Abstract
Objective
Apolipoprotein C-III (apoC-III) is a key regulator of triglyceride (TG) metabolism. Elevated TG rich lipoproteins and apoC-III levels are causally linked to coronary artery disease (CAD) risk. The mechanism(s) through which apoC-III increases CAD risk remains largely unknown. The aim was to confirm the association between apoC-III plasma levels and CAD risk and to explore which lipoprotein subfractions contribute to this relationship between apoC-III and CAD risk.
Approach and Results
Plasma apoC-III levels were measured in baseline samples from a nested case-control study in the prospective EPIC-Norfolk study. The study comprised 2,711 apparently healthy study participants, of whom 832 subsequently developed CAD. We studied the association of baseline apoC-III levels with incident CAD risk, lipoprotein subfractions measured by nuclear magnetic resonance spectroscopy (NMR) and inflammatory biomarkers.
ApoC-III levels were significantly associated with CAD risk (odds ratio 1.91 95% CI 1.48–2.48 for highest compared to lowest quintile), retaining significance after adjustment for traditional CAD risk factors (odds ratio 1.47, 95% CI 1.11–1.94). ApoC-III levels were positively correlated with TG levels, (r=0.39), particle numbers of very-low density lipoprotein (VLDL; r=0.25), intermediate-density lipoprotein (IDL; r=0.23), small dense LDL (r=0.26), and high-sensitivity C-reactive protein (hsCRP) (r=0.15), whereas an inverse correlation was observed with large LDL particle number (r=−0.11), p<0.001 for each. Mediation analysis indicated that the association between apoC-III and CAD risk could be explained by TG-elevation (TG, VLDL and IDL particles), small LDL particle size and hsCRP.
Conclusions
ApoC-III levels are significantly associated with incident CAD risk. Elevated levels of remnant lipoproteins, small dense LDL and low-grade inflammation may explain this association.
Keywords: Apolipoprotein C-III, Remnant cholesterol, Triglycerides, Coronary artery disease
Subject codes: Lipids and Cholesterol, Epidemiology
Introduction
Large prospective epidemiological studies have shown that elevated triglyceride (TG) levels are associated with increased coronary artery disease (CAD) risk,1,2 independent of low density lipoprotein-cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C).3 Triglycerides in plasma are carried in apoB-containing triglyceride-rich lipoproteins (TRL), mainly in very low density lipoproteins (VLDL) and to a lesser extent in intermediate density lipoproteins (IDL). Apolipoprotein C-III (apoC-III) is increasingly recognized as a key regulator of TRL metabolism in human subjects and mediates its effects by both lipoprotein lipase (LPL)-dependent and LPL- independent mechanisms.4,5 ApoC-III has been shown to inhibit LPL mediated hydrolysis of TGs in TRL 6 and to reduce hepatic TRL uptake,7 in particular, those mediated by the LDL receptor and LRP1.8 At higher concentrations, apoC-III also inhibits hepatic lipase activity, which results in reduced conversion of VLDL to IDL and LDL and also affects HDL remodeling.9 Genetic studies have shown that loss-of-function mutations in APOC3 result in a favorable lipid profile characterized by reduced TG and VLDL levels.10 ApoC-III plasma levels are associated with increased risk of CAD and Mendelian randomization studies corroborated causality by directly linking genetic variation in APOC3 to CAD risk.11,12 Collectively, these findings have spurred the development of apoC-III lowering therapies, of which an antisense inhibitor of APOC3 messenger RNA has been shown to potently reduce both plasma apoC-III and TG levels.4,5
In view of the intricate relationship between apoC-III and several pro-atherogenic lipoprotein fractions and it’s recent implication in inflammatory pathways,13 it remains to be established which factors mediate the detrimental impact of elevated apoC-III on CAD risk. Therefore, we assessed the association between apoC-III levels, detailed lipoprotein analyses, inflammatory markers and CAD risk in a large prospective nested case-control study.
Material and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Plasma apoC-III measurements were available for 2,711 participants; 832 cases and 1,879 controls. Baseline characteristics for CAD cases and controls are listed in Table 1. Despite partial loss of matching due to plasma sample availability, age and sex were similar between groups. The prevalence of traditional cardiovascular risk factors was higher in cases compared to controls: more active smokers, higher BMI, elevated blood pressure, total cholesterol, LDL-C, triglycerides and apoB levels, whereas HDL-C and apoA-I levels were lower in cases. ApoC-III levels were indeed higher in cases compared to controls. Figure 1 shows the distribution of apoC-III and triglyceride levels among cases and controls, showing a shifted distribution towards higher apoC-III and triglyceride levels among cases.
Table 1.
Baseline characteristics of controls and cases.
| Controls (n=1,879) | Cases (n=832) | p-value | |
|---|---|---|---|
|
|
|||
| ApoC-III, mg/dL | 4.8 (3.5–6.9) | 5.5 (3.8–7.9) | <0.001 |
|
|
|||
| Age, years | 65.3 ±7.7 | 65.6 ±7.7 | 0.46 |
|
|
|||
| Sex, male | 1174(62.5%) | 520 (62.5%) | 0.99 |
|
|
|||
| Body mass index, kg/m2 | 26.3 ±3.5 | 27.3 ±3.8 | <0.001 |
|
|
|||
| Current smoking | 157 (8.4%) | 127(15.4%) | <0.001 |
|
|
|||
| Systolic blood pressure, mmHg | 139 ±18 | 144 ±19 | <0.001 |
|
|
|||
| Diastolic blood pressure, mmHg | 84 ±11 | 86 ±12 | <0.001 |
|
|
|||
| Diabetes mellitus | 33 (1.8%) | 57 (6.9%) | <0.001 |
|
|
|||
| Lipid-lowering drugs | 20 (1.1%) | 31 (3.7%) | <0.001 |
|
|
|||
| Anti-hypertensive drugs | 329 (17.5%) | 352(42.3%) | <0.001 |
|
|
|||
| Total cholesterol, mmol/L | 6.3 ±1.2 | 6.5 ±1.2 | <0.001 |
|
|
|||
| LDL cholesterol, mmol/L | 4.1 ±1.0 | 4.3 ±1.0 | <0.001 |
|
|
|||
| HDL cholesterol, mmol/L | 1.4 ±0.4 | 1.3 ±0.4 | <0.001 |
|
|
|||
| Triglycerides, mmol/L | 1.7 (1.2–2.3) | 2.0 (1.4–2.8) | <0.001 |
|
|
|||
| Remnant cholesterol, mmol/L | 0.7 (0.5–1.0) | 0.8 (0.6–1.2) | <0.001 |
|
|
|||
| Apolipoprotein A-I, mg/dL | 162.2 ±28.9 | 154.6 ±29.3 | <0.001 |
|
|
|||
| Apolipoprotein B, mg/dL | 129.1 ±31.2 | 137.6 ±32.7 | <0.001 |
|
|
|||
| Lipoprotein(a), mg/dL | 8.4 (6.4–13.6) | 9.5 (6.9–24.7) | <0.001 |
|
|
|||
Continuous variables with a normal distribution are reported as mean ± standard deviation. Continuous variables with a non-normal distribution are reported as median (interquartile range). Dichotomous variables are reported as percentage of total cohort (number). P-values are for student’ s T-test for continuous variables and X2 test for dichotomous variables.
Figure 1.
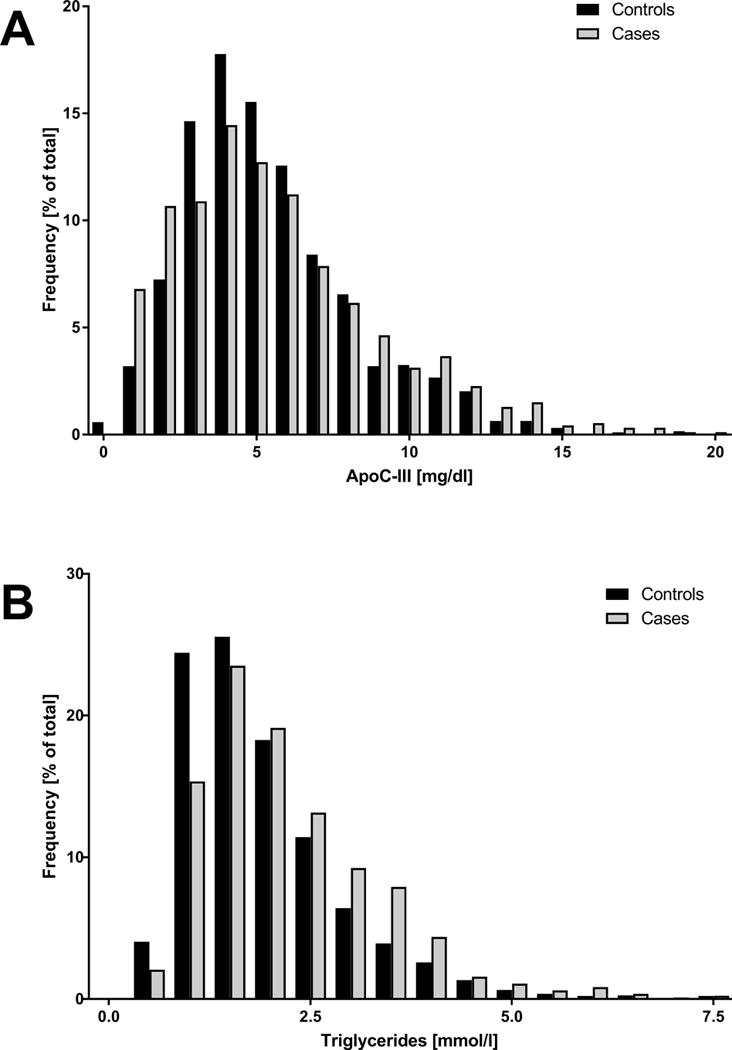
Distribution of apoC-III in mg/dl (panel A) and triglycerides in mmol/l (panel B) as percentage of total cohort for cases (n=832) and controls (n=1,879).
Table 2 and Supplemental Table I show baseline characteristics according to apoC-III quintiles. Age was slightly lower and there were fewer males in the highest quintile compared to the lowest. For a complete overview, baseline characteristics for males and females separately are provided in Supplemental Table II and III. Higher apoC-III levels were associated with increased BMI, use of lipid-lowering drugs, use of anti-hypertensive drugs and increased systolic and diastolic blood pressure. Triglyceride levels were most profoundly associated with apoC-III quintiles.
Table 2.
Baseline characteristics per apoC-III quintile.
| Q1 (n=559) | Q2 (n=526) | Q3 (n=560) | Q4 (n=534) | Q5 (n=532) | P for trend | |
|---|---|---|---|---|---|---|
|
|
||||||
| ApoC-III, mg/dL | 2.5 (1.8–2.9) | 3.9 (3.6–4.1) | 5.1 (4.7–5.5) | 6.7 (6.2–7.2) | 10.2 (8.8–11.9) | <0.001 |
|
|
||||||
| Age, years | 66.2 ±7.7 | 65.4 ±7.5 | 64.8 ±8 | 65.2 ±7.7 | 65.3 ±7.6 | <0.05 |
|
|
||||||
| Sex, male | 374 (66.9%) | 346 (65.8%) | 341(60.9%) | 325(60.9%) | 308(57.9%) | <0.01 |
|
|
||||||
| Body mass index, kg/m2 | 26 ±3.5 | 25.7 ±3.1 | 26.7 ±3.7 | 27 ±3.6 | 27.5 ±4 | <0.001 |
|
|
||||||
| Current smoking | 51(9.2%) | 53(9.2%) | 57(10.3%) | 62(11.7%) | 61(11.6%) | 0.13 |
|
|
||||||
| Systolic blood pressure, mmHg | 140 ±18 | 138 ±18 | 140 ±19 | 140 ±18 | 143 ±18 | <0.001 |
|
|
||||||
| Diastolic blood pressure, mmHg | 83 ±11.1 | 83 ±11.5 | 84 ±11.8 | 84 ±10.9 | 86 ±10.8 | <0.001 |
|
|
||||||
| Diabetes Mellitus | 18(3.2%) | 17(3.2%) | 19(3.4%) | 13(2.4%)_ | 23(4.3%) | 0.56 |
|
|
||||||
| Lipid-lowering drugs | 4(0.7%) | 6(1.1%) | 8(1.4%) | 15(2.8%) | 18(3.4%) | <0.01 |
|
|
||||||
| Anti-hypertensive drugs | 114(20.4%) | 114(21.7%) | 128(22.9%) | 155(29%) | 170(32%) | <0.001 |
|
|
||||||
| Total cholesterol, mmol/L | 6.0 ±1.1 | 6.2 ±1.1 | 6.2 ±1 | 6.5 ±1.1 | 6.8 ±1.4 | <0.001 |
|
|
||||||
| LDL cholesterol, mmol/L | 3.9 ±1 | 4.1 ±1 | 4.1 ±1 | 4.2 ±1 | 4.3 ±1.1 | <0.001 |
|
|
||||||
| HDL cholesterol, mmol/L | 1.4 ±0.4 | 1.4 ±0.4 | 1.3 ±0.4 | 1.3 ±0.4 | 1.3 ±0.4 | <0.01 |
|
|
||||||
| Triglycerides, mmol/L | 1.4 (1–1.8) | 1.5 (1.2–2) | 1.7 (1.2–2.3) | 1.9 (1.4–2.7) | 2.5 (1.8–3.4) | <0.001 |
|
|
||||||
| Remnant cholesterol, mmol/L | 0.6 (0.5–0.8) | 0.7 (0.5–0.9) | 0.8 (0.6–1.0) | 0.8 (0.6–1.2) | 1.1 (0.8–1.4) | <0.001 |
|
|
||||||
| Apolipoprotein A-I, mg/dL | 160.7 ±29.6 | 158.9 ±29.9 | 159.8 ±29.5 | 160.9 ±28.1 | 159.4 ±29.1 | 0.90 |
|
|
||||||
| Apolipoprotein B, mg/dL | 119.7 ±29.1 | 129.7 ±30 | 129.2 ±29 | 136.1 ±31.4 | 145.2 ±34.6 | <0.001 |
|
|
||||||
| Lipoprotein(a), mg/dL | 8.2 (6.5–14.4) | 8.9 (6.7–16.4) | 8.8 (6.7–18.7) | 8.6 (6.5–14.3) | 8.7 (6.4–18.9) | 0.31 |
|
|
||||||
Continuous variables with a normal distribution are reported as mean ± standard deviation. Continuous variables with a non-normal distribution are reported as median (interquartile range). Dichotomous variables are reported as percentage of total cohort (number). ApoC-III range for each quintile was Q1 <3.27, Q2: 3.27–4.45, Q3: 4.45–5.79, Q4: 5.79–7.87, Q5 >7.87 mg/dL.
ApoC-III and lipid metabolism parameters
At baseline, apoC-III levels were correlated with total cholesterol (r=0.23, p<0.001), LDL-C (r=0.13, p<0.001) and triglycerides (r=0.39, p<0.001) Table 3). ApoC-III levels were correlated with VLDL particle number and size (r=0.24 and r=0.27, p<0.001) as well as with large VLDL/chylomicron sized particle number (r= 0.39, p<0.001). There was also a positive correlation with IDL particle number (r=0.23, p<0.001) and similarly, LDL particle number (r=0.26, p< 0.001) and an inverse correlation with LDL particle size (r=−0.22, p<0.001). Similar correlations were found for LDL parameters measured by gradient gel-electrophoresis. Furthermore, apoC-III levels were positively correlated with apolipoprotein A-V levels (r=0.21, p<0.001) and inversely correlated with LPL mass (r=−0.13, p<0.001). Sex-specific Spearman correlations for apoC-III and lipid and non-lipid variables are given in Supplemental Table IV and are similar to the overall group.
Table 3.
Spearman correlation coefficients and p-values of apoC-III levels with lipid and non-lipid parameters.
| Lipids | Spearman correlation coefficient | P-Value | Subjects (N) |
|---|---|---|---|
| Total cholesterol, mmol/L | 0.23 | <0.001 | 2680 |
| LDL cholesterol, mmol/L | 0.13 | <0.001 | 2560 |
| HDL cholesterol, mmol/L | −0.05 | <0.001 | 2559 |
| Triglycerides, mmol/L | 0.39 | <0.001 | 2669 |
| Apolipoprotein A1, mg/dL | −0.01 | 0.73 | 2289 |
| Apolipoprotein B, mg/dL | 0.26 | <0.001 | 2457 |
| Lipoprotein(a), mg/dL | 0.01 | 0.62 | 2662 |
| NMR data | |||
| VLDL particles, nmol/L | 0.24 | <0.001 | 2694 |
| VLDL size, nm | 0.27 | <0.001 | 2694 |
| Large VLDL/chylomicrons, nmol/L | 0.39 | <0.001 | 2694 |
| Medium VLDL, nmol/L | 0.23 | <0.001 | 2694 |
| Small VLDL, nmol/L | 0.13 | <0.001 | 2694 |
| IDL, nmol/L | 0.23 | <0.001 | 2694 |
| LDL particles, nmol/L | 0.26 | <0.001 | 2694 |
| LDL size, nm | −0.22 | <0.001 | 2694 |
| Large LDL, nmol/L | −0.11 | <0.001 | 2694 |
| Small LDL, nmol/L | 0.26 | <0.001 | 2694 |
| HDL particles, nmol/L | 0.20 | <0.001 | 2694 |
| TRL metabolism | |||
| Lipoprotein lipase, ng/ml | −0.13 | <0.001 | 2675 |
| Apolipoprotein A-V, ng/ml | 0.21 | <0.001 | 2616 |
| HDL parameters | |||
| Cholesterol efflux capacity | 0.08 | <0.001 | 2607 |
| LCAT-concentration, mg/L | 0.13 | <0.001 | 2486 |
| CETP-concentration, mg/L | −0.01 | 0.64 | 2479 |
| Inflammatory markers | |||
| C-reactive protein, mg/L | 0.15 | <0.001 | 2681 |
| Glucose metabolism | |||
| HbA1C [%] | 0.15 | <0.001 | 1044 |
Spearman correlations with corresponding p-values.
In line with the correlations with TG-related parameters, apoC-III levels were correlated with HDL particle number (r=0.20, p<0.001) and inversely correlated with HDL particle size (r=−0.23, p<0.001.) There was a weak positive correlation between apoC-III and cholesterol efflux capacity (r=0.08, p<0.001), LCAT mass (r=0.13, p<0.001) but no correlation with CETP mass (r=−0.01, p=0.64). Finally, apoC-III levels were weakly correlated with hsCRP levels (r=0.15, p<0.001). All correlations were additionally tested in a linear regression model adjusted for age, sex, BMI, current smoking, systolic blood pressure, history of diabetes mellitus, lipid-lowering and anti-hypertensive drug use, which did not attenuate significance importantly.
Further analysis of apoC-III levels by quintiles and lipoprotein particle numbers and size measured by NMR spectroscopy shows a progressive increase in all sizes of VLDL particles, IDL and small dense LDL, but a decrease for large LDL, all p<0.001 for trend Figure 2, detailed particle numbers and size per apoC-III quartile are provided in Supplemental Table I.
Figure 2.
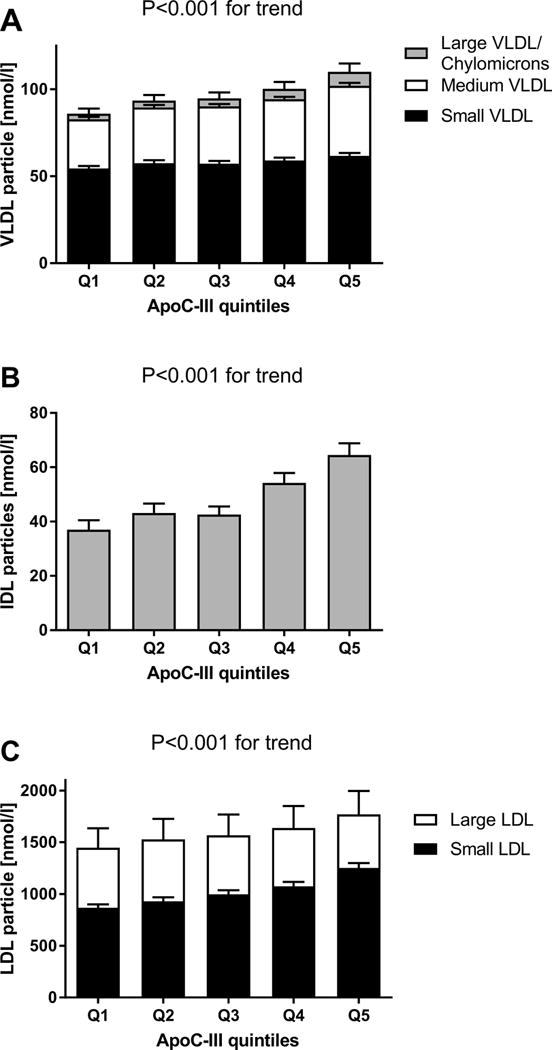
Nuclear magnetic resonance spectroscopy measured lipoprotein subparticle numbers per apoC-III quintile.
Bars display mean particle numbers in nmol/l and corresponding 95% confidence interval. A. VLDL (small, medium, large/chylomicron) particle numbers, total bar height represents total VLDL particle number. B. IDL particle numbers per apoC-III quintile. C. LDL (small and large) particle number per apoC-III, total bar height represents total LDL particle number. P-value for trend < 0.001 for all (sub)particle number associations.
ApoC-III levels and CAD risk
In a logistic regression analysis, adjusted for age and sex, the odds ratio for incident CAD was 1.91 [95%CI: 1.48–2.48] for highest quintile apoC-III compared to lowest quintile, Figure 3 and Supplemental Table V. Following adjustment for cardiovascular risk factors (age, sex, BMI, current smoking, diabetes mellitus, systolic blood pressure, lipid-lowering and anti-hypertensive drug use), apoC-III levels remained significantly associated with CAD risk, (odds ratio 1.47 95%CI 1.11–1.94). This association was attenuated but remained significant after further adjustment for LDL-C (odds ratio 1.35 95%CI 1.01–1.81) or HDL-C (odds ratio 1.41, 95%CI 1.05–1.88), but lost significance after adjustment for triglycerides (odds ratio 1.17 95%CI 0.87–1.58), Table 4.
Figure 3.
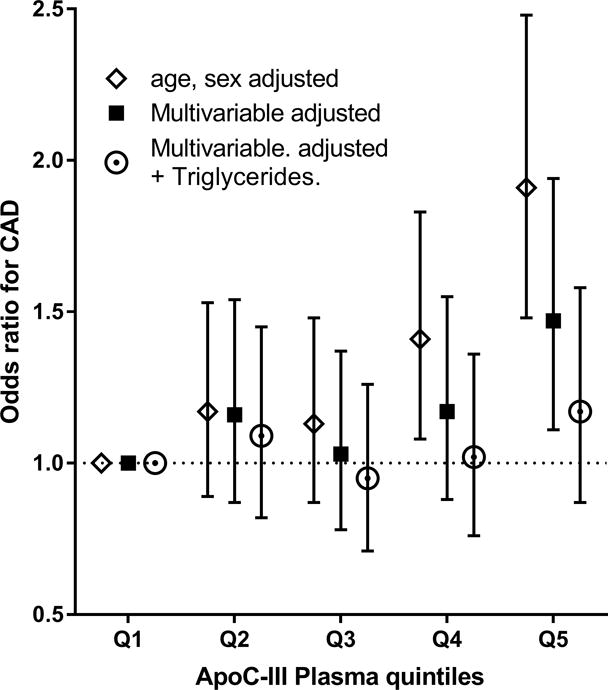
Association of apoC-III quintiles and incident coronary artery disease risk.
Data are odds ratios and corresponding 95% confidence interval. Model 1(open diamond) is matched for age and sex, model 2 (black square) is adjusted for age, sex, BMI, current smoking, diabetes mellitus, use of blood-pressure lowering and lipid lowering drugs. Model 3 (open circle) is model 2 additionally adjusted for triglyceride levels. ApoC-III range for each quintile was Q1 <3.27, Q2: 3.27–4.45, Q3: 4.45–5.79, Q4: 5.79–7.87, Q5 >7.87 mg/dL.
Table 4.
Mediation analysis for apoC-III quintiles and CAD risk
| Model 1 (age, sex adjusted) | Model 2 (multivariable adjusted) | |||||
|---|---|---|---|---|---|---|
| Odds ratio | Percentage explained | P-value | Odds ratio | Percentage explained | P-value | |
| Base Model | 1.91 (1.48–2.48) | 1.47 (1.11–1.94) | ||||
| additionally adjusted for: | ||||||
| +Triglycerides | 1.34 (1.01–1.78) | 55% | <0.001 | 1.17 (0.87–1.58) | 59% | <0.001 |
| +apoB | 1.57 (1.18–2.08) | 31% | <0.001 | 1.24 (0.91–1.68) | 44% | <0.001 |
| +VLDL (NMR) | 1.64 (1.25–2.14) | 24% | <0.001 | 1.26 (0.94–1.68) | 41% | <0.001 |
| +Large VLDL | 1.53 (1.15–2.03) | 35% | <0.001 | 1.33 (0.99–1.80) | 26% | <0.05 |
| +LDL (NMR) | 1.64 (1.25–2.14) | 24% | <0.001 | 1.30 (0.98–1.74) | 31% | <0.001 |
| + Large LDL | 1.88 (1.45–2.44) | – | ns | 1.48 (1.12–1.96) | – | ns |
| + Small LDL | 1.63 (1.25–2.14) | 24% | <0.001 | 1.33 (1.00–1.78) | 25% | <0.01 |
| +CRP | 1.64 (1.26–2.14) | 23% | <0.001 | 1.38 (1.04–1.83) | 16% | <0.001 |
| +LDL-C | 1.71 (1.30–2.24) | 18% | <0.001 | 1.35 (1.00–1.81) | 23% | <0.001 |
| +HDL-C | 1.72 (1.31–2.26) | 17% | <0.001 | 1.41 (1.05–1.88) | 12% | <0.001 |
Odds ratios and corresponding 95% confidence intervals for coronary artery disease for highest quintile compared to lowest apoC-III quintile. ‘+ parameter’ indicates odds ratio for apoC-III (Q5 vs Q1) additionally adjusted for ‘+ parameter’. Percentage explained indicates the proportion of the association of apoC-III with CAD risk that is explained by the mediator. P-value for likelihood ratio test between models, ns is non-significant.
Model 1: ApoC-III highest versus lowest quintile, adjusted for age, sex.
Model 2: additionally adjusted for BMI, current smoking, diabetes mellitus, systolic blood pressure lipid-lowering medication and antihypertensive medication use.
In subgroup analysis, we found that apoC-III levels were not as strongly associated with CAD risk in subjects with LDL-C lower than the median compared to subjects with LDL-C over the median (Supplemental Figure I). Furthermore apoC-III levels were not significantly associated with CAD risk in subjects with triglycerides lower than the median (Supplemental Figure I and II). There was no important difference in CAD risk between males and females.
ApoC-III mediation analysis
Next, we explored which of the lipoprotein subfractions explained part of the association between apoC-III and CAD risk, Table 4. We found that plasma triglyceride levels (55%) explained a major part of the association between apoC-III and CAD risk, followed by apoB levels (31%). VLDL and LDL particle numbers both explained 24%, of the association between apoC-III and CAD risk, and interestingly, the association observed for LDL particles was fully attributable to small dense LDL particles (24%), independent of large LDL particle numbers. Similar effect sizes were found for the multi-variable adjusted model, independent from BMI, smoking status, diabetes mellitus, systolic blood pressure and blood pressure lowering and lipid lowering drugs. Similar results were found for the difference and product method and for apoC-III as a continuous variable (data are shown for difference method and apoC-III quintiles).14
Triglyceride levels and calculated remnant cholesterol levels
In recent literature, triglyceride levels are increasingly acknowledged as a biomarker for the cholesterol content of TRL, known as remnant cholesterol, which is understood to be the relevant contributor to atherosclerosis.15 As LDL-C in our study is calculated using the Friedewald formula both parameters are mathematically related, which is illustrated by the strong correlation between triglycerides and remnant cholesterol levels (R2=0.99, p<0.001). However, to be as thorough and complete as possible in our analyses we did include remnant cholesterol and found identical associations for apoC-III and triglyceride or remnant cholesterol levels.
Discussion
This large, prospective study demonstrates that elevated apoC-III levels are associated with a significantly increased risk of CAD. Furthermore, the risk of elevated apoC-III levels was strongly and quantitatively associated with several parameters of TRL, small LDL particles and inflammation. This study represents the largest study to date to substantiate the association between apoC-III plasma levels and CAD risk, in terms of total subjects studied and CAD case numbers, and provides another level of epidemiologic evidence to support the genetic data of apoC-III as a potential target of therapy.5
Our results are in line with previous reports that report on the association of apoC-III with CAD risk16–18, and recently, with atherosclerotic burden in diabetics19. In those studies, apoC-III has been shown to correlate strongly with plasma TG levels in populations with a wide variation in absolute plasma TG levels.4,5,17 In our study, the correlation was weaker, which perhaps can be explained by the relatively restricted range of TG values and low incidence of diabetes observed in this normal population. Nevertheless, we found that subjects with higher apoC-III levels had higher VLDL particle numbers and larger VLDL mean particle size as well as increased IDL particle numbers. Previous tracer kinetic studies9,20 support a role for apoC-III in inhibiting the rate of clearance of VLDL from plasma, which is suggested to be consistent with inhibition of LPL mediated hydrolysis and/or inhibition of hepatic uptake of TRL. In particular, the presence of apoC-III on light LDL was associated with enhanced conversion to dense LDL.9 These latter observations are perhaps related to our observation that high apoC-III levels are associated with a shift towards predominantly small dense LDL particles. Alternatively, increased levels of TRL particles in hypertriglyceridemia increases the transfer of TG from VLDL to LDL in exchange for cholesteryl ester, mediated by CETP, and in turn the TG enriched in LDL are hydrolyzed via hepatic lipase, further contributing to small dense LDL.21 Similar findings were reported in a smaller genetic study of loss-of-function APOC3 variants that found a clear correlation with VLDL, IDL and LDL subfractions.10
The mechanism linking apoC-III and CAD risk is poorly understood. Triglycerides per se are unlikely causal in atherosclerosis development as TG’s can be degraded by most cell types and do not accumulate in atherosclerotic plaques. However, VLDL and remnant lipoproteins, such as small VLDL and IDL do accumulate in the arterial intima, where up to 40% of particles consist of VLDL and VLDL remnant sized lipoproteins.22 Macrophages within the arterial intima secrete LPL, which can further metabolize these TRL’s to even smaller remnants, which can then be taken up by macrophages, delivering their cholesterol content and generating foam cells.23,24
Thus, hypertriglyceridemia should be regarded as a marker of the cholesterol content in TRLs (VLDL + IDL), so-called remnant-cholesterol. Indeed, calculated remnant cholesterol and genetic variants determining remnant cholesterol levels are strongly associated with CAD risk.15 Using mediation analysis in our study, we found that a substantial part of the increased CAD risk associated with apoC-III levels could be attributed to the effect of TRL ‘remnant’ particle concentrations. Despite the strong effect on TRL’s, part of the association of apoC-III and CAD risk could also be explained by the effect of LDL-particle concentration, which was fully attributable to small dense LDL particles, well known biomarkers of increased CAD risk.25,26
Last, we show that apoC-III is correlated with higher hsCRP levels in our study. Interestingly, elevated remnant cholesterol levels, driven by genetics, are also associated causally with low-grade inflammation.15 Such a low-grade inflammatory state, as assessed by elevated hsCRP, is associated with increased CAD risk.27
Although we show strong correlations between apoC-III levels, CAD risk and lipoprotein particle concentrations, it should be stressed this does not necessarily imply causality and confirmation by intervention studies is warranted. However, we did adjust for BMI and other risk factors in our analyses and genetic studies of APOC3 loss-of function variants do support our findings on lipoprotein particles.10 HbA1C levels were only available for 40% of the study cohort, and were therefore not included in the multivariable adjusted regression models. We did adjust for diabetes status, but cannot rule out that the effect of apoC-III on glucose metabolism might be underestimated in our study.
Currently, apoC-III targeted therapies are in development of which the second-generation antisense oligonucleotide volanesorsen has shown the most promise to date. In 57 high TG patients, 300 mg of weekly volanesorsen resulted in apoC-III reductions up to ~−80%, which translated to equally potent lowering of plasma TG (up to −70.9%) and VLDL-C (up to −70%).5 The strong association between apoC-III, TRLs and CAD in our study holds promise for the potential of these therapies to lower CAD risk, which will need to be tested in future cardiovascular outcome trials. However, our study also indicates that the association of apoC-III and CAD risk is not as strong in subjects with lower LDL-C, supporting LDL-C lowering therapies as the initial CAD risk modifying strategy, as is advocated in current guidelines.
Conclusions
Our results demonstrate the robust association of elevated plasma apoC-III levels with CAD risk, independent of traditional cardiovascular disease risk factors. Furthermore, we show that elevated apoC-III levels are associated with an unfavorable lipoprotein profile, characterized by increased VLDL and IDL particles, and a redistribution of LDL particles towards increased small dense LDL particles and low-grade inflammation. Mediation analysis indicated that the association between apoC-III and CAD risk is likely explained by apoC-III’s association with TG-elevation (TG, VLDL and IDL particles) and small dense LDL.
Supplementary Material
Highlights.
ApoC-III levels are significantly associated with coronary artery disease risk, independent from traditional cardiovascular risk factors.
This study represents the largest study to date to substantiate this association, in terms of total subjects studied and coronary artery disease case numbers
Elevated levels of remnant lipoproteins, small dense LDL and low-grade inflammation may explain the association between apoC-III and coronary artery disease risk.
Acknowledgments
The authors wish to thank the participants and staff of the EPIC-Norfolk prospective population study.
Sources of funding
The EPIC-Norfolk Study is funded by Cancer Research UK grant number 14136 and the Medical Research Council grant number G1000143. JJK is a recipient of the Dutch Heart Foundation Lifetime Achievement Award (2010)# 2010T082. GKH is holder of a Vidi grant [016.156.445] from the Netherlands Organisation for Scientific Research (NWO). ST and JLW are supported by NIH grants R01-HL119828, P01-HL088093, P01 HL055798, R01-HL106579, R01-HL078610, and R01-HL124174. This study was performed independently from IONIS pharmaceuticals and the authors received no financial support from IONIS pharmaceuticals for this study.
Abbreviations
- ApoC-III
Apolipoprotein C-III
- CAD
Coronary artery disease
- CETP
Cholesteryl ester transfer protein
- LCAT
Lecithin–cholesterol acyltransferase
- LPL
Lipoprotein lipase
- NMR
Nuclear magnetic resonance
- TRL
Triglyceride rich lipoprotein
Footnotes
Disclosures
ST and JLW are co-inventors and receive royalties from patents owned by the University of California San Diego on oxidation-specific antibodies. ST currently has a dual appointment at UCSD and as an employee of Ionis Pharmaceuticals. JLW is a consultant to Ionis Pharmaceuticals, Intercept, CymaBay and Prometheus.
References
- 1.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw K-T, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–8. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 2.Emerging Risk Factors Collaboration. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Do R, Willer CJ, Schmidt EM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45:1345–52. doi: 10.1038/ng.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, Geary RS, Baker BF, Graham MJ, Crooke RM, Witztum JL. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med. 2014;371:2200–6. doi: 10.1056/NEJMoa1400284. [DOI] [PubMed] [Google Scholar]
- 5.Gaudet D, Alexander VJ, Baker BF, Brisson D, Tremblay K, Singleton W, Geary RS, Hughes SG, Viney NJ, Graham MJ, Crooke RM, Witztum JL, Brunzell JD, Kastelein JJP. Antisense Inhibition of Apolipoprotein C-III in Patients with Hypertriglyceridemia. N Engl J Med. 2015;373:438–47. doi: 10.1056/NEJMoa1400283. [DOI] [PubMed] [Google Scholar]
- 6.Ginsberg HN, Le NA, Goldberg IJ, Gibson JC, Rubinstein A, Wang-Iverson P, Norum R, Brown WV. Apolipoprotein B metabolism in subjects with deficiency of apolipoproteins CIII and AI. Evidence that apolipoprotein CIII inhibits catabolism of triglyceride-rich lipoproteins by lipoprotein lipase in vivo J Clin Invest. 1986;78:1287–95. doi: 10.1172/JCI112713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sehayek E, Eisenberg S. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J Biol Chem. 1991;266:18259–67. [PubMed] [Google Scholar]
- 8.Gordts PLSM, Nock R, Son N-H, Ramms B, Lew I, Gonzales JC, Thacker BE, Basu D, Lee RG, Mullick AE, Graham MJ, Goldberg IJ, Crooke RM, Witztum JL, Esko JD. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J Clin Invest. 2016;126:2855–66. doi: 10.1172/JCI86610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendivil CO, Zheng C, Furtado J, Lel J, Sacks FM. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler Thromb Vasc Biol. 2010;30:239–45. doi: 10.1161/ATVBAHA.109.197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollin TI, Damcott CM, Shen H, Ott SH, Shelton J, Horenstein RB, Post W, McLenithan JC, Bielak LF, Peyser PA, Mitchell BD, Miller M, O’Connell JR, Shuldiner AR. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science. 2008;322:1702–5. doi: 10.1126/science.1161524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjærg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med. 2014;371:32–41. doi: 10.1056/NEJMoa1308027. [DOI] [PubMed] [Google Scholar]
- 12.TG and HDL Working Group of the Exome Sequencing Project, Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014;371:22–31. doi: 10.1056/NEJMoa1307095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng C, Azcutia V, Aikawa E, Figueiredo J-L, Croce K, Sonoki H, Sacks FM, Luscinskas FW, Aikawa M. Statins suppress apolipoprotein CIII-induced vascular endothelial cell activation and monocyte adhesion. Eur Heart J. 2013;34:615–24. doi: 10.1093/eurheartj/ehs271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.VanderWeele TJ. Mediation Analysis: A Practitioner’s Guide. Annu Rev Public Health. 2016;37:17–32. doi: 10.1146/annurev-publhealth-032315-021402. [DOI] [PubMed] [Google Scholar]
- 15.Varbo A, Benn M, Tybjærg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128:1298–309. doi: 10.1161/CIRCULATIONAHA.113.003008. [DOI] [PubMed] [Google Scholar]
- 16.Wyler von Ballmoos MC, Haring B, Sacks FM. The risk of cardiovascular events with increased apolipoprotein CIII: A systematic review and meta-analysis. J Clin Lipidol. 2015;9:498–510. doi: 10.1016/j.jacl.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Scheffer PG, Teerlink T, Dekker JM, Bos G, Nijpels G, Diamant M, Kostense PJ, Stehouwer CDA, Heine RJ. Increased plasma apolipoprotein C-III concentration independently predicts cardiovascular mortality: the Hoorn Study. Clin Chem. 2008;54:1325–30. doi: 10.1373/clinchem.2008.103234. [DOI] [PubMed] [Google Scholar]
- 18.Pechlaner R, Tsimikas S, Yin X, Willeit P, Baig F, Santer P, Oberhollenzer F, Egger G, Witztum JL, Alexander VJ, Willeit J, Kiechl S, Mayr M. Very-Low-Density Lipoprotein-Associated Apolipoproteins Predict Cardiovascular Events and Are Lowered by Inhibition of APOC-III. J Am Coll Cardiol. 2017;69:789–800. doi: 10.1016/j.jacc.2016.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qamar A, Khetarpal SA, Khera AV, Qasim A, Rader DJ, Reilly MP. Plasma apolipoprotein C-III levels, triglycerides, and coronary artery calcification in type 2 diabetics. Arterioscler Thromb Vasc Biol. 2015;35:1880–8. doi: 10.1161/ATVBAHA.115.305415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borén J, Watts GF, Adiels M, Söderlund S, Chan DC, Hakkarainen A, Lundbom N, Matikainen N, Kahri J, Vergès B, Barrett PHR, Taskinen M-R. Kinetic and Related Determinants of Plasma Triglyceride Concentration in Abdominal Obesity: Multicenter Tracer Kinetic Study. Arterioscler Thromb Vasc Biol. 2015;35:2218–24. doi: 10.1161/ATVBAHA.115.305614. [DOI] [PubMed] [Google Scholar]
- 21.Chapman MJ, Le Goff W, Guerin M, Kontush A. Cholesteryl ester transfer protein: at the heart of the action of lipid-modulating therapy with statins, fibrates, niacin, and cholesteryl ester transfer protein inhibitors. Eur Heart J. 2010;31:149–64. doi: 10.1093/eurheartj/ehp399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rapp JH, Harris HW, Hamilton RL, Krupski WC, Reilly LM, Ehrenfeld WK, Stoney RJ, Goldstone J, Kane JP. Particle size distribution of lipoproteins from human atherosclerotic plaque: a preliminary report. J Vasc Surg. 1989;9:81–8. [PubMed] [Google Scholar]
- 23.Ylä-Herttuala S, Lipton BA, Rosenfeld ME, Goldberg IJ, Steinberg D, Witztum JL. Macrophages and smooth muscle cells express lipoprotein lipase in human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991;88:10143–7. doi: 10.1073/pnas.88.22.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindqvist P, Ostlund-Lindqvist AM, Witztum JL, Steinberg D, Little JA. The role of lipoprotein lipase in the metabolism of triglyceride-rich lipoproteins by macrophages. J Biol Chem. 1983;258:9086–92. [PubMed] [Google Scholar]
- 25.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–81. [PubMed] [Google Scholar]
- 26.El Harchaoui K, van der Steeg WA, Stroes ESG, Kuivenhoven JA, Otvos JD, Wareham NJ, Hutten BA, Kastelein JJP, Khaw K-T, Boekholdt SM. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:547–53. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, JUPITER Study Group Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


