Abstract
Chemical investigation of a halophilic actinomycete strain belonging to the genus Nocardiopsis inhabiting a hypersaline saltern led to the discovery of new 18-membered macrolides with nitrile functionality, borrelidins C–E (1–3), along with a previously reported borrelidin (4). The planar structures of borrelidins C–E, which are new members of the rare borrelidin class of antibiotics, were elucidated by NMR, mass, IR, and UV spectroscopic analyses. The configurations of borrelidines C–E were determined by the interpretation of ROESY NMR spectra, J-based configuration analysis, a modified Mosher’s method, and CD spectroscopic analysis. Borrelidins C and D displayed inhibitory activity, particularly against the Gram-negative pathogen Salmonella enterica, and moderate cytotoxicity against the SNU638 and K562 carcinoma cell lines.
Keywords: antibacterial, saltern, halophilic actinomycetes, borrelidin, Salmonella enterica
1. Introduction
Chemical studies of marine-derived actinomycetes in the past 20 years have resulted in the discovery of structurally and biologically diverse secondary metabolites [1,2]. Salinity is obviously a distinct factor that continuously promotes the metabolic adaptation of marine-derived actinomycetes to marine environments [3]. Salterns are the most extreme habitats with respect to salinity and can harbor truly halophilic actinomycetes [4]. However, the chemistry of actinobacteria in these hypersaline environments had been mostly neglected until our report of the salternamides as the first secondary metabolites from a saltern-derived actinomycete (Streptomyces sp.) in 2015 [5,6]. A subsequent biological study of salternamide A revealed that this chlorinated metabolite suppresses the hypoxia-induced accumulation of HIF-1α and induces apoptosis in human cancer cells [7]. Our continuing study of saltern-derived actinomycetes led to the discovery of indolosesquiterpenoids, called xiamycins C–E, from a halophilic Streptomyces sp. Xiamycin D displayed potent antiviral activity against porcine epidemic diarrhea virus (PEDV) [8]. These promising results indicated that saltern-derived actinobacteria can be utilized as prolific sources of bioactive secondary metabolites with pharmaceutical potential, leading us to expand our research program from the common genus Streptomyces to diverse phylogenetic groups in the phylum Actinobacteria that inhabit salterns.
Halophilic actinomycete strains were selectively isolated from topsoil samples collected from a saltern located in Jeungdo, Jeollanam-do, Republic of Korea. From among the isolated actinomycete strains, strain HYJ128, belonging to the genus Nocardiopsis, was cultivated in liquid medium. Chemical profiling based on an LC/MS analysis indicated that this strain produces a series of secondary metabolites that commonly display a UV spectrum with an absorption maximum at λmax 257 nm, are expected to incorporate a conjugation of at least two double bonds and have an [M − H]− molecular ion at m/z 504. This initial evaluation by UV spectroscopy and MS prompted the production of large cultures and chromatographic purification to obtain three new polyketide-derived macrolides, borrelidins C–E (1–3), which are new members of the borrelidin class of antibiotics, originally isolated from Streptomyces spp. [9] (Figure 1). Herein, we report the structures of borrelidins C–E from the rare halophilic actinomycete Nocardiopsis sp. HYJ128, along with the antibacterial and cytotoxic biological activities of these compounds.
Figure 1.
The structures of borrelidins C–E (1–3) and borrelidin (4).
2. Results
2.1. Structural Elucidation
Borrelidin C (1) was obtained as a white powder that was determined to have the molecular formula C28H43NO7 with an unsaturation number of eight based on the analysis of negative ion mode high-resolution fast atom bombardment (HR-FAB) mass spectrometry data (obsd. [M − H]− at m/z 504.2959, calcd. [M − H]− 504.2961). The 1H NMR spectrum of 1 in pyridine-d5 displayed 3 double-bond protons (δH 6.83, 6.66, and 6.33), 4 oxymethine protons (δH 5.65, 4.79, 4.57, and 4.37), 20 aliphatic methine or methylene protons (δH 3.44~0.95), and 12 protons in four methyl groups (δH 1.28, 0.97, 0.91, and 0.85; Table 1). In the 13C NMR spectrum, 2 carbonyl carbons (δC 179.4 and 172.4), 5 resonances in the double bond region (δC 143.7, 139.1, 127.7, 120.1, and 118.3), 4 oxygen-bearing carbons (δC 76.7, 72.4, 72.2, and 70.8), and 17 aliphatic carbons (δC 48.3~15.4) were identified. The interpretation of the HSQC spectrum assigned all the 13C-1H one-bond correlations. Because the number of carbons in the double bond region was five and the molecular weight was an odd number, one of the five carbons between 118.3 and 143.7 should be associated with a nitrogen atom as an imine or nitrile functional group. An absorption peak at 2218 cm−1 in the IR spectrum and a 13C chemical shift of 118.3 ppm were observed, characteristically supporting the existence of nitrile functionality [10]. This nitrile functional group explains two of the eight double bond equivalents inherent in the molecular formula. In addition, the two carbonyl groups and two double bonds accounted for the unsaturation number of 4, thus revealing that borrelidin C (1) is a bicyclic compound.
Table 1.
NMR data for 1–3 in pyridine-d5.
| C/H | 1 | 2 | 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| δH a | Mult (J in Hz) | δC b | δH a | Mult (J in Hz) | δC b | δH a | Mult (J in Hz) | δC b | |
| 1 | – | – | 172.4 s | – | – | 172.7 s | – | – | 172.7 s |
| 2 | 2.79 | m | 39.3 t | 2.82 | m | 41.1 t | 2.92 | m | 41.3 t |
| 2.76 | m | 2.80 | m | 2.79 | m | ||||
| 3 | 4.37 | m | 70.8 d | 4.34 | m | 71.2 d | 4.44 | m | 69.8 d |
| 4 | 1.98 | m | 36.5 d | 1.92 | m | 36.9 d | 1.96 | m | 36.8 d |
| 5 | 1.34 | m | 43.9 t | 1.26 | m | 44.9 t | 1.72 | m | 41.5 t |
| 0.95 | m | 0.94 | m | 1.66 | m | ||||
| 6 | 2.10 | m | 27.6 d | 2.00 | m | 27.9 d | 2.37 | m | 32.2 d |
| 7 | 1.07 | m | 48.1 t | 1.05 | m | 48.6 t | 3.26 | br d (9.5) | 81.5 d |
| 1.00 | m | 0.95 | m | ||||||
| 8 | 1.73 | m | 26.8 d | 1.72 | m | 27.1 d | 2.01 | m | 34.4 d |
| 9 | 1.41 | ddd (13.0, 13.0, 2.5) | 37.9 t | 1.38 | ddd (13.0, 13.0, 2.5) | 38.1 t | 1.37 | m | 36.3 t |
| 0.98 | m | 0.99 | m | 1.15 | m | ||||
| 10 | 2.28 | m | 35.6 d | 2.30 | m | 36.0 d | 2.36 | m | 36.1 d |
| 11 | 4.57 | d (9.5) | 72.2 d | 4.58 | d (9.5) | 72.6 d | 4.62 | d (9.5) | 72.7 d |
| 12 | – | – | 120.1 s | – | – | 120.6 s | – | – | 120.6 s |
| 13 | 6.83 | d (11.0) | 143.7 d | 6.76 | d (11.5) | 143.2 d | 6.83 | d (11.0) | 143.4 d |
| 14 | 6.66 | dd (14.0, 11.0) | 127.7 d | 6.68 | dd (14.0, 11.5) | 128.1 d | 6.71 | dd (14.0, 11.0) | 127.9 d |
| 15 | 6.33 | m | 139.1 d | 6.27 | ddd (14.0, 11.0, 4.0) | 139.5 d | 6.33 | ddd (14.0, 11.0, 4.0) | 139.9 d |
| 16 | 2.60 | m | 36.2 t | 2.57 | m | 36.6 t | 2.57 | m | 37.0 t |
| 2.57 | m | 2.49 | m | 2.5 | m | ||||
| 17 | 5.65 | m | 76.7 d | 5.37 | m | 76.8 d | 5.38 | m | 76.8 d |
| 18 | 3.22 | m | 44.7 d | 3.49 | m | 45.1 d | 3.09 | m | 46.8 d |
| 19 | 2.42 | m | 39.1 t | 2.26 | m | 40.8 t | 1.90 | m | 30.3 t |
| 1.80 | m | 1.60 | m | 1.27 | m | ||||
| 20 | 4.79 | m | 72.4 d | 4.59 | m | 73.3 d | 1.79 | m | 26.2 t |
| 1.62 | m | ||||||||
| 21 | 2.46 | m | 41.6 t | 2.38 | m | 40.8 t | 2.14 | m | 32.3 t |
| 2.41 | m | 2.20 | m | 1.98 | m | ||||
| 22 | 3.44 | ddd (8.5, 8.5, 8.5) | 48.3 d | 3.07 | ddd (8.0, 8.0, 8.0) | 49.7 d | 2.89 | ddd (7.5, 7.5, 7.5) | 50.8 d |
| 23 | – | – | 179.4 s | – | – | 181.9 s | – | – | 181.2 s |
| 24 | 0.85 | d (7.0) | 18.3 q | 0.89 | d (7.0) | 18.9 q | 0.92 | d (7.0) | 17.9 q |
| 25 | 0.97 | d (6.5) | 18.6 q | 0.92 | d (6.5) | 18.7 q | 1.18 | d (6.5) | 11.6 q |
| 26 | 0.91 | d (6.5) | 20.6 q | 0.90 | d (6.5) | 21.0 q | 1.31 | d (6.5) | 16.7 q |
| 27 | 1.28 | d (6.5) | 15.4 q | 1.30 | d (6.5) | 15.7 q | 1.37 | d (6.5) | 15.8 q |
| 28 | – | – | 118.3 s | – | – | 118.6 s | – | – | 118.6 s |
a 600 MHz; b 150 MHz.
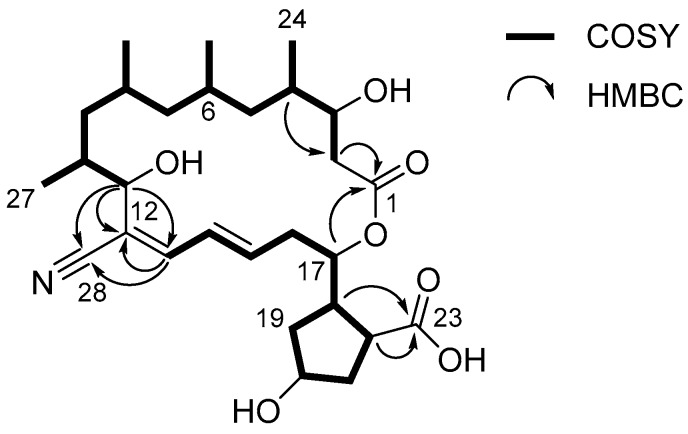
Examination of the 1H-1H COSY NMR spectrum of borrelidin C (1) identified two spin systems (Figure 2). H2-2 methylene began the first spin system by connecting C-2 and C-3 with a H2-2/H-3 COSY correlation. This chain was sequentially extended to C-11 by consecutive 1H-1H couplings from H-3 to H-11. Four branched methyl groups—C-24, C-25, C-26, and C-27—were respectively assigned at C-4, C-6, C-8, and C-10 by their COSY correlations with the corresponding methine protons. Second, H-13 (δH 6.83) displayed a correlation with H-14 at 6.66 ppm. H-14 extended the spin system to H-15 (δH 6.33) by a three-bond 1H-1H coupling between H-14 and H-15, thus constructing a diene moiety. The H-15/H2-16 (δH 2.60 and 2.57) COSY correlation extended the chain structure from the diene moiety to the aliphatic methylene C-16 (δC 36.2). A homonuclear correlation between H2-16 and H-17 (δH 5.65) connected the oxymethine C-17 (δC 76.7) to C-16. Additionally, an array of COSY correlations was observed from H-17 to H-22 through consecutive methylenes H2-19, H2-20, and H2-21, identifying a cyclopentane and extending the carbon framework from C-17 to C-22 (δC 48.3).
Figure 2.
The key COSY and HMBC correlations of borrelidin C (1).
Despite the comprehensive analysis of the COSY NMR spectrum of 1, two carbonyl carbons (δC 179.4 and 172.4), one olefinic carbon (δC 120.1), and a nitrile carbon (δC 118.3) were not assigned. These unassigned moieties were connected to the first and/or second spin systems by HMBC correlations. The C-1 carbonyl carbon (δC 172.4) was assigned next to C-2 (δC 39.3) by an HMBC correlation from H2-2 to C-1. The other carbonyl carbon (C-23) correlated with H-18 (δH 3.22) and H-22 (δH 3.44) in the HMBC NMR spectrum, confirming connectivity of this carbon to C-22 (δC 48.3). The olefinic quaternary carbon C-12 at 120.1 ppm was located between C-11 (δC 72.2) and C-13 (δC 143.7), thus linking the two spin systems based on H-11/C-12 and H-13/C-12 HMBC correlations. This connectivity was further secured by an HMBC correlation from H-11 (δH 4.57) to C-13. The nitrile functional group was assigned to C-12 based on three-bond heteronuclear couplings from H-11 and H-13 to the nitrile carbon C-28 (δC 118.3). Finally, a macrocyclic lactone ring was constructed based on a key HMBC correlation from H-17 (δH 5.65) to C-1, assigning C-1 as an ester carbon and subsequently proposing the C-23 carboxylic acid functional group. Therefore, the remaining two double bond equivalents of borrelidin C (1) were explained by the cyclopentane and the macrocyclic ring, and the planar structure of borrelidin C (1) was elucidated as a new member of the borrelidin class. Comparison of the structure of 1 with that of borrelidin (4) revealed that borrelidin C (1) has an additional hydroxyl group at C-20 in the cyclopentane ring.
Borrelidin D (2) was purified as a white powder with the molecular formula C28H43NO7 based on the analysis of HR-FAB-MS (obsd. [M − H]− at m/z 504.2958, calcd. [M − H]− 504.2961). The IR, UV, and mass spectra of this compound were analogous to those of borrelidin C (1). Because the 1H and 13C NMR and COSY, HSQC, HMBC, and ROESY NMR spectra were also very similar to those of 1, the planar structure of borrelidin D (2) could be readily elucidated as a structure identical to that of borrelidin C (1) based on these NMR spectra, as shown in Figure 1. A careful analysis of the NMR spectra of 2 indicated that the difference between 1 and 2 could originate from the stereochemistry of the hydroxyl group at C-20 (δC 73.3) because the 1H and 13C chemical shifts of the cyclopentane exhibited noticeable differences.
Borrelidin E (3) was isolated as a white powder. The molecular formula was deduced as C28H43NO7 based on the HR-FAB-MS analysis (obsd. [M − H]− at m/z 504.2966, calcd. [M − H]− 504.2961). Because the spectroscopic data—including the 1D and 2D NMR, mass, UV, and IR spectra—displayed analogous features to those of borrelidins C and D (1–2), the planar structure of borrelidin E (3) could be determined by comparing the spectra with those of 1 and 2. In the 13C NMR spectrum of 3, a relatively deshielded oxygen-bearing carbon was detected at 81.5 ppm, which was not observed in borrelidins C and D. A comprehensive analysis of the 1D and 2D NMR spectra revealed that this oxygenated carbon was at C-7 (δC 81.5), not C-20 (δC 26.2), which is different from borrelidins C and D. Therefore, the structure of borrelidin E was established as a new congener of borrelidin with a hydroxyl group at C-7.
The configurations of the stereogenic centers of borrelidins C–E (1–3) were deduced to be identical to those of borrelidin (4) based on the high degree of similarity of the NMR and CD spectroscopic data (Figure S28) and the common biosynthetic origin of 1–4. However, the configurations of the additional hydroxyl groups at C-20 in 1–2 and at C-7 in 3 required further analysis. In the ROESY NMR spectrum of borrelidin C (1), the H-17/H-22 correlation indicated that these protons are on the same face. More importantly, the H-18/H-20 correlation showed a syn-configuration between H-18 and H-20 on the face opposite from H-17 and H-22, thus establishing the relative configuration of the cyclopentane (Figure 3a). In contrast, in the ROESY NMR data of borrelidin D (2), H-20 correlated with H-22, which in turn correlated with H-17, demonstrating that these three protons are facing the same direction (Figure 3b). Therefore, the relative configuration of C-20 in borrelidin D was opposite to that in borrelidin C as expected vide supra.
Figure 3.
Key ROESY correlations around the cyclopentane of borrelidins C-D (1–2).
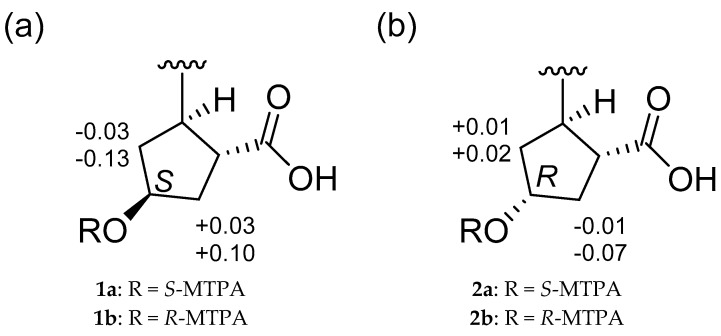
The absolute configuration of C-20 in borrelidin C (1) was determined by a modified Mosher’s method [11]. Bis-S- and R-MTPA esters (1a and 1b) were obtained by derivatizing borrelidin C with R- and S-α-methoxy-α-(trifluoromethyl)phenylacetyl chloride (MTPA-Cl), respectively. Fortunately, the hydroxyl group at C-20 in 1 was successfully esterified with MTPA, thus enabling an analysis of the 1H chemical shifts. The 1H chemical shifts of 1a and 1b were assigned by analyzing their COSY NMR spectra. The ΔδS-R values of the 1H chemical shifts around the stereogenic center (C-20) were calculated (Figure 4a). The consistent distribution of the ΔδS-R value signs finally determined the 20S configuration. The absolute configuration of C-20 in borrelidin D (2) was also established by the modified Mosher’s method. As expected based on the relative configuration, the absolute configuration of C-20 in 2 was determined as R, which is opposite to that of C-20 in 1 (Figure 4b).
Figure 4.
ΔδS-R values in ppm of (a) 1a and 2b and (b) 2a and 2b in pyridine-d5.
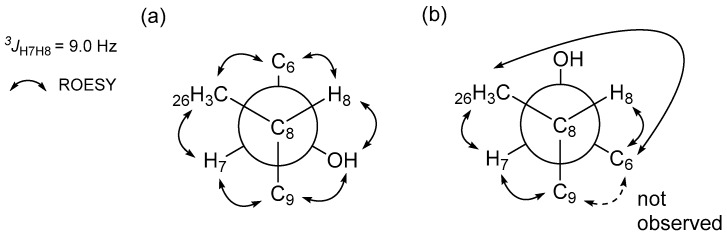
To investigate the configuration of C-7 in borrelidin E (3), a homo-decoupling 1H experiment was performed to obtain the 1H-1H coupling constants of H-7 (δH 3.26) and H-8 (δH 2.01), which were not clearly measurable in the ordinary 1H NMR spectrum because H-7 (broad doublet) coupled with H-6 as well. When H-6 at 2.38 ppm was irradiated, the coupling constant between H-7 and H-8 was successfully measured as 9.0 Hz (Figure S27). The anti-relationship between H-7 and H-8 was assigned based on this large 3JH7H8 value in the J-based configuration analysis (Figure 5) [12]. Further analysis of ROESY correlations with this H-7/H-8 anti-relationship enabled us to select of the correct rotamer and assign its 7S configuration based on the previously-established 8S configuration in borrelidin (4) [9] (Figure 5a).
Figure 5.
J-based configuration analysis for the configuration of borrelidin E (3) at C-7 and C-8. Two rotamers were selected with 3JH7H8 (9.0 Hz). (a) The rotamer satisfies the observed ROESY correlations; (b) The rotamer cannot satisfy the observed H-6/H3-26 ROESY correlation.
2.2. Bioactivities of the Borrelidins
Because borrelidin was initially reported as an antibacterial compound, the bioactivities of the new borrelidin family members (1–3) and borrelidin (4) were first evaluated in antimicrobial assays against phylogenetically diverse pathogenic bacterial strains, including Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 19433, Enterococcus faecium ATCC 19434, Proteus hauseri NRBC 3851, Klebsiella pneumoniae ATCC 10031, Salmonella enterica ATCC 14028, and Escherichia coli ATCC 25922, using ampicillin as a positive control compound (Table 2). Borrelidins C and D (1–2) displayed moderate antibacterial activity specifically against S. enterica ATCC 14028 with minimum inhibitory concentration (MIC) values of 16 µM and 63 µM, respectively, while borrelidin (4) broadly inhibited most of the tested pathogens except S. aureus and E. coli. Borrelidin (4) was also most active against S. enterica, with an MIC value of 0.51 µM, which is approximately three times more potent than the positive control ampicillin (MIC = 1.4 µM). However, borrelidin E (3) did not exhibit significant inhibitory activity against the tested bacteria. These results revealed that hydroxylation at C-20 significantly reduces antibacterial activity, and hydroxylation at C-7 can even abolish the activity. The specific inhibitory activities of 1–4 against S. enterica indicated that the borrelidin class might be a particularly useful scaffold for antibiotics against S. enterica, which causes salmonellosis mainly via food contamination [13].
Table 2.
Antibacterial activities of the borrelidins (1–4) against pathogenic bacteria.
| MIC in µM | Gram-Positive | Gram-Negative | |||||
|---|---|---|---|---|---|---|---|
| S. aureus | E. faecalis | E. faecium | P. hauseri | K. pneumoniae | S. enterica | E. coli | |
| Borrelidin C (1) | >250 | 250 | >250 | >250 | >250 | 16 | >250 |
| Borrelidin D (2) | >250 | >250 | >250 | >250 | >250 | 63 | >250 |
| Borrelidin E (3) | 250 | >250 | >250 | >250 | >250 | 250 | >250 |
| Borrelidin (4) | >260 | 33 | 65 | 16 | 65 | 0.51 | 260 |
| Ampicillin | 0.37 | 5.7 | 5.7 | 0.37 | >367 | 1.4 | 23 |
Additionally, the cytotoxicities of borrelidins C–E (1–3) and borrelidin (4) against various human carcinoma cell lines—such as A549 (lung cancer), HCT116 (colon cancer), SNU638 (stomach cancer), SK-HEP1 (liver cancer), MDA-MB231 (breast cancer), and K562 (leukemia)—were measured (Table 3). Borrelidins C and D (1–2) showed significant cytotoxicity against the tested cancer cell lines. In particular, borrelidin C (1) exhibited considerable cytotoxic activity against SNU638 and K562 with IC50 values of 5.5 μM and 5.7 μM, respectively. Borrelidin D (2) displayed comparable cytotoxicity against these cancer cell lines with IC50 values of 8.7 μM and 6.7 μM, respectively, whereas borrelidin E (3) did not display inhibitory activity against any of the tested cancer cell lines. Among the borrelidins, borrelidin (4) exhibited the most potent cytotoxicity against all the cell lines that were tested (IC50 = 0.8 µM).
Table 3.
Cytotoxicities of the borrelidins (1–4) against cancer cell lines.
| IC50 in µM | A549 | HCT116 | SNU638 | SK-HEP1 | MDA-MB231 | K562 |
|---|---|---|---|---|---|---|
| Borrelidin C (1) | 9.1 | 10 | 5.5 | 63 | 96 | 5.7 |
| Borrelidin D (2) | 12 | 15 | 8.7 | 71 | 64 | 6.7 |
| Borrelidin E (3) | >100 | >100 | >100 | >100 | >100 | >100 |
| Borrelidin (4) | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Etoposide | 0.68 | 14 | 0.57 | 8.7 | 5.4 | 1.5 |
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured with a Jasco P-1020 polarimeter using a 1-cm cell. UV spectra were obtained using a Perkin Elmer Lambda 35 UV/VIS spectrophotometer (Perkin Elmer, Waltham, MA, USA). CD spectra were recorded using an Applied Photophysics Chirascan-Plus circular dichroism spectrometer (Applied Photophysics Ltd., Leatherhead, Surrey, UK). IR spectra were acquired using a Thermo Nicolet iS10 spectrometer (Thermo, Madison, CT, USA). 1H, 13C, and 2D NMR spectra were obtained using a Bruker Avance 600-MHz (National Center for Interuniversity Research Facilities (NCIRF) at Seoul National University) spectrometer (Bruker, Billerica, MA, USA). Electrospray ionization (ESI) low-resolution LC/MS data were acquired with an Agilent Technologies 6130 quadrupole mass spectrometer coupled with an Agilent Technologies 1200-series HPLC (Agilent Technologies, Santa Clara, CA, USA) using a reversed-phase C18 column (Phenomenex Luna, 100 × 4.6 mm) (Phenomenex, Torrence, CA, USA). HR-FAB mass spectra were obtained using a Thermo Scientific Q high-resolution mass spectrometer (Thermo, Madison, CT, USA) at the NCIRF at Seoul National University.
3.2. Isolation, Cultivation, and Extraction of the Halophilic Actinomycete Strain
A saltern topsoil sample was collected on Jeung-do Island in Shinan-gun, Jeollanamdo, Korea. The sample (1 g) was diluted in 10 mL of sterilized artificial saline water (3× the saline concentration of seawater) and vortexed. The mixture was spread onto actinomycete isolation agar, A4 medium (1 L of artificial seawater, 18 g of agar, and 100 mg/L cycloheximide), A5 medium (750 mL of artificial seawater, 250 mL of distilled H2O, 18 g of agar, and 100 mg/L cycloheximide), A6 medium (1 L of artificial seawater, 18 g of agar, and 5 mg/L polymyxin B sulfate), A7 medium (1 L of artificial seawater, 18 g of agar, and 5 mg/L kanamycin), and chitin-based agar (1 L of artificial seawater, 4 g of chitin, 0.75 g of K2HPO4, 0.5 g of MgSO4∙7H2O, 3.5 g of KH2PO4, 10 mg of FeSO4∙7H2O, 10 mg of MnCl2∙4H2O, 10 mg of ZnSO4∙7H2O, 18 g of agar, and 100 mg/L cycloheximide). The strain HYJ128 was isolated on actinomycete isolation agar medium. Colonies were repeatedly inoculated onto fresh agar plates to obtain single strains. HYJ128 16S rDNA sequence analysis data obtained from COSMO Co., Ltd. (Cosmogenetech co. Ltd., Seoul, Korea) revealed that HYJ128 is most closely related to Nocardiopsis lucentensis (99% identity), identifying the strain as a Nocardiopsis sp. (GenBank accession number: LC013479). The HYJ128 strain was cultured in 50 mL of A1+ mannitol medium (4 g of yeast extract, 10 g of starch, 2 g of peptone, and 4 g of mannitol in 1 L of artificial seawater) in a 125-mL Erlenmeyer flask. After the strain was cultivated for five days on a rotary shaker at 200 rpm at 30 °C, 10 mL of the culture was inoculated into 200 mL of YEME medium in a 500-mL Erlenmeyer flask. The strain HYJ128 was further cultivated for five days, and 10 mL of the culture was inoculated into 1 L of the same medium in 2.8-L Fernbach flasks. These large cultures (36 L) were incubated at 200 rpm at 30 °C. After five days, the entire culture volume was extracted twice using ethyl acetate. The ethyl acetate layer was separated and dried over anhydrous sodium sulfate. The ethyl acetate extract was enriched in vacuo to yield 7 g of dried material.
3.3. Isolation of the Borrelidins
The dried extract was adsorbed on celite, loaded onto a 20-g C18 resin, and fractionated with 200 mL each of 20, 40, 60, 80, and 100% MeOH in H2O and 1:1 MeOH/CH2Cl2. The borrelidins (1–4) were found in the 60% MeOH/H2O fraction. To purify 1–4, the 60% fraction was chromatographed using reversed-phase HPLC with a C18 column (Kromasil, 5 μm, 250 × 10 mm) under gradient conditions (45–80% MeOH/water with UV detection at 254 nm and a flow rate of 2 mL/min). Finally, borrelidin C (1) (13 mg), borrelidin D (2) (7 mg), borrelidin E (3) (7 mg), and borrelidin (4) (25 mg) eluted as pure compounds at retention times of 45, 47, 31, and 51 min, respectively.
3.3.1. Borrelidin C (1)
White powder; +20 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 257 (4.50) nm; CD (MeOH) (Δ ε) 225 (3.54), 259 (2.70) nm; IR (neat) νmax 3404, 2214, 1717, 1566, 1275 cm−1; 1H and 13C NMR data, see Table 1; HR-FAB-MS m/z 504.2959 [M − H]− (calcd. for C28H42NO7 504.2961).
3.3.2. Borrelidin D (2)
White powder; +15 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 257 (4.50) nm; CD (MeOH) (Δ ε) 223 (3.55), 261 (0.98) nm; IR (neat) νmax 3402, 2216, 1718, 1566, 1265 cm−1; 1H and 13C NMR data, see Table 1; HR-FAB-MS m/z 504.2958 [M − H]− (calcd. for C28H42NO7 504.2961).
3.3.3. Borrelidin E (3)
White powder; +12 (c 0.1, MeOH); UV (MeOH) λmax (log ε) 257 (4.50) nm; CD (MeOH) (Δ ε) 225 (2.23), 255 (2.89) nm; IR (neat) νmax 3444, 2204, 1710, 1555, 1273 cm−1; 1H and 13C NMR data, see Table 1; HR-FAB-MS m/z 504.2966 [M − H]− (calcd. for C28H42NO7 504.2961).
3.4. MTPA Esterification of Borrelidins C–D (1–2)
Each compound (1 mg) was prepared in two 40-mL vials and dried for eight hours in vacuo. A volume of 1 mL of anhydrous pyridine was injected under argon gas into each vial to dissolve the compound. Each vial was stirred for 15 min at room temperature. After 15 min, R-MTPA chloride and S-MTPA chloride (20 μL) were added separately to the reaction vials. The reactions were terminated after 4 h by adding MeOH. The esterified products were purified via HPLC using a C18 column (Kromasil, 5 μm, 250 × 10 mm) with a gradient of 50% MeOH/water to 100% MeOH over 45 min, at a flow rate of 2 mL/min, and using UV absorbance at 254 nm for detection. Bis-MTPA esters (1a, 1b, 2a, and 2b) eluted at the retention times of 50, 51, 52, and 54 min, respectively. 1H NMR, COSY, and TOCSY NMR experiments were performed to calculate ΔδS-R values.
3.4.1. Bis-S-MTPA Ester (1a) of Borrelidin C (1)
1H NMR (600 MHz, pyridine-d5) δ 7.78–7.72 (m, 4H), 7.50–7.42 (m, 6H), 7.06–6.99 (m, 2H), 6.40 (m, 1H), 5.75 (d, J = 11.0, 1H), 5.73 (d, J = 11.0, 1H), 5.31 (m, 1H), 5.06 (m, 1H), 3.62 (s, 3H), 3.60 (s, 3H), 2.90 (m, 1H), 2.85 (s, 1H), 2.83 (m, 1H), 2.8 (m, 1H), 2.66 (m, 1H), 2.62 (m, 1H), 2.57 (m, 1H), 2.56 (m, 1H), 2.42 (m, 1H), 2.33 (m, 1H), 2.16 (dd, J = 14.0, 7.0, 1H), 1.72–1.62 (m, 3H), 1.61–1.53 (m, 3H), 1.42 (m, 1H), 1.23–1.13 (m, 2H), 1.03 (d, J = 6.0, 3H), 1.01 (d, J = 6.5, 3H), 0.95 (d, J = 6.0, 3H), 0.92 (d, J = 5.5, 3H).
3.4.2. Bis-R-MTPA Ester (1b) of Borrelidin C (1)
1H NMR (600 MHz, pyridine-d5) δ 7.80–7.73 (m, 4H), 7.51–7.41 (m, 6H), 7.08–7.01 (m, 2H), 6.38 (m, 1H), 5.74 (d, J = 11.0, 1H), 5.70 (m, 1H), 5.40 (m, 1H), 5.01 (m, 1H), 3.78 (s, 3H), 3.62 (s, 3H), 2.87–2.78 (m, 4H), 2.67 (m, 1H), 2.65 (m, 1H), 2.57 (m, 1H), 2.56 (m, 1H), 2.39 (m, 1H), 2.30 (m, 1H), 2.06 (dd, J = 14.0, 7.0, 1H), 1.74–1.64 (m, 2H), 1.63–1.56 (m, 3H), 1.54 (m, 1H), 1.40 (t, J = 12.5, 1H), 1.20–1.14 (m, 2H), 1.01 (d, J = 7.0, 3H), 0.92 (d, J = 6.0, 3H), 0.90 (d, J = 6.0, 3H), 0.74 (m, 3H).
3.4.3. Bis-S-MTPA Ester (2a) of Borrelidin D (2)
1H NMR (600 MHz, pyridine-d5) δ 7.85–7.82 (m, 2H), 7.79–7.76 (m, 2H), 7.57–7.44 (m, 6H), 7.23 (m, 1H), 6.85 (dd, J = 14.0, 11.0, 1H), 6.43 (ddd, J = 14.0, 11.0, 5.0, 1H), 5.91 (d, J = 11.0, 1H), 5.73 (d, J = 11.0, 1H), 5.23 (m, 1H), 4.93 (m, 1H), 3.74 (s, 3H), 3.66 (s, 3H), 3.18 (dd, J = 17.0, 2.0, 1H), 2.69 (dd, J = 17.0, 11.0, 1H), 2.62 (m, 1H), 2.56–2.54 (m, 2H), 2.40 (m, 1H), 2.31 (m, 1H), 2.13 (m, 1H), 1.98–1.94 (m, 2H), 1.87 (d, J = 11.0, 1H), 1.67 (m, 1H), 1.56 (dd, J = 13.0, 3.0, 1H), 1.42 (m, 1H), 1.30–1.25 (m, 2H), 1.13–1.08 (m, 2H), 1.07 (d, J = 6.0, 3H), 1.04 (dd, J = 8.5, 2.0, 1H), 1.02 (d, J = 1.5, 1H), 0.97 (d, J = 7.0, 3H), 0.90 (d, J = 6.5, 3H), 0.87 (d, J = 6.0, 3H).
3.4.4. Bis-R-MTPA Ester (2b) of Borrelidin D (2)
1H NMR (600 MHz, pyridine-d5) δ 7.93–7.89 (m, 2H), 7.85–7.82 (m, 2H), 7.54–7.46 (m, 6H), 7.26 (m, 1H), 6.90 (dd, J = 15.0, 11.0, 1H), 6.44 (m, 1H), 5.81 (d, J = 11.0, 1H), 5.76 (d, J = 11.0, 1H), 5.27 (m, 1H), 4.91 (m, 1H), 3.78 (s, 3H), 3.76 (s, 3H), 3.21 (dd, J = 17.5, 2.0, 1H), 2.75 (dd, J = 17.5, 11.0, 1H), 2.66 (m, 1H), 2.53–2.48 (m, 2H), 2.41 (m, 1H), 2.31 (m, 1H), 2.12 (m, 1H), 1.97 (d, J = 11.0, 1H), 1.94–1.85 (m, 2H), 1.65 (m, 1H), 1.56 (dd, J = 13.0, 3.0, 1H), 1.43 (m, 1H), 1.25–1.19 (m, 2H), 1.08–1.00 (m, 4H), 0.95 (d, J = 7.0, 3H), 0.89 (d, J = 7.0, 3H), 0.88 (d, J = 7.0, 3H), 0.74 (d, J = 6.5, 3H).
3.5. Antibacterial Activity Assay
Gram-positive bacteria (S. aureus ATCC 25923, E. faecalis ATCC 19433, E. faecium ATCC 19434) and Gram-negative bacteria (P. hauseri NRBC 3851, K. pneumoniae ATCC 10031, S. enterica ATCC 14028, E. coli ATCC 25922) were used for antimicrobial activity tests. Bacteria were grown overnight in Luria Bertani (LB) broth (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) at 37 °C, harvested by centrifugation, and then washed twice with sterile distilled water. Stock solutions of the borrelidins (1–4) were prepared in DMSO. Each stock solution was diluted with m Plate Count Broth (Difco) to prepare serial two-fold dilutions in the range of 50 to 0.8 μg/mL. Ten microliters of the broth, containing approximately 105 colony-forming units (cfu)/mL of test bacteria, was added to each well of a 96-well microliter plate. The culture plates were incubated for 12 h at 37 °C. The MIC values were determined as the lowest concentration of test compound that inhibited bacterial growth. Ampicillin was used as a reference compound.
3.6. Cytotoxicity Assay
The effect of borrelidins (1–4) on cell proliferation was measured by the sulforhodamine B (SRB) cellular protein-staining method with some modifications. In brief, A549 (lung cancer), HCT116 (colon cancer), SNU638 (stomach cancer), SK-HEP1 (liver cancer), MDA-MB231 (breast cancer), and K562 (leukemia) cells (1 × 104 cells in 190 μL of complete DMEM) were seeded in 96-well plates with various concentrations of borrelidins (1–4) and incubated at 37 °C in a humidified atmosphere with 5% CO2. After 72 h of borrelidin (1–4) treatment, the cells were fixed with 10% TCA solution for 1 h, and cellular proteins were stained with a solution of 0.4% SRB in 1% acetic acid. The stained cells were dissolved in 10 mM Tris buffer (pH 10.0). The effect of borrelidins (1–4) on cell viability was calculated as a percentage relative to a solvent-treated control, and the IC50 values were calculated using a nonlinear regression analysis (percent survival versus concentration). Etoposide was used as a positive control.
4. Conclusions
Chemical investigation of the rare saltern-derived actinomycete Nocardiopsis sp. strain HYJ128 resulted in the discovery of new members of the borrelidin class of antibiotics. Borrelidins C and D (1, 2) and borrelidin (4) displayed antibacterial inhibitory activity, particularly against the Gram-negative pathogen S. enterica, indicating that the borrelidin class could be a useful scaffold to develop antibiotics against S. enterica. Moreover, additional hydroxylation at C-20 or C-7 of borrelidin, resulting in borrelidins C–E, significantly reduced or even abolished antibacterial activity. Borrelidins C–D exhibited significant cytotoxic effects against the SNU638 and K562 carcinoma cell lines, whereas borrelidin E did not show any significant activity in these assays. Borrelidins C–E are rare members of the borrelidin class of antibiotics. Although borrelidin was initially reported in a terrestrial Streptomyces sp. in the early days of antibiotic discovery in 1949 [9], only one additional member, borrelidin B, in which the nitrile functional group was reduced to a primary amine, has been reported, and this member was isolated from a marine sediment-derived Streptomyces sp. in 2014 [14]. The discovery of borrelidins C–E as the first secondary metabolites from a saltern-derived rare actinomycete genus Nocardiposis emphasizes the unexplored potential of halophilic rare actinomycetes inhabiting extremely saline saltern environments.
Acknowledgments
This work was supported by a National Research Foundation (NRF) of Korea grant funded by the Korean government (Ministry of ICT and Future Planning; 2009-0083533) and a grant from the Marine Biotechnology Program (Genome Analysis of Marine Organisms and Development of Functional Applications, PJT200620).
Supplementary Materials
The following are available online at www.mdpi.com/1660-3397/15/6/166/s1. Figures S1–S6: 1H, 13C, COSY, HSQC, HMBC, and ROESY NMR data of 1 in pyridine-d5, Figures S7–S12: 1H, 13C, COSY, HSQC, HMBC, and ROESY NMR data of 2 in pyridine-d5, Figures S13–S18: 1H, 13C, COSY, HSQC, HMBC, and ROESY NMR data of 3 in pyridine-d5, Figures S19 and S20: 1H and COSY NMR data of S-MTPA ester (1a) of 1 in pyridine-d5, Figures S21 and S22: 1H and COSY NMR data of R-MTPA ester (1b) of 1 in pyridine-d5, Figures S23 and S24: 1H and COSY NMR data of S-MTPA ester (2a) of 2 in pyridine-d5, Figures S25 and S26: 1H and COSY NMR data of S-MTPA ester (2b) of 2 in pyridine-d5, Figure S27: 1H NMR data of homo-decoupling experiment by irradiation 1H at δ 2.38 for borrelidin E (3) in pyridine-d5, Figure S28: Experimental CD spectra of 1–4.
Author Contributions
Jungwoo Kim, Seong-Hwan Kim, Daniel Shin, Wanki Park, Yoonho Shin, Won Kyung Kim, Sang Kook Lee, Ki-Bong Oh, Jongheon Shin and Dong-Chan Oh conceived and designed the experiments. Jungwoo Kim and Seong-Hwan Kim isolated and cultivated the bacterial strain, isolated the compounds, performed the chemical experiments, and analyzed the spectroscopic data. Daniel Shin analyzed the spectroscopic and biological data. Wanki Park performed the antibacterial tests. Yoonho Shin and Won Kyung Kim evaluated cytotoxicities. Jungwoo Kim, Daniel Shin, Sang Kook Lee, Ki-Bong Oh, Jongheon Shin, and Dong-Chan Oh wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jensen P.R., Moore B.S., Fenical W. The marine actinomycete genus Salinispora: A model organism for secondary metabolite discovery. Nat. Prod. Rep. 2015;32:738–751. doi: 10.1039/C4NP00167B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenical W., Jensen P.R. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat. Chem. Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]
- 3.Jensen P.R., Gontang E., Mafnas C., Mincer T.J., Fenical W. Culturable marine actinomycete diversity from tropical Pacific Ocean sediments. Environ. Microbiol. 2005;7:1039–1048. doi: 10.1111/j.1462-2920.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 4.Duangmal K., Suksaard P., Pathom-aree W., Mingma R., Matsumoto A., Takahashi Y. Actinopolyspora salinaria sp. nov., a halophilic actinomycete isolated from solar saltern soil. Int. J. Syst. Evol. Microbiol. 2016;66:1660–1665. doi: 10.1099/ijsem.0.000926. [DOI] [PubMed] [Google Scholar]
- 5.Kim S.-H., Shin Y., Lee S.K., Shin J., Oh D.-C., Lee S.-H., Oh K.-B. Salternamides A-D from a halophilic Streptomyces sp. actinobacterium. J. Nat. Prod. 2015;78:836–843. doi: 10.1021/acs.jnatprod.5b00002. [DOI] [PubMed] [Google Scholar]
- 6.Kim S.-H., Shin Y., Lee S.K., Shin J., Oh D.-C. Salternamide E from a saltern-derived marine actinomycete Streptomyces sp. Nat. Prod. Sci. 2015;21:273–277. doi: 10.20307/nps.2015.21.4.273. [DOI] [Google Scholar]
- 7.Bach D.-H., Kim S.-H., Hong J.-Y., Park H.J., Oh D.-C., Lee S.K. Salternamide A suppresses hypoxia-induced accumulation of HIF-1α and induces apoptosis in human colorectal cancer cells. Mar. Drugs. 2015;13:6962–6976. doi: 10.3390/md13116962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S.-H., Ha T.-K.-Q., Oh W.K., Shin J., Oh D.-C. Antiviral indolosesquiterpenoid xiamycins C-E from a halophilic actinomycete. J. Nat. Prod. 2016;79:51–58. doi: 10.1021/acs.jnatprod.5b00634. [DOI] [PubMed] [Google Scholar]
- 9.Berger J., Jampolsky L.M., Goldberg M.W. Borrelidin, a new antibiotic with antiborrelia activity and penicillin-enhancement properties. Arch. Biochem. 1949;22:476–478. [PubMed] [Google Scholar]
- 10.Pretsch E., Bȕhlmann P., Affolter C. Structure Determination of Organic Compounds—Tables of Spectral Data. Springer; New York, NY, USA: 2000. [Google Scholar]
- 11.Freire F., Seco J.M., Quiñoá E., Riguera R. Determining the absolute stereochemistry of secondary/secondary diols by 1H NMR: Basis and applications. J. Org. Chem. 2005;70:3778–3790. doi: 10.1021/jo048643a. [DOI] [PubMed] [Google Scholar]
- 12.Matsumori N., Kaneno D., Murata M., Nakamura H., Tachibana K. Stereochemical determination of acyclic structures based on carbon-proton spin-coupling constants. A method of configuration analysis for natural products. J. Org. Chem. 1999;64:866–876. doi: 10.1021/jo981810k. [DOI] [PubMed] [Google Scholar]
- 13.Hurley D., McCusker M.P., Fanning S., Martins M. Salmonella-host interactions—Modulation of the host innate immune system. Front. Immunol. 2014;5:481. doi: 10.3389/fimmu.2014.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulze C.J., Bray W.M., Loganzo F., Lam M.-H., Szal T., Villalobos A., Koehn F.E., Linington R.G. Borrelidin B: Isolation, biological activity, and implications for nitrile biosynthesis. J. Nat. Prod. 2014;77:2570–2574. doi: 10.1021/np500727g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.