Abstract
Background
Severe traumatic injury is associated with bone marrow dysfunction that manifests as impaired erythropoiesis and prolonged hematopoietic progenitor cell (HPC) mobilization from the bone marrow. Extramedullary erythropoiesis, the development of red blood cells outside the bone marrow, has not been studied following severe injury and critical illness. This study examined the influence of lung contusion/hemorrhagic shock (LCHS) followed by chronic stress (CS) on the rodent spleen and to investigate the involvement of the splenic erythropoietin/erythropoietin receptor and BMP4 signaling.
Methods
Male Sprague-Dawley rats were subjected to LCHS and LCHS/CS. Animals underwent two hours of daily restraint stress until the day of sacrifice. On day seven the spleen was assessed for weight, growth of splenic CFU-GEMM, BFU-E, and CFU-E colonies, the presence of HPCs, and splenic mRNA expression of bone morphogenetic protein 4 (BMP4), erythropoietin and its receptor. Data was presented as mean±SD; *p<0.05 vs. naïve and **p<0.05 vs. LCHS by t-test.
Results
On day seven, the addition of CS to LCHS increased spleen weight by 22%. LCHS/CS increased splenic growth of CFU-GEMM, BFU-E and CFU-E colonies by 28–39% vs. LCHS alone. Seven days after LCHS/CS, splenic HPCs increased from 0.60% to 1.12 % compared to naïve animals. Following LCHS/CS, both BMP4 and EPO expression increased significantly in the spleen. Splenic EPOr expression decreased following LCHS/CS in the presence of a persistent moderate anemia.
Conclusions
Extramedullary erythropoiesis, manifest by increased splenic weight, splenic erythroid colony growth, splenic HPCs, BMP4, and EPO expression, is present in the spleen after LCHS/CS. Splenic EPOr expression was significantly decreased following LCHS/CS. Extramedullary erythropoiesis may play a key role in identifying new therapies to aid the recovery from acute anemia following severe trauma and chronic stress.
Keywords: Erythropoiesis, hematopoietic progenitor cells, spleen, anemia, erythropoietin
Background
Following severe trauma, there a norepinephrine-mediated hypercatecholamine state that is associated with bone marrow dysfunction due to both prolonged hematopoietic progenitor cell (HPC) mobilization and decreased growth of bone marrow erythroid progenitor cells.1–6 Previous work has demonstrated that HPC mobilization to the peripheral blood can persist up to seven days in rodents and up to fourteen days in severely injured humans.3–4 Injury-associated anemia is multifactorial in nature and likely secondary to a combination of acute blood loss, multiple phlebotomies, nutritional abnormalities, and bone marrow dysfunction, resulting in inadequate production of erythrocytes.5–8 While this anemia appears early following traumatic injury, it often persists for months regardless of blood transfusions.5–8 Previously, we have demonstrated that a rodent model of lung contusion, hemorrhagic shock, and chronic stress mimics what is seen in critical illness after injury.4, 6 The addition of chronic stress to lung contusion and hemorrhagic shock leads to prolonged HPC mobilization and decreased bone marrow erythroid progenitor cell growth and is associated with a persistent injury-associated anemia.4, 6
In the bone marrow, erythropoiesis produces erythrocytes at a steady state; however, at times of stress, erythropoiesis can occur in other organs that were once embryonic sites of erythropoiesis such as the liver and spleen.9–11 Erythropoiesis that occurs outside of the bone marrow is known as extramedullary erythropoiesis.12 Various hematopoietic stressors, including infection, anemia, pregnancy, and myocardial infarction, can all trigger extramedullary erythropoiesis or stress erythropoiesis.13–21 In the presence of anemia, hypoxia induces erythropoietin (EPO) production in the kidney, which can lead to extramedullary erythropoiesis in the spleen.9, 12, 20, 21 The adult spleen contains a unique microenvironment that is favorable for the rapid expansion of erythroid progenitors in response to stressful stimuli.20, 21 In the spleen, extramedullary erythropoiesis resulted in accelerated red blood cell production in an attempt to keep up with the demand.22 Aside from occurring in different organs, homeostatic erythropoiesis that occurs in the bone marrow and extramedullary erythropoiesis that occurs in the spleen also differ in the signals to which they respond. Extramedullary erythropoiesis in the spleen uniquely requires bone morphogenetic protein 4 (BMP4) in order to expand erythroid progenitor cells into mature erythroblasts.20
Extramedullary erythropoiesis has been well studied following phenylhydralazine-induced anemia in mice.9, 10, 20–23 However, the erythropoietic effects of severe trauma and chronic stress on the spleen have not been previously reported. Therefore, this study sought to examine the influence of lung contusion/hemorrhagic shock followed by chronic stress on the rodent spleen and to investigate the involvement of the splenic erythropoietin/erythropoietin receptor and BMP4 signaling.
Methods
Rodents
Male Sprague-Dawley rats (Charles River, Wilmington, MA), weighing 275 to 300g were housed in pairs with free access to food and water during daily night/day cycles of 12 hours each. Female animals were excluded due to estrous cycle variability and its impact after hemorrhagic shock. The animal protocol was approved by the University of Florida Institutional Animal Care and Use Committee.
Experimental Rodent Models
Rodents were randomly assigned into one of the three groups (n=6/group): 1) naïve control; 2) lung contusion followed by hemorrhagic shock (LCHS); 3) LCHS followed by daily chronic stress (LCHS/CS). Naïve rodents underwent daily handling. All rodents were weighed daily using a Kent Scientific animal weighing scale (Torrington, CT, USA). Rodents who underwent LCHS and LCHS/CS were sacrificed after seven days. Immediately after sacrifice, spleens were harvested and weighed using a Mettler Toledo balance (Columbus, OH, USA). Spleen and peripheral blood were collected and stored at −80°C freezer.
Lung Contusion and Hemorrhagic Shock
As previously described, after intra-peritoneal pentobarbital, a unilateral lung contusion was made by a percussive nail gun (Sears Brand, Chicago, IL) applied directly to a 12mm metal plate that was placed in the right axilla of the rodent.4 Ten minutes after lung contusion, the right femoral artery and internal jugular vein were cannulated using heparinized saline (10units/ml). The femoral artery tubing was then connected to a continuous blood pressure monitoring device (BP-2 Digital Blood Pressure Monitor; Columbus Instruments, Columbus, Ohio) for measurement of mean arterial pressure and heart rate. Blood was then withdrawn from the internal jugular to maintain a mean arterial pressure of 30–35 mmHg for 45 minutes. After shock shed blood is reinfused.
Chronic Restraint Stress
Daily restraint stress was utilized to recreate chronic stress. Rodents undergoing LCHS/CS were placed in nose cone holders (Kent Scientific Corporation, Torrington, CT, USA) for two hours daily for six days. During the two hours, rodents were repositioned in the cone every 30 minutes to prevent habituation and during which an alarm sounded for two minutes. Rodents in the LCHS/CS model were given 24 hours to recover from the injury prior to starting restraint stress. Those rodents in LCHS alone model were restricted from food and water during daily restraint stress.
Spleen Erythroid Progenitor Colonies
Spleen erythroid progenitor colony growth assays, including colony-forming units-granulocyte-, erythrocyte-, monocyte- megakaryocyte (CFU-GEMM), burst-forming unit-erythroid (BFU-E), and colony-forming unit-erythroid (CFU-E), were performed. The spleen was shredded and placed through a 70μm filter over a plate containing 1ml Iscove’s Modified Dulbecco’s Medium (IMDM). The sample was then collected, counted, and a solution of spleen mononuclear cells was prepared to yield a concentration of 20×106 cells/mL in IMDM. Cells were plated in duplicate (1×106 per plate) with Methocult media (Stemcell, Vancouver, BC, Canada). Plates were further supplemented with 1.3 U/mL rhEpo and 6 U/mL rhIL-3 for BFU-E and CFU-E or 3 U/mL rhGM-CSF for CFU-GEMM. Plates were then incubated at 37°C in 5% CO2 incubator. A blinded reader assessed colony growth of CFU-E on day 7, BFU-E colonies on day 14, and CFU-GEMM colonies on day 17.
Flow Cytometry
Cell suspensions from harvested spleens were prepared as described above. 1×106 cells/mL were stained to identify HPCs that were counted by flow cytometry using CD-71 and c-kit (CD-117) antibodies. CD-71, also known as transferrin receptor-1, is a marker for one of the earliest erythroid progenitor cells precursors, BFU-E cells.24 C-kit, also known as CD 117, is a receptor also expressed on early progenitor cells such a CFU-GEMM, BFU-E, and CFU-E.25 Briefly, spleen cells were incubated with mouse anti-rat CD71 (BD Biosciences, San Jose, CA) antibody conjugated with fluorescein isothiocyanate and rat anti-mouse CD117 (c-kit) (Southern Biotech, Birmingham, AL, USA) antibody conjugated to phycoerythrin (BD Biosciences). Cells were also stained with a Live/Dead Fixable stain kit and DyeCycle Ruby (ThermoFisher Scientific, Waltham, MA, USA) to evaluate for viability and cell cycle. The samples were run on BD LSR II flow cytometer equipped with FACSDiva software (BD Biosciences) to quantitate CD117 and CD71 positive proliferating cells.
Erythropoietin, Erythropoietin Receptor and BMP4 Expression
Spleen RNA were isolated used RNeasy Mini Kit (Qiagen, Germantown, MD, USA). Complementary DNA (cDNA) was then prepared from the RNA using a cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). EPO (32 cycles, annealing temperature 50°C), erythropoietin receptor (EPOr) (32 cycles, annealing temperature 60°C) and BMP4 (32 cycles, annealing temperature 56 °C) polymerase chain reaction (PCR) was performed using the following primers: EPO: 5-AGTCGCGTTCTGGAGAGGTA-3, 5-TGCAGAAAGTATCCGCTG-3, EPOr: 5-GCTCCTATGACCACCCACAT-3, 5-GGTGGTGAAGAGACCCTCAA-3, and BMP4: 5-TGATACCTGAGACCGGGAAG-3, 5-TCCTCACAGTGTTTGGCTCTG-3. The following β-actin primers (annealing temperature 60°C) were used: 5-AGCCATGTACGTAGCCATCC-3, 5-CTCTCAGCTGTGGTGGTGAA-3. Amplified bands were visualized with 1.5% agarose gels and densitometry was measured using Image Processing and Analysis (ImageJ-NIH, Bethesda, MD).
Hemoglobin Analysis
Heparinized blood samples were used for the analysis of hemoglobin levels using a VetScan HM5 Hematology Analyzer (Abaxis, CA, USA).
Statistical Analysis
Graph Pad Prism Version 6 (San Diego, CA, USA) was used to perform all statistical analysis. All data was presented as mean ± standard deviation. Normally distributed variables were compared using t-tests while Mann-Whitney U-test was used to compare all other variables. Significance was defined as *p < 0.05 vs. naïve controls and **p < 0.05 vs. untreated counterpart.
Results
Impact on Spleen Weight
Seven days following LCHS alone, spleen weight was similar to naïve animals (Figure 1A). However, the addition of CS to LCHS significantly increased weight by 29% (**p=0.015) versus LCHS (Figure 2A).
Figure 1.
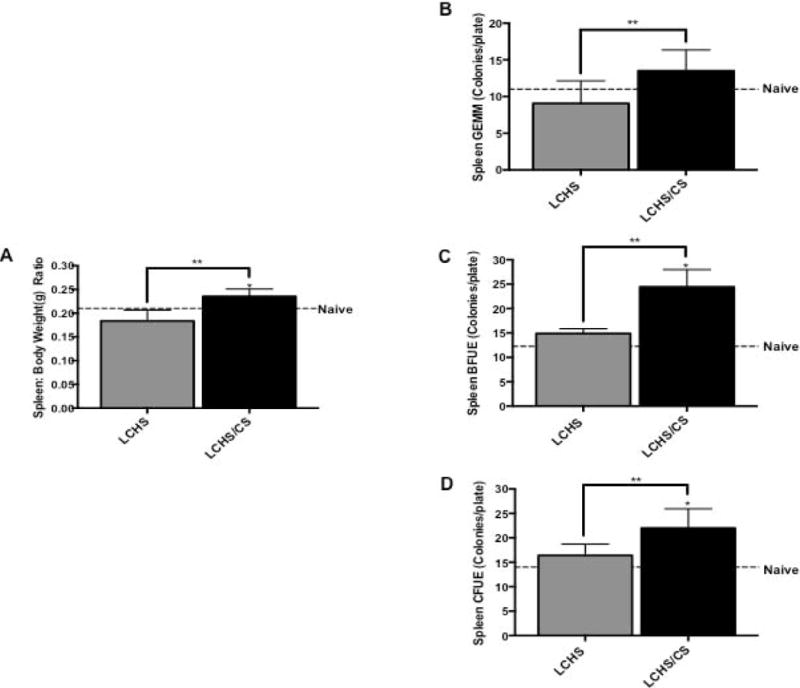
Spleen size and erythroid progenitor colony growth following LCHS and LCHS/CS. 1A. LCHS/CS significantly increased spleen weight in relation to total body weight compared to LCHS alone.1B–D. Daily CS exposure following LCHS resulted in increased growth in all three erythroid progenitor colonies in the spleen. LCHS= lung contusion and hemorrhagic shock; LCHS/CS= lung contusion, hemorrhagic shock and chronic restraint stress; *p <0.05 vs. naïve; **p <0.05 vs. LCHS.
Figure 2.
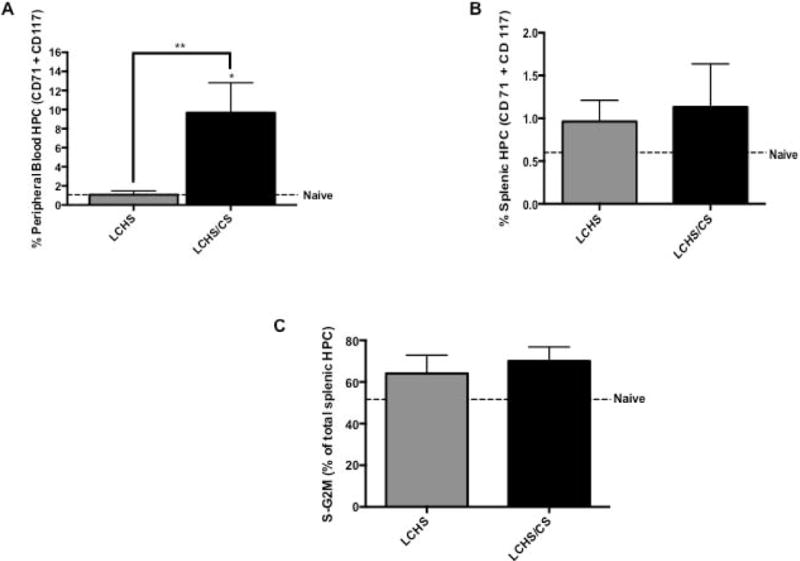
Mobilization of HPCs to peripheral blood and spleen following LCHS and LCHS/CS. 2A. The addition of CS to LCHS results in significantly increased early HPC mobilization to the peripheral blood. 2B. Following LCHS/CS, there was an increase in early HPCs in the spleen compared to naive. 2C. The addition of CS following LCHS resulted in a high number of proliferating cells. LCHS= lung contusion and hemorrhagic shock; LCHS/CS= lung contusion, hemorrhagic shock and chronic restraint stress; ; S-G2M= Synthesis-Gap2 and Mitosis phase of the cell cycle; *p <0.05 vs. naïve; **p <0.05 vs. LCHS.
Impact on Spleen Erythroid Progenitor Colony growth
Spleen erythroid progenitor colony growth was assessed to evaluate erythropoiesis occurring in the spleen. On day seven, there were no significant differences in spleen CFU-GEMM, BFU-E, and CFU-E erythroid progenitor colonies following LCHS in comparison to naïve (Figure 1B–D). LCHS/CS increased growth of spleen CFU-GEMM, BFU-E and CFU-E colonies as compared to naïve animals. The addition of CS to LCHS increased spleen CFU-GEMM, BFU-E, and CFU-E colony growth by 49%, 61% and 34%, respectively (**p=0.0002, **p=0.0163, **p=0.0163) when compared with LCHS alone (Figure 1B–D).
Hematopoietic Progenitor Cells in the Spleen
The addition of CS to LCHS increased the percent of CD71/CD117 double positive HPCs in the peripheral blood nine-fold in comparison to naïve and LCHS rodents (Figure 2A). In the spleen on day seven, LCHS increased the percent of CD71/CD117 double positive splenic HPCs from 0.60 to 0.96% compared to naïve animals (Figure 2B). With the addition CS to LCHS, the percent of CD71/CD117 double positive splenic HPCs increased 88% when compared to naïve animals (LCHS/CS: 1.13±0.5 vs. naïve: 0.60±0.50) (Figure 2B). Following LCHS and LCHS/CS, 64% and 70% of splenic HPCs detected were actively proliferating cells (Figure 2C).
Impact on Splenic BMP4, EPO, and EPOr expression
In the spleen, stress erythropoiesis requires BMP4 to differentiate immature erythroid progenitor cells in to erythroblasts.20 Seven days following LCHS, spleen BMP4 expression was similar to naïve (Figure 3). With the addition of daily CS to LCHS, we demonstrated enhanced expression of BMP4. LCHS/CS BMP4 expression increased 18% compared to naïve rodents (Figure 3).
Figure 3.
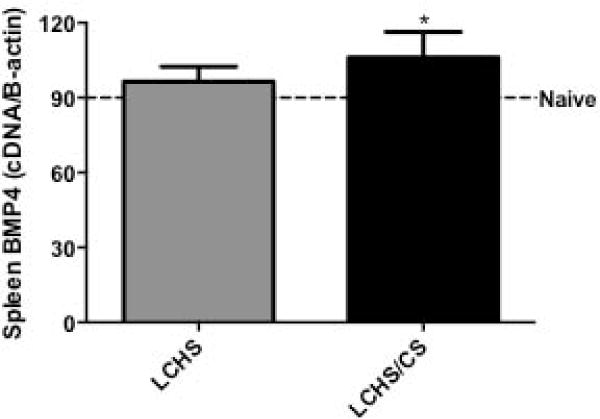
The addition of CS following LCHS significantly increased spleen BMP4 expression. LCHS= lung contusion and hemorrhagic shock; LCHS/CS= lung contusion, hemorrhagic shock and chronic restraint stress; *p <0.05 vs. naïve
LCHS alone did not cause any significant changes in splenic EPO or EPOr expression (Figures 4A and 4B). Seven days following LCHS/CS, EPO expression increased 23% (Figure 4A) while there was significant suppression of EPOr expression (27±5** vs. 42±6, p=0.0077) compared to LCHS alone (Figure 4B). Despite evidence of splenic erythropoiesis, seven days following LCHS/CS there was a persistent-injury associated anemia with a hemoglobin of 10.8 ± 0.8 g/dL (Figure 4C). There was no significant change in the number of circulating red blood cells (LCHS: 6.1±0.7 vs. LCHS: 6.0±0.5 1012/l).
Figure 4.
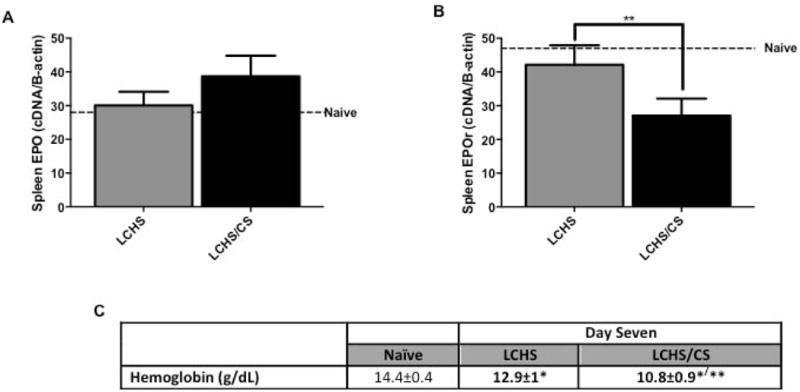
Splenic EPO and EPOr expression and hemoglobin levels following LCHS and LCHS/CS. 4A. LCHS/CS increased EPO expression in the spleen. 4B. LCHS/CS resulted in significantly decreased EPOr expression compared to LCHS alone. 4C. LCHS/CS resulted in a persistent-injury associated anemia on day seven. LCHS= lung contusion and hemorrhagic shock; LCHS/CS= lung contusion, hemorrhagic shock and chronic restraint stress; *p <0.05 vs. naïve; **p <0.05 vs. LCHS.
Discussion
Following severe trauma, critically injured patients develop a persistent-injury associated anemia within a week of hospitalization that can last for months.27, 28 Although the etiology of persistent injury-associated anemia is complex and multifactorial, previous studies have demonstrated that hypercatecholaminemia associated with severe injury suppressed bone marrow erythroid colony growth and resulted in prolonged HPC mobilization.3, 5 Currently, there are no data on the role of extramedullary erythropoiesis following severe traumatic injury. This is the first study to establish that following lung contusion/hemorrhagic shock and chronic stress extramedullary erythropoiesis occurs in the spleen as demonstrated by increased spleen weight, splenic erythroid progenitor colony growth, splenic HPCs, BMP4 and EPO expression. This study also demonstrated decreased expression of splenic EPOr and despite evidence of extramedullary erythropoiesis a persistent injury-associated anemia remained.
Increased splenic erythroid colony growth and EPO are necessary for extramedullary erythropoiesis.22, 23, 29 Inra et al.23 demonstrated that mice injected with cyclophosphamide and granulocyte colony stimulating factor to induce HPC mobilization had increased extramedullary erythropoiesis with increased spleen size and splenic erythroid progenitor cell growth, including growth of CFU-GEMM and BFU-E. Bennet et al.14 also demonstrated that severely anemic mice develop significantly enlarged spleens, 3–4 times the number of cells compared to naïve mice and splenic erythroid progenitor cell colony growth increased twenty times compared to normal spleens. In this study, seven days after LCHS with daily CS there was a significant increase in spleen weight as well as splenic erythroid colony growth. This study demonstrated the importance of stress following traumatic injury and the addition of CS following LCHS was key to the activation of extramedullary erythropoiesis which was not seen in rodents subject to LCHS alone. The initial sympathetic response following trauma is a necessary physiologic response but when this stressful state is prolonged it impacts many organs. This finding is supported by the work of Vignjevic et al.30 who demonstrated that psychological stress alone activates extramedullary erythropoiesis in adult mice.
In periods of homeostasis when HPCs mobilize from the bone marrow, they tend to migrate to sites of erythropoiesis such as the spleen.9 However, after trauma we previously demonstrated that HPCs (CD71 and CD117 positive cells) mobilize from the bone marrow to the peripheral blood and to sites of injury to aid in tissue repair.4 This prolonged HPC mobilization after injury was associated with decreased bone marrow cellularity and decreased bone marrow erythroid colony growth.6 In this study, the addition of CS to LCHS increased splenic HPCs on day seven. This is similar to work by Perry et al.29 where phenylhydralazine-induced anemic mice increased late splenic HPCs (Ter119 positive cells), a marker for mature erythroid cells. Yet, chronic stress alone in mice did not increase the total number of Ter119 positive cells after seven days.30 It took 14 days of restraint stress to increase the number of Ter119 positive cells suggesting that chronic stress led to a delay in terminal erythroid differentiation.30 This confirms that extramedullary erythropoiesis in the spleen works rapidly to produce mature erythroid cells. Similarly, in our model of LCHS/CS, 70% of the HPCs in the spleen were viable and proliferating cells, suggesting that the majority of HPCs in the spleen are maturing.
Previous work has demonstrated that extramedullary erythropoiesis is activated by acute anemia as a rapid response to HPC mobilization and a need for mature red blood cells.22 In the spleen during extramedullary erythropoiesis, HPCs differentiate to BFU-E cells in the presence of BMP4 and EPO.20 In this study, we found that the expression of BMP4 in the spleen is increased following LCHS/CS. Millot et al.20 demonstrated that anemic mice who received combined zymosan and EPO injections had a 4 fold increase in spleen BMP4 expression. Both the combination of EPO and a pro-inflammatory state played a role in the increased expression of BMP4 and erythroblast maturation in the spleen.20
In the bone marrow, EPO and its receptor, EPOr, are vital for the maturation of BFU-E and CFU-E to pro-erythroblasts31. However, splenic BFU-Es have the capability to mature in the presence of EPO alone.22 Previous work by Lenox et al.22 demonstrated that splenic BFU-Es in phenyl-hydralazine induced anemic mice grew 2–3 times more with EPO alone as compared to bone marrow BFU-Es. Bone marrow exposed to only EPO formed very few BFU-Es.22 Likewise, our current work demonstrated that seven days following LCHS/CS, spleen EPO expression increased and splenic BFU-Es had the most significant colony growth as compared to CFU-GEMM and CFU-E. There is growing evidence that splenic erythroid progenitors are distinct from those in the bone marrow.22 Further analysis of extramedullary erythropoiesis has demonstrated decreased expression of splenic EPOr following LCHS/CS. This is supported by findings of decreased EPOr and glucocorticoid receptor expression in the spleen following chronic psychological stress.30 The glucocorticoid receptor cooperates with EPOr to enhance the proliferation of splenic erythroid progenitors demonstrating the importance of sympathetic activation in this process.
Following LCHS/CS, anemia persisted regardless of elevated splenic EPO expression and increased extramedullary erythropoiesis in the spleen. This finding is supported by Bennett et al.14 and Millot et al.20 where mice remained anemic despite evidence of extramedullary erythropoiesis. The reduced hemoglobin following LCHS/CS may be the result of a low level of circulating iron.26 Hepcidin sequesters iron within macrophages preventing the maturation of red blood cells. In addition, while iron deficiency is associated with an increased rate of erythropoiesis, erythroid precursors have been shown to proliferate and then become quiescent without completing the maturation cycle.32 The lack of improvement in anemia may also be related to the severity of bone marrow dysfunction caused by LCHS/CS. The burden of producing mature functional erythrocytes and restoring hemoglobin levels may exceed the capacity of the spleen following severe trauma and chronic stress. Although extramedullary erythropoiesis does not provide enough mature erythrocytes to improve anemia following LCHS/CS, it does provide insight on the erythropoietic response after injury.
In conclusion, chronic stress following lung contusion/hemorrhagic shock activated extramedullary erythropoiesis in the spleen leading to increased splenic weight, increased splenic erythroid progenitor colony growth, and increased proliferating splenic HPCs. In addition, the splenic expression of EPO and BMP4 were associated with extramedullary erythropoiesis. Additional studies are needed to examine the connection between persistent injury-associated anemia and extramedullary erythropoiesis to further delineate the impact of chronic stress on the erythropoietic response following trauma. Extramedullary erythropoiesis may play a key role in identifying new therapies to aid the recovery from acute anemia following severe trauma and chronic stress.
Acknowledgments
This research was supported by the National Institutes of Health. AMM was supported by NIH NIGMS grant R01 GM105893-01A1. PAE was supported by P30 AG028740 from the National Institute on Aging and by the NIH NIGMS grant R01 GM113945-01. Finally, AMM and PAE were all supported by P50 GM111152-01 (NIGMS). TJL was supported by a post-graduate training grant (T32 GM-08721) in burns, trauma and perioperative injury by NIGMS.
Footnotes
Level of Evidence: Level II, prognostic study
To be presented at the 30th Annual Meeting of Eastern Association for the Surgery of Trauma in Hollywood, FL, January 10–14, 2017
The authors have no relevant conflicts of interest and nothing to disclose.
Authors’ contributions
IGA contributed to the formation of the experimental rodent model design, performed rodent survival surgeries, assisted with tissue collection/processing, hematology and protein analysis, did significant writing and statistical analysis of the manuscript.
KBK assisted with the formation of the experimental rodent model design, hematology, and protein analysis, performed tissue collection/processing, and protein isolation.
TJL assisted with performing rodent survival surgeries and tissue collection/processing.
HR performed sample preparations, tissues collecting/processing, protein and isolation.
PAE provided assistance with the research question, interpretation of the data, statistical analysis, and writing of the manuscript.
AMM conceived the concept for the experimental rodent model design, research question, interpretation of data, and contributed significantly to the writing of the manuscript and final edits. All authors have read and approved the final manuscript.
References
- 1.Fonseca RB, Mohr AM, Wang L, Clinton E, Sifri ZC, Rameshwar P, Livingston DH. Adrenergic modulation of erythropoiesis following severe injury is mediated through bone marrow stroma. Surg Infect. 2004;5(4):385–93. doi: 10.1089/sur.2004.5.385. [DOI] [PubMed] [Google Scholar]
- 2.Fonseca RB, Mohr AM, Wang L, Sifri ZC, Rameshwar P, Livingston DH. The impact of a hypercatecholamine state on erythropoiesis following severe injury and the role of IL-6. J Trauma. 2005;59(4):884–9. doi: 10.1097/01.ta.0000187653.64300.f5. [DOI] [PubMed] [Google Scholar]
- 3.Bible LE, Pasupuleti LV, Alzate WD, Gore AV, Song KJ, Sifri ZC, Livingston DH, Mohr AM. Early propranolol administration to severely injured patients can improve bone marrow dysfunction. J Trauma Acute Care Surg. 2014;77(1):54–60. doi: 10.1097/TA.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bible LE, Pasupuleti LV, Gore AV, Sifri ZC, Kannan KB, Mohr AM. Daily propranolol prevents prolonged mobilization of hematopoietic progenitor cells in a rat model of lung contusion, hemorrhagic shock, and chronic stress. Surgery. 2015;158(3):595–601. doi: 10.1016/j.surg.2015.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston DH, Anjaria D, Wu J, Hauser CJ, Chang V, Deitch EA, Rameshwar P. Bone marrow failure following severe injury in humans. Ann Surg. 2003;238(5):748–53. doi: 10.1097/01.sla.0000094441.38807.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bible LE, Pasupuleti LV, Gore AV, Sifri ZC, Kannan KB, Mohr AM. Chronic restraint stress after injury and shock is associated with persistent anemia despite prolonged elevation in erythropoietin levels. J Trauma Acute Care Surg. 2015;79(1):91–6. doi: 10.1097/TA.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bateman AP, McArdle F, Walsh TS. Time course of anemia during six months follow up following intensive care discharge and factors associated with impaired recovery of erythropoiesis. Crit Care Med. 2009;37(6):1906–12. doi: 10.1097/CCM.0b013e3181a000cf. [DOI] [PubMed] [Google Scholar]
- 8.Corwin HL, Gettinger A, Rodriguez RM, Pearl RG, Gubler KD, Enny C, Colton T, Corwin MJ. Efficacy of recombinant human erythropoietin in the critically ill patient: a randomized, double-blind, placebo-controlled trial. Crit Care Med. 1999;27(11):2346–50. doi: 10.1097/00003246-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Lenox LE, Shi L, Hegde S, Paulson RF. Extramedullary erythropoiesis in the adult liver requires BMP-4/Smad5-dependent signaling. Exp Hematol. 2009;37(5):549–58. doi: 10.1016/j.exphem.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulson RF, Shi L, Wu DC. Stress erythropoiesis: new signals and new stress progenitor cells. Curr Opin Hematol. 2011;18(3):139–45. doi: 10.1097/MOH.0b013e32834521c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ploemacher RE, van Soest PL. Morphological investigation on phenylhydrazine-induced erythropoiesis in the adult mouse liver. Cell Tissue Res. 1977;178(4):435–61. doi: 10.1007/BF00219567. [DOI] [PubMed] [Google Scholar]
- 12.Johns JL, Christopher MM. Extramedullary hematopoiesis: a new look at the underlying stem cell niche, theories of development, and occurrence in animals. Vet Path. 2012;49(3):508–23. doi: 10.1177/0300985811432344. [DOI] [PubMed] [Google Scholar]
- 13.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465(7299):793–7. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett M, Pinkerton PH, Cudkowicz G, Bannerman RM. Hemopoietic progenitor cells in marrow and spleen of mice with hereditary iron deficiency anemia. Blood. 1968;32(6):908–21. [PubMed] [Google Scholar]
- 15.Burberry A, Zeng MY, Ding L, Wicks I, Inohara N, Morrison SJ, Nunez G. Infection mobilizes hematopoietic stem cells through cooperative NOD-like receptor and Toll-like receptor signaling. Cell Host Microbe. 2014;15(6):779–91. doi: 10.1016/j.chom.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheshier SH, Prohaska SS, Weissman IL. The effect of bleeding on hematopoietic stem cell cycling and self-renewal. Stem Cells Dev. 2007;16(5):707–17. doi: 10.1089/scd.2007.0017. [DOI] [PubMed] [Google Scholar]
- 17.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, Iwamoto Y, Thompson B, Carlson AL, Heidt T, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–9. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowler JH, Nash DJ. Erythropoiesis in the spleen and bone marrow of the pregnant mouse. Dev Biol. 1968;18(4):331–53. doi: 10.1016/0012-1606(68)90045-6. [DOI] [PubMed] [Google Scholar]
- 19.Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, Takeichi M, Wendt GR, Morrison SJ. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555–8. doi: 10.1038/nature12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millot S, Andrieu V, Letteron P, Lyoumi S, Hurtado-Nedelec M, Karim Z, Thibaudeau O, Bennada S, Charrier JL, Lasocki S, et al. Erythropoietin stimulates spleen BMP4-dependent stress erythropoiesis and partially corrects anemia in a mouse model of generalized inflammation. Blood. 2010;116(26):6072–81. doi: 10.1182/blood-2010-04-281840. [DOI] [PubMed] [Google Scholar]
- 21.Porayette P, Paulson RF. BMP4/Smad5 dependent stress erythropoiesis is required for the expansion of erythroid progenitors during fetal development. Dev Biol. 2008;317(1):24–35. doi: 10.1016/j.ydbio.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenox LE, Perry JM, Paulson RF. BMP4 and Madh5 regulate the erythroid response to acute anemia. Blood. 2005;105(7):2741–8. doi: 10.1182/blood-2004-02-0703. [DOI] [PubMed] [Google Scholar]
- 23.Inra CN, Zhou BO, Acar M, Murphy MM, Richardson J, Zhao Z, Morrison SJ. A perisinusoidal niche for extramedullary haematopoiesis in the spleen. Nature. 2015;527(7579):466–71. doi: 10.1038/nature15530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong HY, Wilkes S, Yang H. CD71 is selectively and ubiquitously expressed at high levels in erythroid precursors of all maturation stages: a comparative immunochemical study with glycophorin A and hemoglobin A. Am J Surg Pathol. 2011;35(5):723–32. doi: 10.1097/PAS.0b013e31821247a8. [DOI] [PubMed] [Google Scholar]
- 25.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90(4):1345–64. [PubMed] [Google Scholar]
- 26.Alamo IG, Kannan KB, Smith MA, Efron PA, Mohr AM. Characterization of erythropoietin and hepcidin in the regulation of persistent injury-associated anemia. J Trauma Acute Care Surg. 2016;81(4):705–12. doi: 10.1097/TA.0000000000001163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS, Shapiro MJ. The CRIT Study: Anemia and blood transfusion in the critically ill–current clinical practice in the United States. Crit Care Med. 2004;32(1):39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 28.Napolitano LM. Scope of the problem: epidemiology of anemia and use of blood transfusions in critical care. Crit Care. 2004;8(Suppl 2):S1–8. doi: 10.1186/cc2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry JM, Harandi OF, Paulson RF. BMP4, SCF, and hypoxia cooperatively regulate the expansion of murine stress erythroid progenitors. Blood. 2007;109(10):4494–502. doi: 10.1182/blood-2006-04-016154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vignjevic S, Budec M, Markovic D, Dikic D, Mitrovic O, Mojsilovic S, Duric SV, Koko V, Cokic BB, Cokic V, Jovcic G. Chronic psychological stress activates BMP4-dependent extramedullary erythrpoiesis. J Cell Mol Med. 2014;1:91–103. doi: 10.1111/jcmm.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nalbant D, Saleh M, Goldman FD, Widness JA, Veng-Pedersen P. Evidence of receptor-mediated elimination of erythropoietin by analysis of erythropoietin receptor mRNA expression in bone marrow and erythropoietin clearance during anemia. J Pharmacol Exp Ther. 2010;333(2):528–32. doi: 10.1124/jpet.109.163568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Ginzburg YZ. Crosstalk between iron metabolism and erythropoiesis. Adv Hematol. 2010;2010:605435. doi: 10.1155/2010/605435. [DOI] [PMC free article] [PubMed] [Google Scholar]


