentrations were determined by LCMS analysis. A significant effect of dose is indicated by *.
4. Discussion
These findings show that the vaccine previously shown to alter locomotor and thermoregulatory responses to methamphetamine (MA), as well as to alter intravenous self-administration of MA, in male rats (Miller et al., 2015; Miller et al., 2013b) is also efficacious in attenuating locomotor stimulation induced by MA in female rats. Protection against the locomotor stimulation produced by intraperitoneal administration of MA in female rats was dose dependent, with a complete attenuation of locomotor elevation at the 0.25 mg/kg dose and a partial protection at 0.5 mg/kg. The fact that protection in MH6-KLH rats was present at low doses and could be overcome at high doses, in an essentially monotonic relationship, accords well with the mechanism of action of anti-drug vaccination. It contrasts, however, with a prior study of mouse locomotor activity in which the anti-MA vaccine resulted, curiously, in an enhanced locomotor response to low dose MA and a protective effect at high doses (Shen et al., 2013). The mechanisms underlying such biphasic effects are as yet unknown but interpretation of the results of the present study is much more straightforward in comparison.
The lack of protection against vapor-inhalation effects of MA was most likely because of the high MA dose that was delivered, as indicated by the plasma exposure data (Figure 6). The inhalation conditions for this study were originally selected based on the behavioral effects observed in a prior study in which intracranial self-stimulation reward thresholds and locomotor activity responses were similar across i.p. injection (0.5–1.0 mg/kg) and vapor inhalation (12.5–100 mg/mL; 30–40 minutes) methods of delivering MA (Nguyen et al., 2016a). Similar to those prior behavioral findings the maximum locomotor stimulation observed in this study after MA vapor inhalation was within the range produced by the i.p. injection of MA. Nevertheless, the analysis of plasma MA levels showed that almost an order of magnitude higher MA exposure was obtained by vapor inhalation of MA (12.5, 100 mg/mL; 40 minutes) compared with i.p. injection (0.25, 1.0 mg/kg) in unvaccinated rats at the point in time post-administration when activity was maximal. Since either the 12.5 or 100 mg/mL inhalation concentration used for the plasma study resulted in blood levels in excess of levels found after injection of the highest i.p. dose used in the telemetry study (for which no group differences were observed), it is unsurprising that no vaccine group differences were found in the inhalation challenges. Although the similar magnitude of behavioral effects does not seem to correlate with peak plasma levels, it is likely that the time-course differences associated with inhalation over 40 min versus a single i.p. injection resulted the more-similar behavioral profile.
An alternative potential explanation for the difference between the initial vapor-inhalation and the i.p. result may be a low titer during the initial vapor study, given that no direct evidence for the titer levels were obtained during the initial study. Nevertheless, the antibody titer was confirmed to be high in the blood samples drawn at the end of the i.p. challenges where efficacy against MA was observed. The vapor inhalation was repeated within a week and there were again no group differences identified (this time with 20 or 40 minutes of inhalation). Thus it may be the case that protection against vapor inhalation of MA might be observed in vaccinated animals with inhalation parameters that resulted in a plasma MA level more similar to that found after 0.25 mg/kg, i.p..
Although this study only focused on spontaneous locomotor activity as measured by radiotelemetry, active stimulant doses of MA have been shown to alter other motor behaviors including, but not limited to, wheel running activity and stereotypy. The doses tested in both routes of administration were congruent with doses previously tested in our lab that elicit behavioral changes indexed by those measurements (Miller et al., 2015; Nguyen et al., 2016a). In conclusion, this study therefore confirms the efficacy of the MH6-KLH anti-methamphetamine vaccine in female rats for the first time, which supports continued development of such approaches for eventual human therapeutic use.
Highlights.
Active immunization produces antibodies which can sequester drug in the blood
Such anti-drug immunotherapy has not been broadly tested in female rats
A vaccine prevented methamphetamine-induced hyperlocomotion in female rats
The MH6-KLH candidate vaccine is therefore effective in both sexes
Acknowledgments
This is manuscript #29436 from The Scripps Research Institute.
Role of Funding Source. The study was conducted under the support of USPHS grants (R01 DA024705; R01 DA024105; R01 DA042211). The NIH/NIDA had no role in study design, collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: MAT designed the study with significant input from JDN. KMC, SAV and JDN collected and organized the in vivo data, and completed initial data analyses. KDJ contributed to the original design of the MH6 conjugate vaccine and oversaw PTB and KCC who created and validated the MH6 conjugate vaccine; PTB performed antibody titer assessments. CSH performed plasma MA analyses. JDN and MAT conducted literature searches and provided summaries of previous related work. MAT and JDN undertook the statistical analysis and created figures and drafted the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest. The authors have no conflicts of interest to report for this study.
References
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA. In vivo potency and efficacy of the novel cathinone alpha-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: Self-administration and locomotor stimulation in male rats. Psychopharmacology (Berl) 2015;232:3045–3055. doi: 10.1007/s00213-015-3944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–140. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno R, Matthews AJ, Topp L, Degenhardt L, Gomez R, Dunn M. Can the severity of dependence scale be usefully applied to ‘ecstasy’? Neuropsychobiology. 2009;60:137–147. doi: 10.1159/000253550. [DOI] [PubMed] [Google Scholar]
- Byrnes-Blake KA, Carroll FI, Abraham P, Owens SM. Generation of anti-(+)methamphetamine antibodies is not impeded by (+)methamphetamine administration during active immunization of rats. Int Immunopharmacol. 2001;1:329–338. doi: 10.1016/s1567-5769(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, Janda KD. Suppression of psychoactive effects of cocaine by active immunization. Nature. 1995;378:727–730. doi: 10.1038/378727a0. [DOI] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das-Douglas M, Colfax G, Moss AR, Bangsberg DR, Hahn JA. Tripling of methamphetamine/amphetamine use among homeless and marginally housed persons, 1996–2003. J Urban Health. 2008;85:239–249. doi: 10.1007/s11524-007-9249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito EE, Babuscio TA, Nich C, Ball SA, Carroll KM. Gender differences in clinical outcomes for cocaine dependence: Randomized clinical trials of behavioral therapy and disulfiram. Drug Alcohol Depend. 2014;145:156–167. doi: 10.1016/j.drugalcdep.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duryee MJ, Bevins RA, Reichel CM, Murray JE, Dong Y, Thiele GM, Sanderson SD. Immune responses to methamphetamine by active immunization with peptide-based, molecular adjuvant-containing vaccines. Vaccine. 2009;27:2981–2988. doi: 10.1016/j.vaccine.2009.02.105. [DOI] [PubMed] [Google Scholar]
- Fox HC, Morgan PT, Sinha R. Sex differences in guanfacine effects on drug craving and stress arousal in cocaine-dependent individuals. Neuropsychopharmacology. 2014;39:1527–1537. doi: 10.1038/npp.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile CN, Kosten TA, Shen XY, O’Malley PW, Winoske KJ, Kinsey BM, Wu Y, Huang Z, Lykissa ED, Naidu N, Cox JA, Arora R, Kosten TR, Orson FM. Altered methamphetamine place conditioning in mice vaccinated with a succinyl-methamphetamine-tetanus-toxoid vaccine. Am J Addict. 2015;24:748–55. doi: 10.1111/ajad.12307. [DOI] [PubMed] [Google Scholar]
- Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W, Shoptaw S. Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2010;109:20–29. doi: 10.1016/j.drugalcdep.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T, Domingo C, Orson F, Kinsey B. Vaccines against stimulants: Cocaine and MA. Br J Clin Pharmacol. 2014;77:368–374. doi: 10.1111/bcp.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Domingo CB. Can you vaccinate against substance abuse? Expert Opin Biol Ther. 2013;13:1093–1097. doi: 10.1517/14712598.2013.791278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Aarde SM, Moreno AY, Creehan KM, Janda KD, Taffe MA. Effects of active anti-methamphetamine vaccination on intravenous self-administration in rats. Drug Alcohol Depend. 2015;153:29–36. doi: 10.1016/j.drugalcdep.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Creehan KM, Angrish D, Barlow DJ, Houseknecht KL, Dickerson TJ, Taffe MA. Changes in ambient temperature differentially alter the thermoregulatory, cardiac and locomotor stimulant effects of 4-methylmethcathinone (mephedrone) Drug Alcohol Depend. 2013a;127:248–253. doi: 10.1016/j.drugalcdep.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Moreno AY, Aarde SM, Creehan KM, Vandewater SA, Vaillancourt BD, Wright MJ, Jr, Janda KD, Taffe MA. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiatr. 2013b;73:721–728. doi: 10.1016/j.biopsych.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Aarde SM, Cole M, Vandewater SA, Grant Y, Taffe MA. Locomotor stimulant and rewarding effects of inhaling methamphetamine, MDPV, and mephedrone via electronic cigarette-type technology. Neuropsychopharmacology. 2016a;41:2759–2771. doi: 10.1038/npp.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Aarde SM, Vandewater SA, Grant Y, Stouffer DG, Parsons LH, Cole M, Taffe MA. Inhaled delivery of Delta(9)-tetrahydrocannabinol (THC) to rats by e-cigarette vapor technology. Neuropharmacology. 2016b;109:112–120. doi: 10.1016/j.neuropharm.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Bremer PT, Ducime A, Creehan KM, Kisby BR, Taffe MA, Janda KD. Active vaccination attenuates the psychostimulant effects of alpha-PVP and MDPV in rats. Neuropharmacology. 2016c;116:1–8. doi: 10.1016/j.neuropharm.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohia-Nwoko O, Kosten TA, Haile CN. Animal models and the development of vaccines to treat substance use disorders. Int Rev Neurobiol. 2016;126:263–291. doi: 10.1016/bs.irn.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Gonzales R, Obert JL, McCann MJ, Brethen P. Methamphetamine use among treatment-seeking adolescents in Southern California: Participant characteristics and treatment response. J Subst Abuse Treat. 2005;29:67–74. doi: 10.1016/j.jsat.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Ruedi-Bettschen D, Wood SL, Gunnell MG, West CM, Pidaparthi RR, Carroll FI, Blough BE, Owens SM. Vaccination protects rats from methamphetamine-induced impairment of behavioral responding for food. Vaccine. 2013;31:4596–4602. doi: 10.1016/j.vaccine.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XY, Kosten TA, Lopez AY, Kinsey BM, Kosten TR, Orson FM. A vaccine against methamphetamine attenuates its behavioral effects in mice. Drug Alcohol Depend. 2013;129:41–48. doi: 10.1016/j.drugalcdep.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P. Biologic approaches to treat substance-use disorders. Trends Pharmacol Sci. 2015;36:628–635. doi: 10.1016/j.tips.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Creehan KM, Vandewater SA. Cannabidiol fails to reverse hypothermia or locomotor suppression induced by Delta(9) -tetrahydrocannabinol in Sprague-Dawley rats. Br J Pharmacol. 2015;172:1783–1791. doi: 10.1111/bph.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uosukainen H, Tacke U, Winstock AR. Self-reported prevalence of dependence of MDMA compared to cocaine, mephedrone and ketamine among a sample of recreational poly-drug users. Int J Drug Policy. 2015;26:78–83. doi: 10.1016/j.drugpo.2014.07.004. [DOI] [PubMed] [Google Scholar]
- Westermeyer J, Boedicker AE. Course, severity, and treatment of substance abuse among women versus men. Am J Drug Alcohol Abuse. 2000;26:523–535. doi: 10.1081/ada-100101893. [DOI] [PubMed] [Google Scholar]
- Wood E, Stoltz JA, Zhang R, Strathdee SA, Montaner JS, Kerr T. Circumstances of first crystal methamphetamine use and initiation of injection drug use among high-risk youth. Drug Alcohol Rev. 2008;27:270–276. doi: 10.1080/09595230801914750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, Vandewater SA, Parsons LH, Houseknecht KL, Dickerson TJ, Taffe MA. Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PLoS One. 2012;7:e44652. doi: 10.1371/journal.pone.0044652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewska-Kaszubska J. Is immunotherapy an opportunity for effective treatment of drug addiction? Vaccine. 2015;33:6545–6551. doi: 10.1016/j.vaccine.2015.09.079. [DOI] [PubMed] [Google Scholar]


