Abstract
Reduction of salivary nitrate to nitrite by oral microbes expressing nitrate-reductase has emerged as a crucial pathway in systemic NO homeostasis in humans and other mammals. Selective depletion of oral microbes prevents dietary nitrate-dependent lowering of blood pressure, inhibition of platelet aggregation and ischemic injury. To date, most studies interrogate enterosalivary nitrate reduction by following changes in saliva or plasma nitrite and NO-signaling (functional) end points. Little is known about whether, and if so how, nitrate-reductase enzymatic activity per se (i.e. independent of nitrate levels) is a variable and may account for any individual to individual variation. Here, we describe a minimally invasive protocol that allows for NR activity determination from human, rat and mouse tongue scrapes/swabs. We validate this method using selective application of antiseptic agents to the distal tongue surface which decreased NR activity by >80% and show that bacterial number is a significant variable in measured NR activities between males and females. Also, we show that NR activity is >80% lower in smokers (humans) and after bromine gas exposure (mice), suggesting that exposure to inhaled reactive substances inhibit NR activity identifying a potentially new mechanism by which environmental toxicants promote dysfunction in NO-bioavailability. The described method will facilitate studies testing whether NR specific activity is a variable in different pathophysiologic settings, and in turn how this activity modulates enterosalivary nitrate-reduction.
Keywords: nitric oxide, antiseptic, chlorohexidine, microbiome, smoking, halogen, nitrite, oral, saliva, inhaled irritants
Introduction
While the major source of nitric oxide (NO) in mammals are the nitric oxide synthases (NOS), recent studies have shown alternate sources also exist. Specifically, dietary nitrate can be reduced to nitrite, which in turn is reduced to NO and other nitrosating species at low oxygen tensions and low pH ensuring NO-homeostasis in hypoxic tissues (1, 2). Endogenously formed nitrate or dietary nitrate, is first concentrated into the saliva reaching millimolar concentrations. There, orally residing nitrate reducing bacteria, concentrated on the dorsal tongue, reduce nitrate to nitrite(3). The nitrite is swallowed after which it can be further reduced to stimulate NO-dependent signaling processes by several putative mechanisms involving metalloproteins (4, 5). Functionality of this enterosalivary nitrate circuit has been demonstrated in human and experimental models with compelling data showing that nitrate consumption lowers blood pressure, inhibits platelet aggregation, improves exercise performance and inhibits inflammatory tissue injury (6–9). Most importantly, these effects are abrogated with prior depletion of the oral microbiome using antiseptic mouthwash.
Key components of the enterosalivary circuit of NO formation are the oral facultative anaerobes expressing active nitrate reductases. Several studies have demonstrated that nitrate reductase activity is concentrated in the posterior dorsal region of the tongue with at least 15 species of bacteria expressing nitrate reductase identified from human samples (10, 11). To date, the majority of measures used to follow enterosalivary nitrate-reduction are changes in saliva and plasma nitrite levels after nitrate administration, together with functional end points (e.g. blood pressure, exercise performance or platelet aggregation). Few studies have directly measured nitrate reductase (NR) activity. In one protocol, human volunteers held potassium nitrate solution in the mouth and then nitrite formation measured after a given time. Kanady and Jones et al swabbed tongues, then added nitrate and followed nitrite formation ex vivo (12). However, to our knowledge no systematic and reproducible protocol has been described assessing oral NR activity. Our objective was to further develop a method for NR activity determination, with our primary goal being to measure NR specific activity, controlling for bacterial number. We show that under normal conditions, NR activity may vary from individual to individual, but this effect is mediated by varying amounts of bacteria collected in tongue scrapes. Our secondary goal was to develop a protocol that can be used with mice with a view to test if NR activity changes in different pathophysiological conditions. Using human and animal models, we demonstrate that NR activity is lower in instances of exposure to inhaled toxicants (such as cigarette smoke and bromine) and suggest that this may play a role in the development of diseases associated with environmental toxins.
Materials and Methods
Materials
Sterile wood cotton tipped applicators (6cm length) were purchased from Fisher (Cat. No. 23-400-115). Sterile disposable multifunction Lab spatulas were purchased from Sigma Aldrich (Z677787-100EA). 20% Chlorhexidine digluconate solution was purchased from Sigma Aldrich (C9394-25ML). Brain heart infusion (BHI) broth and tryptic soy blood agar (TSBA) was purchased from anaerobes systems (Morgan Hill, CA). Ketamine HCL 100 mg/ml and Xylazine 100 mg/ml solutions were purchased from VetOne (Boise, ID). Male and female C57/Bl6 mice (10–12 weeks, 20–25g) and Sprague Dawley rats (8–10weeks, 200–300g) were purchased from Envigo (Indianapolis, IN). All protocols involving animals were reviewed and approved by the UAB IACUC committee.
Human Subjects
Healthy human subjects who were non-smokers or active smokers (>20 cigarettes per week) were enrolled. Subjects were identified after response to fliers or using the UAB Lung Health Center database. All enrolled subjects did not use antiseptic or over the counter mouthwash (at least 1 month), were not currently or recently (last 3 months) on antibiotics. All procedures were according to UAB Institutional Review Board approved procedures.
Collection of posterior tongue swab
Human
Scrapings from the posterior tongue were collected from male and female adults using the narrow end of a sterile disposable lab spatula. Five gentle scrapes were performed in one direction left to right, followed by a further 5 scrapes in the other direction. Scrape volumes of approximately 30μl were collected. 70μl of normal saline was used to rinse the spatula and total volume (100μl) collected into eppendorf tubes. 400μL of BHI was then added to the samples which were then vortex mixed for 30–45 seconds. All protocols were approved by institutional review board.
Mice
Male and female C57/Bl6 mice (10–12weeks) were briefly anesthetized by intramuscular injection of xylazine and ketamine (7 and 70 mg/kg body weight respectively) into the lower left femoris region. The oral cavity was exposed by suspending mice from the top jaw. Tongues were gently pulled out from the oral cavity using blunted forceps. Sterile cotton tipped applicators soaked in 200 μL of sterile saline were then used to swab the posterior dorsum of the tongue with 10 identical strokes from back to front. Care was taken to swab only the posterior tongue. Mice were then returned back to cages and typically awoke within 20–30min. Applicators were placed into 1 mL of BHI and briefly vortex mixed for 30–45 seconds. After mixing, applicators were removed and placed in to empty Eppendorf tubes and centrifuged at 5,000 rpm for 45 seconds to remove excess culture liquid from the cotton fibers. Any excess culture liquid obtained was then transferred to the original 1 mL of BHI culture broth.
Rat
Male and female Sprague Dawley rats were anesthetized by intraperitoneal injection of xylazine and ketamine (4.5 and 45 mg/kg body weight respectively) and tongue swabs collected as described for mice.
All procedures involving animals were approved by institutional IACUC.
Human nitrate reductase activity
After mixing of tongue scrapes in BHI broth, two aliquots (100μl each) were incubated at 37°C for 10min. Ten μl was then taken to measure baseline nitrite levels. Water (vehicle control) was added to one aliquot, and sodium nitrate (varying concentrations) added to the other and both incubated at 37°C. At indicated times, samples were vortex mixed (~5sec) to ensure homogenous sampling and 10μl collected to measure time dependent changes in nitrite. Nitrite was measured by triiodide based reduction coupled with ozone chemiluminesence as previously described using a Sievers 280i Nitric Oxide analyzer (GE analytical instruments, Boulder, CO) (13). Nitrite levels were determined by comparison with standard curves measured daily; detection limits were 1–10pmol. In parallel, bacterial load was measured on tongue scrapes after initial dilution into BHI broth (described below). Nitrate reductase activity was calculated by normalizing initial rates of nitrite formation with corresponding colony forming units.
Mouse and rat nitrate reductase activity
Initial studies failed to show detectable changes in nitrite formation after nitrate addition to freshly collected mouse or rat tongue swabs. For this reason, swabs were split into two (each of 500 μl) and cultured in a total of 1.5 ml of BHI broth in either aerobic (21% O2) or anaerobic (0.5% O2) incubators at 37°C. For aerobic conditions the broth was cultured in a rotary shaker (200 rpm, 37°C). For anaerobic growth, medium was placed in an air sealed chamber and the inner O2 of the chamber depleted by passing N2 gas through the chamber till the O2 meter reached <0.5%. The whole chamber was then placed in the 37°C incubator. Incubation times were varied as described in results. After indicated incubation times, aliquots were taken and CFU determined, and sodium nitrate-dependent nitrite formation measured as described above.
Bacterial counts
CFU were determined using TSBA agar by the drop plate method. For human samples, 10 μl BHI containing tongues scrapes were serially diluted in sterile normal saline until 10−3 and 10−4 dilutions. For rat and mice, 10 μl were collected from BHI containing bacteria after varying culture times (0, 6, 12, 18h) and 10−5 and 10−6 dilutions then plated on TSBA agar medium. For 0h and 6h, lower dilutions (10−1 – 10−3) were also tested. For each sample, a minimum of 3 drops (10μl each) of each dilution were plated and incubated overnight at 37°C under aerobic or anaerobic conditions. CFU were then counted and averaged for the 3 drops, for each dilution.
Bacterial Growth curve measurement
Posterior tongue swabs were collected with a cotton tip applicator from C57/Bl6 mice as described above. The cotton tip was then placed into 3 ml of BHI, vortex mixed and removed. The medium was then incubated at 37°C for 24h, at 21%O2. At various times over 24h the absorbance of the culture medium was measured at 600nm. Fresh culture medium was used as blank.
Chlorohexidine administration
0.2% chlorohexidine solution prepared from a 20% stock solution. After anesthesia, 10μl of 0.2% chlorohexidine was administered topically to the posterior tongue. Administration frequency (once or twice a day), and number of days was varied to determine optimal conditions as described in results. For twice a day protocol, mice were anesthetized each time and administered chlorohexidine 6–8h apart (between 8.30–9.30am and 4.30–5.30pm). Tongue swabs were collected as described above and at least 2h after the last dose of chlorohexidine. All experiments included water or saline vehicle control.
Dietary nitrate administration
C57Bl/6 mice were provided water or water supplemented with nitrate (3mg/L) ad libitum for 2 weeks. Water was replaced every 3 days. During the last 7 days, mice were also randomized to receive water or chlorohexidine (0.2%. 10ul), twice a day, applied topically to the posterior tongue as outlined above.
Bromine exposure
C57/Bl6 male and female mice (10–12 weeks) were exposed to bromine (600 ppm, 30 min) or air as previously described (14). Mice tongue swab was collected 6h post exposure followed by NR activity measurement and CFU counting as explained above.
Smoke effect on ex-vivo nitrate reductase activity
9 healthy non-smoking human adults (age, 20–45) and 5 healthy smoking adults (age 30–60) were enrolled and tongue scrapes collected for ex vivo NR activity assessment. Inclusion criteria included no gum disease, no gingivitis, no use of antibiotics in the last 3 months, and no use over the counter mouthwash. All procedures were performed according to IRB approved protocols.
Statistical analysis
All data are presented as mean ± S.E.M. Statistical analyses were performed using Student’s t test or 1-way or 2-way ANOVA analyses as described in figure legends. Data outlier tests were performed using the ROUT (Q=5%) analysis function in GraphPad Prism software and indicated where applied in figure legends. Significance was set at p<0.05.
Results
Nitrate reductase activity assessment from the human tongue
Samples were collected by scraping the posterior tongue from 3 healthy adult volunteers. Supplementary Figure 1A shows that nitrate addition led to a time dependent increase in nitrite formation; minimal changes in nitrite were observed with vehicle control. Figure 1A shows that nitrite was formed in a nitrate dose-dependent manner which was linear over a large range (0–10mM). Supplementary Figure 1B shows the nitrate-dependent nitrite formation at lower initial concentrations and also demonstrates significant nitrite formation at the lowest (100μM) nitrate concentration tested. Based on experimental sensitivity to discern changes in nitrite, reported levels of nitrate in the saliva, and to ensure nitrate-dependent nitrite formation was in the linear range, we chose 1mM nitrate as a dose to test further. This also allowed comparison to nitrate-reductase activity from mouse and rat tongues (see below). Figure 1B shows time dependent nitrite formation from tongue scrapes collected from 4 males and 4 females; supplementary Figure 2 shows data from each individual donor (F1–F5 and M1–M5). In all cases, nitrite formation remained low in the absence of added nitrate. Addition of nitrate resulted in nitrite formation, the kinetics of which showed significant donor to donor variation (~3–4 fold between donors). A notable exception was M3. This sample showed no change in nitrite formation after addition of nitrate, but nitrite levels were at 1mM suggesting that either nitrate-reductase activity was extremely low (no nitrate-dependent change in nitrite) or extremely high with complete reduction of added nitrate to nitrite within time taken to mix and sample the first point. This profile was only observed once (see discussion) and therefore excluded from the cumulative data shown in Fig 1C that plots the average levels of nitrite formation versus time, and shows no differences in nitrate reduction between males and female samples. Interestingly, Fig 1C shows that nitrate reduction per bacteria (CFU counts are shown in supplementary Figure 2) was higher in female samples.
Figure 1. Ex-vivo nitrate reductase (NR) activity of human posterior tongue.
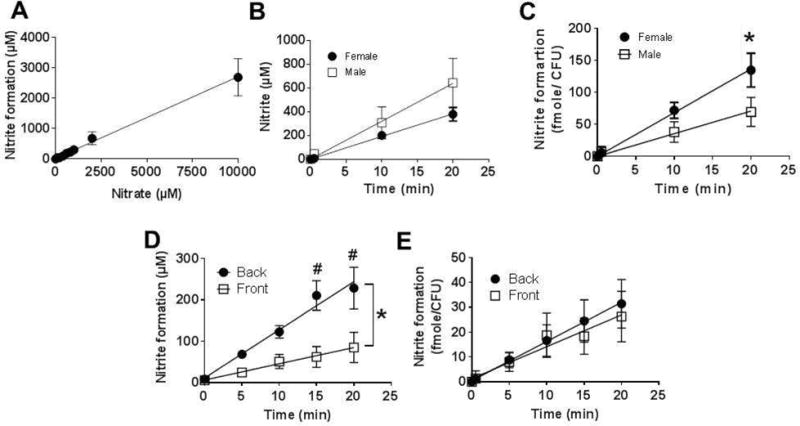
Panel A: Tongue scrapes were collected from 3 volunteers and incubated for 10min in BHI broth, 37°C. Nitrate (at indicated doses) was then added and nitrite formation measured after 40min. Data are mean ± SEM (n=4, except for nitrate doses 200–800μM, where n=3). Panel B: Average time-dependent nitrite formation (after nitrate addition) in male (□) and female (●) samples. Data are mean ± SEM (n=5 (female) and n=4 (male)), Panel C: Average time-dependent nitrite formation (after nitrate addition) normalized to CFU in male and female samples. Data are mean ± SEM (n=5 (female) and n=4 (male)) *P<0.05 by 2-way ANOVA. Panel D: Nitrate reduction to nitrite was measured in paired tongue scrapes collected from the front and back of the tongue. Data show mean ± SEM (n=5 different donors). *P<0.05 by 2-way ANOVA and #P<0.05 by Sidak’s Multiple comparison test. Panel E: data from Panel D normalized to CFU.
To further validate this method, we compared nitrate reductase activity from scrapes collected from the front third and back third of the tongue. Figure 1D shows that nitrite formation was 3–4 fold greater in posterior tongue scrapes consistent with previous studies showing that nitrate reductase activity is concentrated in the posterior compartment (15). Figure 1E shows this difference is due to the number of bacteria, as no difference in nitrate-reduction was observed after normalizing to CFU.
Nitrate reductase activity assessment from the mouse and rat tongue
To our knowledge, oral nitrate reductase activity has not been measured from the mouse tongue. To test this, we collected tongue swabs from male C57/Bl6 mice and assessed nitrate-reductase activity as described in Figure 1. No nitrate-dependent nitrite formation was detectable on freshly collected samples (Figure 2A, time zero). We reasoned this may be due to a combination of limited experimental sensitivity to detect very small changes in nitrite, together with the limited number of bacteria owing to the smaller sampling size from a mouse tongue. To test this, swabs were incubated at 37°C in BHI broth under aerobic or anaerobic conditions for varying times. Nitrate reductase activity was still not detectable at 6h, however was detectable at 12h and 18h which also coincided with an increase in bacterial number (Figure 2B). No differences between aerobic and anaerobic cultures were observed. Supplementary Figure 3 shows growth curves for bacteria isolated from mouse tongues. Using 18h culture conditions, a time where bacteria were in late log phase of growth, Figure 3A shows the nitrate-dependence on nitrite formation. Nitrite formation was minimal at low nitrate concentrations but when above 400μM, nitrite formation was linear with nitrate. Therefore, we decided to use 1mM nitrate and 18hr incubation period for mice tongue swab for determining NR activity. Supplementary Figure 4 shows time dependent nitrite formation from tongue swabs collected from 5 male mice, after culturing under aerobic of anaerobic conditions, together with CFU counts. No nitrite was formed in the absence of added nitrate. Fig 3B–C show nitrate reduction was similar in males compared to females with aerobic and anaerobic cultures, although rates were slightly higher with males under aerobic conditions. Normalizing nitrate reductase activity to CFU (Fig 3D–E) showed no differences between aerobic and anaerobic cultures. We also tested this method with rats and observed a similar requirement for culturing swabs for >12h before significant NR activity was measurable (not shown).
Figure 2. Ex-vivo nitrate reductase activity in posterior tongue swabs from mice.
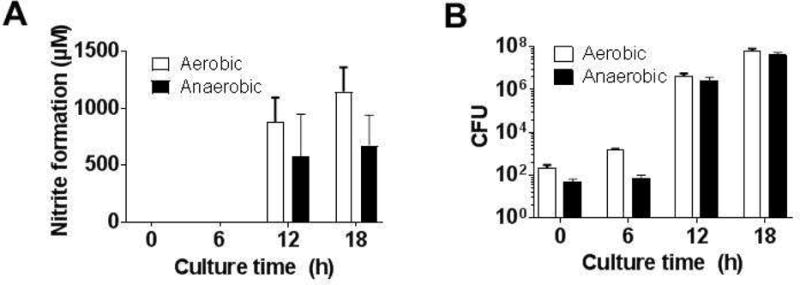
Panel A. Nitrate (1mM) was added to tongue swabs immediately after collection or after culturing for the indicating times in BHI at 37°C under aerobic or anaerobic conditions. Panel B: CFU counts from Panel A. Data are mean ± SEM (n=2 for 0 and 6h group; n=4 for 12 and 18h groups) p<0.05 by 1-way ANOVA for significant time dependent changes for aerobic and anaerobic cultures.
Figure 3. Ex-vivo nitrate reductase activity in posterior tongue swabs from male and female mice.
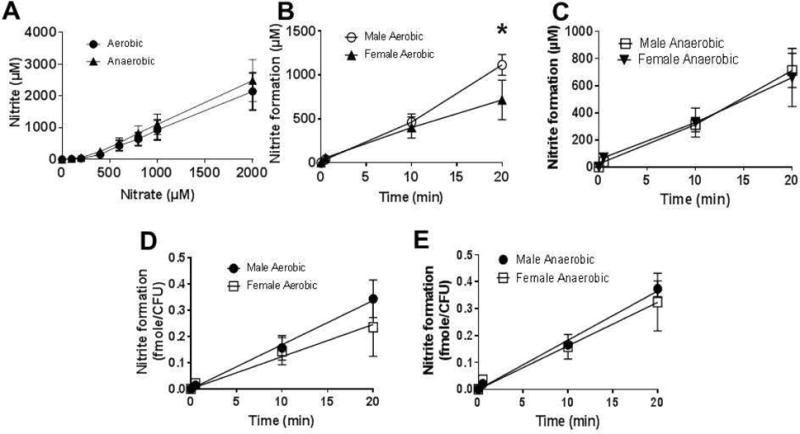
Panel A: Nitrate (at indicated doses) was added to 18h aerobic or anaerobic cultures and nitrite formation measured after 40min. Data are mean ± SEM (n=3). Panel B: average nitrite formation versus time traces for aerobic cultures. *P<0.05 by 2-way ANOVA with Sidak’s post-test (n=4 (females), n=5 (males)). Error bars show SEM. Panel C: average nitrite formation versus time traces for anaerobic cultures (n=4 (females), n=4 (males). Panel D: Nitrate formation normalized to CFU for aerobic cultures. Data are mean ± SEM, n=4 (females), n=5 (males). Panel E: Nitrite formation normalized to CFU for anaerobic cultures. Data are mean ± SEM, n=4 (females), n=4 (males).
Effects of chlorohexidine on oral nitrate reductase activity
To test and validate the assay, 0.2% chlorohexidine was topically administered to the posterior tongue of male mice with the frequency and duration of administration varied into 3 groups: 3 days twice daily, 7 days once daily, and 7 days twice daily. Nitrate reductase activity was then determined as outlined in Figure 2–3. Figure 4A–C respectively show nitrate reduction versus time, CFU and initial rate of nitrate-reductase activity normalized to CFU. Compared to control, administration of 0.2% chlorohexidine twice daily for 3 days increased nitrate reductase activity, whereas treatment for 7 days twice daily decreased nitrate reductase activity. Normalizing to bacterial number indicated that only the twice daily 7 days regimen led to inhibition of nitrate-reductase, by ~90% (Fig 4C). Despite nitrate reductase activity being lower in chlorhexidine treated groups, surprisingly this was not due to lower bacterial number. Figure 4B shows similar CFU counts across all groups. However, these measurements were made after 18h of bacterial growth in BHI. Figure 4D shows CFU counts from tongue swabs collected from control or chlorohexidine treated mice at the time of collection and after 18h of culturing in BHI broth. Bacterial counts were significantly (p < 0.05) lower at the time of collection in chlorohexidine groups compared to control, whereas at 18h, bacterial counts were similar.
Figure 4. Effects of chlorohexidine on nitrate reductase activity in mice.
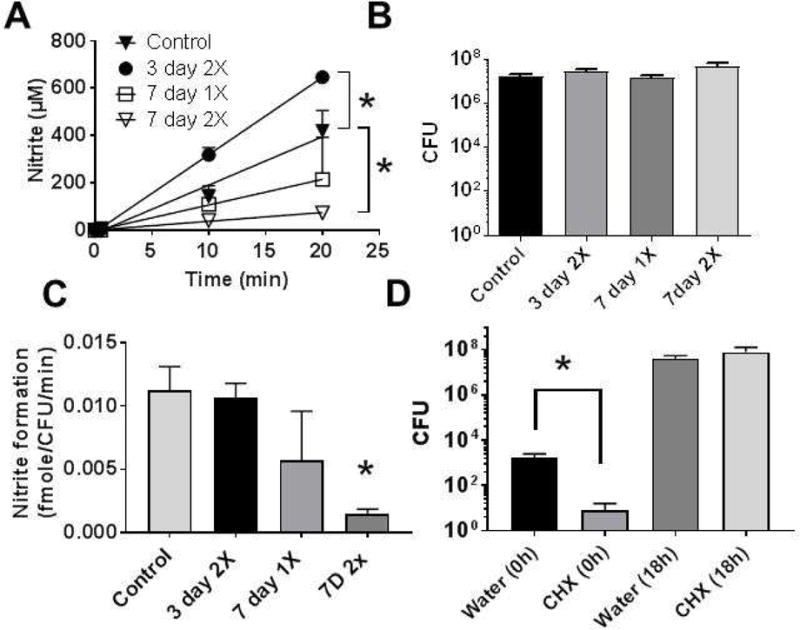
Chlorohexidine (0.2%) was administered directly onto the posterior tongue in a 10μl volume either once daily, 7d, or twice daily 3d or 7d and then nitrate reductase activity measured (Panel A), CFU measured after 18h culture in BHI (Panel B) and initial rate of nitrate reductase activity normalized to CFU calculated (Panel C). Data are mean ± SEM (n=3 for 3 days twice daily; n=3 for 7 days once daily; n=6 for 7 days twice daily, n=7 for vehicle control). Panel D shows CFU counts from vehicle (water) or chlorohexidine treated mice (2× day, 7days), upon collection of tongue swabs or after 18 culturing in BHI broth. Data show mean ± SEM (n=5 in each group). *P<0.05 by unpaired t-test. Two data points from 7 days twice daily and one data point from vehicle control were excluded by ROUT analysis.
To test if chlorohexidine dependent inhibition of NR activity was sufficient to inhibit enterosalivary nitrate-reduction, mice were provided control or nitrate-supplemented drinking water for 2 weeks, with chlorohexidine being administered for the last week. No differences in water consumption or weight gain were observed (not shown). Figure 5A–C respectively show NR activity, CFU and NR activity normalized to CFU. Consistent with Figure 4, chlorohexidine decreased NR activity by ~60–70% (Fig 5A). Interestingly, nitrate supplementation also led to an inhibition of oral NR activity by ~50% compared to control. Figure 5B shows bacterial counts did not change significantly. When the bacterial number was accounted for, NR specific activity was the same in control and nitrate supplemented groups, albeit with a trend towards lower activity per bacteria in the nitrate supplemented group noted. Chlorohexidine treatment inhibited activity in all groups (Figure 5C). Figure 5D shows the effect of nitrate supplementation and chlorohexidine on plasma nitrite levels. Chlorohexidine alone had no effect on plasma nitrite. Nitrate-supplementation increased nitrite ~two-fold relative to control, which was prevented in chlorohexidine treated mice.
Figure 5.
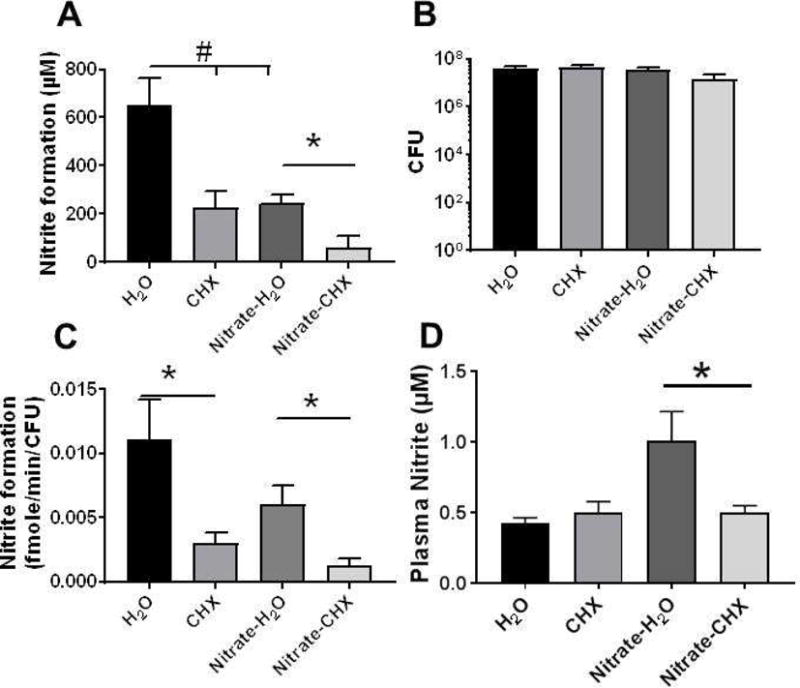
Male C57Bl/6 male mice received water or water supplemented with nitrate for 2 weeks. Chlorohexidine (0.2%) was administered directly onto the posterior tongue in a 10μl volume twice daily for the last 7d. Panel A: Oral nitrate reductase activity was measured as described in Figure 3. Data show nitrite levels 20min after addition of nitrate to tongue swabs Panel B: CFU measured after 18h culture in BHI. Panel C: Initial rate of nitrate reductase activity normalized to CFU. All data are mean ± SEM (H2O n=8, CHX n=8, Nitrate-H2O n=7, Nitrate-CHX n=5). *p<0.05 by unpaired t-test; #p<0.05 by 1-way ANOVA with Tukey post-test. One outlier from Nitrate-H2O and two outliers from nitrate-CHX removed by ROUT analysis. Panel D shows the nitrite levels in plasma. Data are mean ± SEM (n=8 except for H2O group where n=9). *p<0.05 by 1-way ANOVA with Tukeys post-test.
Altered NR activity after exposure to inhaled irritants and toxicants
Developing an NR activity assay may facilitate testing of whether this component of the enterosalivary nitrate-reduction cycle varies in different pathophysiological settings. We hypothesized that NR activity may be lower in instances of inhaled irritant exposure, specifically when the oral cavity is exposed to reactive substances that may inhibit NR activity and/promote oral dysbiosis. To test this, we first assessed if cigarette smoking is associated with altered NR activity. Cigarette smoking exposes the oral cavity to numerous reactive substances and is associated with systemic inflammation characterized by diminished NO-bioavailability. Posterior lingual samples were collected from current adult smokers, who are otherwise healthy. NR activity was measured and compared to NR activities measured from healthy non-smokers shown in Figure 1. Fig 6A shows that the NR activity in smokers was decreased ~70%, whereas bacterial number was higher (Fig 6B). NR activity per CFU was almost 90% lower in smokers compared to non-smokers (Fig 6C).
Figure 6.
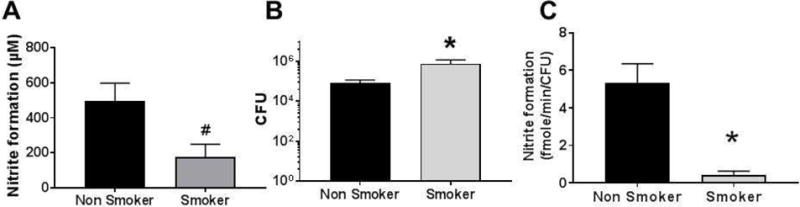
Scrapes from the posterior tongue were collected from healthy adult non-smokers (n=9; 4 males and 5 females, age range 20–45y as shown in Fig 1), or smokers (n=5; 3 male and 2 female, age range 31–60y). NR activity (Panel A), bacterial count (Panel B), and initial rate of nitrate activity normalized with CFU (Panel C) was calculated. Data are mean ± SEM. #p=0.05, *p<0.05 unpaired t-test.
To test if exposure to reactive substances inhibits NR activity in mice, we used a Br2 gas exposure model as recently described (14). Mice were exposed to air or Br2 (600ppm, 30 min), and then brought back to room air. Tongue swabs were collected 6h thereafter, cultured for 18h and then NR activity determined. Similar to the case with human smokers, Figure 7A shows that NR activity was inhibited by >70%, despite similar levels of bacteria (Fig 7B). Expressing NR activity per CFU showed an even greater inhibition in Br2 exposed mice compared to air-exposed mice (Fig 7C).
Figure 7.
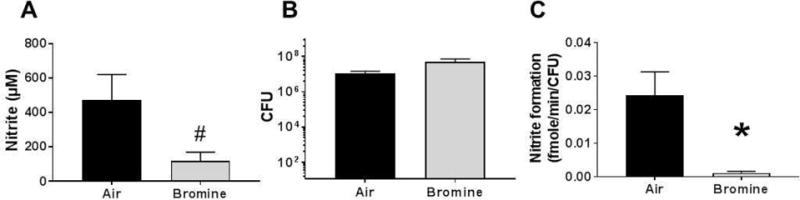
C57/Bl6 mice were exposed to bromine (600 ppm) for 30 min and then brought back to room air. 6h thereafter, tongue swab was collected and cultured in BHI for 18h. NR activity was then determined by adding nitrate (1mM). Panel A shows nitrite formed after 20 min. Panel B shows CFU counts and Panel C corresponding rate of nitrate reductase activity over 20 min normalized to CFU. Data are mean ± SEM (n=7 for Air and n=6 for bromine). #p=0.05, *P < 0.05 by unpaired t-test. One outlier from air and two outlier from bromine group removed by ROUT analysis.
Discussion
The enterosalivary nitrate reduction system has emerged as a key player in oral and systemic NO-homeostasis. Most studies testing this pathway rely on manipulating nitrate as the variable (by providing nitrate or appropriate control in the diet), or removing oral cavity bacteria (by antiseptic mouthwash). The oral microbiome diversity has also gained interest in this regard and as indicated by recent studies, multiple bacterial species may be responsible for nitrate reduction and may change under different environmental and dietary regimens (10, 11). However, few studies have directly measured nitrate-reductase activity in humans and even less in widely used mouse or rat based experimental models (3, 12). In this study, we further developed methods for measuring nitrate reductase activity in humans and then optimized these for use in mice and rats.
Nitrate addition to human tongue swabs demonstrated a dose-dependent increase in nitrite formation over a wide nitrate concentration range (0–2mM). This encompasses reported nitrate levels in saliva (μM–mM), suggesting that substrate saturation is unlikely in any dietary nitrate scenario. Also consistent with previous reports, nitrate reductase activity was concentrated on the back of the tongue being 3–4-fold greater compared to the front of the tongue. This was due to more nitrate-reductase containing bacteria residing on the posterior tongue, as no differences in rates normalized to CFU were observed with anterior and posterior swabs. Importantly, significant donor to donor heterogeneity in nitrite formation rates was observed within males or females. Also notable was that CFU counts were lower in females. However, after normalizing to bacterial number, nitrate reductase activities were higher in females. How this translates to the efficacy of nitrate-dependent modulation of NO-signaling is now known. Interestingly, a lower efficacy for nitrate in preventing platelet aggregation and blood pressure lowering in females has been reported (16). Collectively, these data suggest that the number of bacteria represent a donor to donor variable in the enterosalivary nitrate reduction pathway. In addition, we show that NR activity was lower in smokers compared to non-smokers, which was not due to total bacteria collected in swabs suggesting altered bacterial diversity or selective inhibition of NR activity. Whether lower NR activity in smokers introduces a limitation in the enterosalivary cycle is not known and we appreciate the limitations associated with low sample size and possible influence of other demographic factors in these data. We present these data to show that NR activity may be lower in certain settings and speculate that lower NR activity may underlie, in part, decreased NO-bioavailability in smokers. In addition, a recent paper demonstrates cigarette smoking attenuated nitrate-dependent lowering of blood pressure via increases in thiocyanate, the latter competing with nitrate transport into the saliva(17). Collectively, these observations underscore the potential for cigarette smoking to inhibit the enterosalivary nitrate-reductase system.
Even less is known about mouse or rat oral nitrate reduction. Dietary nitrate or nitrate administered by gavage results in lowering of blood pressure and improves angiogenesis (18, 19). This effect is attenuated by oral antiseptic administration supporting a role for oral microbes. While this suggests a similar enterosalivary nitrate reduction circuit in rodents compared to humans, recent studies also indicate nitrate is not concentrated into the saliva and that in rodents other nitrate-reducing mechanisms involving xanthine oxidoreductase for example, may work in parallel with oral nitrate-reductase expressing bacteria to control nitrite levels and NO-signaling (20). Our data show that nitrate-reductase activity was undetectable in freshly collected tongue swabs from mice. However, bacteria were present and upon culturing for >12h, activity was detectable suggesting that lack of detection at baseline is due to limited sensitivity due to low bacteria levels in swabs collected. Similar results were observed with rats (data not shown).
Validation of the method was provided by the effects of chlorohexidine administration locally to the posterior tongue. Interestingly, this decreased bacterial number at the time of collection, but did not affect bacterial number after 18h culturing. Despite the similar numbers, nitrate-reductase activity was inhibited by >90% however. This suggests that nitrate-reductase expressing bacteria were less abundant and/or less able to grow in culture after chlorohexidine exposure compared to other bacteria and raises interesting questions regarding whether nitrate-reductase expressing bacteria are more sensitive to oral antiseptics compared to other oral microbes. Moreover, despite inhibiting nitrate reductase activity no changes in plasma nitrite were observed. This may indicate that oral nitrate reduction is not limiting in the enterosalivary system and that higher levels of inhibition are required before plasma nitrite levels are affected. Alternatively, these data could reflect a minimal role for endogenous nitrate and oral nitrate-reduction in regulating plasma nitrite levels in mice under basal conditions. A role for oral nitrate reduction was evident however, when mice were supplemented with dietary nitrate suggesting that this pathway is more prominent in regulating plasma nitrite at higher nitrate levels. Surprisingly, we also observed that nitrate-supplementation led to a decrease in oral nitrate-reductase activity (Fig 5A). After normalization to bacterial number however, no significant difference was observed (Fig 5C), suggesting nitrate supplementation affected bacterial number rather than specific activity. Little is known regarding how dietary factors regulate the oral nitrate-reducing microbiome. While our data with mice indicate bacterial levels are lower, a recent study with hypercholesterolemic patients indicated that dietary nitrate exposure enriches for nitrate-reductase containing bacteria as assessed by microbiome sequencing (21).
Our results also underscore a limitation and the potential introduction of artifacts when culturing bacteria collected by swabs, and then using the bacterial number thereafter to normalize nitrate-reductase activity. Indeed, a major hurdle in oral nitrate-reductase activity assessments is lack of understanding of which bacteria express nitrate-reductase activity, and what is the optimal method to normalize for the number of bacteria present. Despite these limitations, as proof-of concept studies to test if this method could observe changes in NR activity, we exposed mice to Br2 gas. Bromine is a reactive halogen that causes direct airway injury leading to acute lung injury and systemic inflammation (14). Our data indicate that Br2 exposure significantly attenuated NR activity measured several hours after the exposure. Similar to the effects of chlorohexidine, bacterial number was not different suggesting that Br2 exposure caused dysbiosis or selective inhibition of NR containing bacteria. Further studies testing whether Br2 or cigarette smoke inhibit NR activity and/or promote growth of bacteria unable to reduce nitrate are warranted. Interestingly, our previous studies have shown that the related halogen, Cl2 gas, induces systemic injury characterized by loss of NO-bioavailability (22). We speculate that loss of NR activity and disruption of the enterosalivary nitrate reduction circuit may contribute to these systemic effects of halogen gas exposure.
There are several other limitations that require attention. Most significant and one that applies to any study sampling the lingual microbes, is that the method tested relies on sampling of tongue by swabbing or gentle scraping. NR expressing bacteria reside deep in the crypts of the tongue, and to what extent sampling captures deeper residing bacteria is not known. One approach to possible overcome this limitation is to administer a solution of nitrate, hold it in the oral cavity for some time (mins) and then recover this solution and measure nitrite produced (3). The other is to sample bacteria directly from the tongue, add nitrate and measure nitrite formation ex vivo(12). We opted to utilize the latter approach as it more amenable to clinical and experimental testing requiring a relatively simple swab collection, allows for more detailed kinetic evaluation of nitrite formation, and bacteria can be collected and characterized using biochemical, molecular or genetic approaches, and this can be directly linked to nitrate-reductase activity. Related to these considerations is to how redundant is NR activity. In other words, is NR activity a limiting step in enterosalivary nitrate-dependent stimulation of NO-signaling, or to what extent does NR activity have to be inhibited before inhibition of nitrate-dependent NO-signaling is observed. It is clear that flux through this pathway can be increased by providing substrate nitrate. We suggest that future studies, incorporate NR activity assessments with other measures of nitrite formation, NO-signaling end points to evaluate these key questions.
Additional salient considerations regarding measuring NR activity ex vivo include observations that nitrite may be formed before addition of nitrate requiring its subtraction from nitrate-dependent increases in nitrite. Also, in some cases time dependent formation of nitrite appeared to be autocatalytic for both human and mouse samples, which was most evident after 30min incubation resulting in increases in nitrite levels greater than the amount of nitrate added (e.g. see M1 and F2, supplementary Fig 2). This is likely due changes in bacterial metabolism and growth under these conditions leading to formation of nitrite independent of any nitrate-dependent nitrite formation. Assuming this is a variable, any method evaluating NR activity ex vivo, necessitates time dependent measures of nitrite formation both pre- and post-nitrate addition. We recommend assessing nitrite at 0, 5, 10min pre-addition of nitrate, and then 5, 10, 15, 20 min post nitrate addition. Rates of nitrite formation pre- and post-nitrate are determined, the former subtracted to calculate nitrate-dependent initial rates of nitrite formation followed by normalization to CFU. We found this approach greatly decreases variance between donors suggesting bacterial amount is the major variable. Another interesting consideration is to what extent is the oral microbiome depleted by sampling itself. This is likely to be more a consideration with mice, where each sampling is likely to deplete a greater percent of the total bacteria, due to lower initial numbers. Indeed, we observed that repeated daily assessment of mouse NR activity showed decreased NR activity each successive day (not shown). We suggest effects of sampling on basal NR activity be considered as a variable in future study designs.
In summary, we present a method and associated limitations for measuring lingual NR activity in humans and small rodent models. We show that bacterial number is a significant variable in NR activity, and that exposure to inhaled reactive substances is associated with lower NR activities. Future studies evaluating the impact of varied NR activities on nitrate-dependent nitrite formation, and subsequent NO-bioavailability are required to assess whether ex vivo NR activity could be an effective biomarker for the functionality of the enterosalivary nitrate-reduction circuit.
Supplementary Material
Highlights.
Method for measuring oral nitrate-reductase activity from humans and mice is developed and tested
Bacterial content is a significant variable in nitrate-reductase activity ex vivo
Cigarette smoking or exposure to bromine gas inhibits nitrate-reductase activity
Acknowledgments
This research was supported by the CounterACT Program, National Institutes of Health, Office of the Director, and the National Institute of Environmental Health Sciences, Grant Number U01ES023759; U01 ES02645802 and 1U01ES027697-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR, Jr, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 3.Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci. 2005;113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 4.Lundberg JO, Gladwin MT, Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov. 2015;14:623–641. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 5.Hezel MP, Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015;21:7–16. doi: 10.1111/odi.12157. [DOI] [PubMed] [Google Scholar]
- 6.Bailey SJ, Varnham RL, DiMenna FJ, Breese BC, Wylie LJ, Jones AM. Inorganic nitrate supplementation improves muscle oxygenation, O(2) uptake kinetics, and exercise tolerance at high but not low pedal rates. J Appl Physiol (1985) 2015;118:1396–1405. doi: 10.1152/japplphysiol.01141.2014. [DOI] [PubMed] [Google Scholar]
- 7.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapil V, Weitzberg E, Lundberg JO, Ahluwalia A. Clinical evidence demonstrating the utility of inorganic nitrate in cardiovascular health. Nitric Oxide. 2014;38:45–57. doi: 10.1016/j.niox.2014.03.162. [DOI] [PubMed] [Google Scholar]
- 9.Kapil V, Haydar SM, Pearl V, Lundberg JO, Weitzberg E, Ahluwalia A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med. 2013;55:93–100. doi: 10.1016/j.freeradbiomed.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A, Van Eijl S, Sagi-Kiss V, Chowdhury TA, Curtis M, Kuhnle GG, Wade WG, Ahluwalia A. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2016;103:25–38. doi: 10.3945/ajcn.115.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyde ER, Andrade F, Vaksman Z, Parthasarathy K, Jiang H, Parthasarathy DK, Torregrossa AC, Tribble G, Kaplan HB, Petrosino JF, Bryan NS. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PLoS One. 2014;9:e88645. doi: 10.1371/journal.pone.0088645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanady JA, Aruni AW, Ninnis JR, Hopper AO, Blood JD, Byrd BL, Holley LR, Staker MR, Hutson S, Fletcher HM, Power GG, Blood AB. Nitrate reductase activity of bacteria in saliva of term and preterm infants. Nitric Oxide. 2012;27:193–200. doi: 10.1016/j.niox.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang JD, Jr, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, Liu Y, Jhala N, Crowe DR, Smith AB, Cross RC, Frenette L, Kelley EE, Wilhite DW, Hall CR, Page GP, Fallon MB, Bynon JS, Eckhoff DE, Patel RP. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal S, Lam A, Bolisetty S, Carlisle MA, Traylor A, Agarwal A, Matalon S. Heme Attenuation Ameliorates Irritant Gas Inhalation-Induced Acute Lung Injury. Antioxid Redox Signal. 2016;24:99–112. doi: 10.1089/ars.2015.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1:546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 16.Velmurugan S, Kapil V, Ghosh SM, Davies S, McKnight A, Aboud Z, Khambata RS, Webb AJ, Poole A, Ahluwalia A. Antiplatelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex. Free Radic Biol Med. 2013;65:1521–1532. doi: 10.1016/j.freeradbiomed.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey SJ, Blackwell JR, Wylie LJ, Holland T, Winyard PG, Jones AM. Improvement in blood pressure after short-term inorganic nitrate supplementation is attenuated in cigarette smokers compared to non-smoking controls. Nitric Oxide. 2016;61:29–37. doi: 10.1016/j.niox.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro LC, Amaral JH, Ferreira GC, Portella RL, Ceron CS, Montenegro MF, Toledo JC, Jr, Tanus-Santos JE. Gastric S-nitrosothiol formation drives the antihypertensive effects of oral sodium nitrite and nitrate in a rat model of renovascular hypertension. Free Radic Biol Med. 2015;87:252–262. doi: 10.1016/j.freeradbiomed.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 19.Hendgen-Cotta UB, Luedike P, Totzeck M, Kropp M, Schicho A, Stock P, Rammos C, Niessen M, Heiss C, Lundberg JO, Weitzberg E, Kelm M, Rassaf T. Dietary nitrate supplementation improves revascularization in chronic ischemia. Circulation. 2012;126:1983–1992. doi: 10.1161/CIRCULATIONAHA.112.112912. [DOI] [PubMed] [Google Scholar]
- 20.Montenegro MF, Sundqvist ML, Nihlen C, Hezel M, Carlstrom M, Weitzberg E, Lundberg JO. Profound differences between humans and rodents in the ability to concentrate salivary nitrate: Implications for translational research. Redox Biol. 2016;10:206–210. doi: 10.1016/j.redox.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A, Van Eijl S, Sagi-Kiss V, Chowdhury TA, Curtis M, Kuhnle GG, Wade WG, Ahluwalia A. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2015 doi: 10.3945/ajcn.115.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honavar J, Samal AA, Bradley KM, Brandon A, Balanay J, Squadrito GL, MohanKumar K, Maheshwari A, Postlethwait EM, Matalon S, Patel RP. Chlorine gas exposure causes systemic endothelial dysfunction by inhibiting endothelial nitric oxide synthase-dependent signaling. Am J Respir Cell Mol Biol. 2011;45:419–425. doi: 10.1165/rcmb.2010-0151OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


