Abstract
Human dendritic cells (DCs) play a fundamental role in the initiation of long-term adaptive immunity during vaccination against influenza. Understanding the early response of human DCs to vaccine exposure is thus essential to determine the nature and magnitude of maturation signals that have been shown to strongly correlate with vaccine effectiveness. In 2009, the H1N1 influenza epidemics fostered the commercialization of the non-adjuvanted monovalent H1N1 California vaccine (MIV-09) to complement the existing non-adjuvanted trivalent Fluzone®09–10 vaccine (TIV-09). In retrospective studies, MIV-09 displayed lower effectiveness than TIV-09. Here, we show that TIV-09 induces monocyte-derived DCs (moDCs), blood conventional DCs (cDCs) and plasmacytoid DCs (pDCs) to express CD80, CD83, and CD86, and secrete cytokines. TIV-09 stimulated the secretion of type I interferons (IFN) IFN-α and IFN-β and type III IFN IL-29 by moDCs and cDCs subsets. The vaccine also induced the production of IL-6, TNF and the chemokines IP-10 and MIP-1β. Conversely, MIV-09 did not induce the production of type I IFN in moDCs and blood cDCs. Furthermore, it inhibited the TIV-09-induced secretion of type I IFN by these DCs. Yet, both vaccines induced pDCs to secrete type I IFN indicating that different influenza vaccines activate distinct molecular signaling pathways in DC subsets. These results suggest that subtypes of non-adjuvanted influenza vaccines trigger immunity through different mechanisms and that the ability of a vaccine to induce an IFN response in DCs may offset the absence of adjuvant and increase vaccine efficacy.
INTRODUCTION
Vaccination is the most efficient way to protect humans against influenza. Several influenza vaccines are currently available including split subunit vaccines and attenuated viral vaccines (1). Split vaccines, which are most commonly used, are prepared by culture of the influenza virus in hen eggs or cell cultures, followed by purification and treatment with detergents (2). In the US, split vaccines are used without adjuvants while in Europe, some vaccines contain adjuvants such as MF59 or AS03 (3, 4). Because the composition of influenza vaccines varies annually based on results from global influenza surveillance data, the determination of vaccine effectiveness remains a continuous challenge.
The need for rapid implementation of a vaccination program during the outbreak of the pandemic influenza A(H1N1)pdm09 virus in 2009 led initial studies to focus on immunogenicity rather than overall efficacy of the vaccine. Several influenza vaccines were then marketed, including the trivalent non-adjuvanted seasonal flu vaccine Fluzone®09–10 (hereafter TIV-09) containing A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2)-like and B/Brisbane/60/2008 viral strains and the monovalent H1N1 vaccine (hereafter MIV-09) containing A/California/7/2009 (H1N1)-like virus. The monovalent vaccine licensed in Europe and Canada was adjuvanted while the one licensed in Australia and the US was not. Subsequent vaccine trials performed with TIV-09 or the adjuvanted forms of MIV-09 demonstrated efficacy (5–9). In contrast, the non-adjuvanted form of MIV-09 displayed lower effectiveness, low seroprotection in healthy children (35%) and suboptimal response in solid organ transplant (SOT) recipients and children with systemic lupus erythematosus (SLE) (9–13). It is unclear why non-adjuvanted TIV-09 and MIV-09, two vaccines produced by the same manufacturer (Sanofi-Pasteur), display such disparate efficacy levels.
Traditionally, vaccine efficacy has been measured by hemagglutinin inhibiting antibody response as a correlate of humoral immunity (14). Some studies suggested that assessment of T-cell responses might provide a better correlate of vaccine protection against influenza, especially in the elderly (15). Other studies suggested that influenza vaccines might differentially activate subpopulations of immune cells (16, 17). We and others demonstrated that administration of seasonal influenza vaccines to healthy volunteers induces global transcriptional changes in whole blood that can be followed at discrete time points (18–23). In particular, a transcriptional signature of interferon (IFN)-inducible genes was observed between 12 and 36h post-vaccination, followed by a plasmablast signature at day 7 (23). This early signature may represent an important correlate of vaccine efficacy, as type I IFNs enhance antigen cross-presentation to CD8+ T cells, foster T-helper 1 differentiation, support B cell differentiation into antibody-producing plasmablasts and induce dendritic cell (DC) maturation (24–26). DCs play an essential role in vaccination, by detecting and presenting foreign antigens to adaptive immune cells (27, 28). We recently showed that different DC subsets, including monocyte-derived DCs (moDCs) and blood-derived conventional DCs (cDCs), respond differently to various bacterial and viral vaccines (29).
To better understand the biological mechanisms underlying the difference in effectiveness of non-adjuvanted TIV-09 and MIV-09, we compared their ability to activate human DC subsets. Our results suggest that in the absence of adjuvant, TIV-09 activates a broader range of DC subsets than MIV-09 in a type I IFN-dependent manner, possibly explaining the increased efficacy of the former vaccine.
RESULTS
TIV-09 but not MIV-09 activates moDCs and induces type I IFN secretion
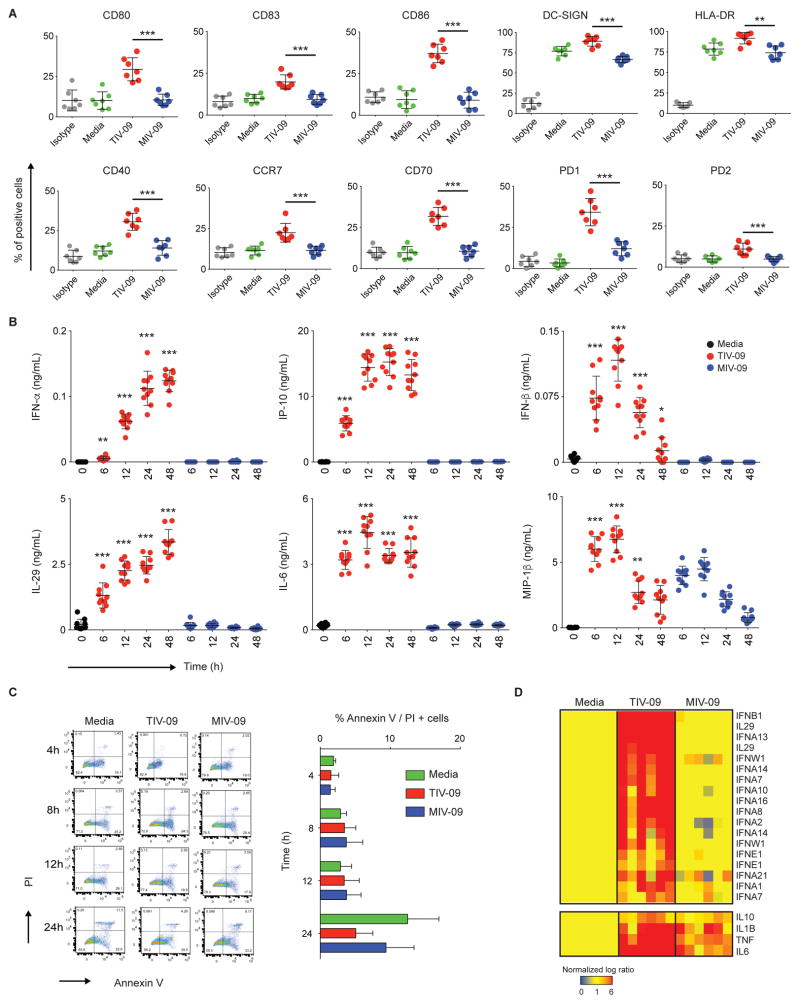
To assess the effects of TIV-09 and MIV-09 on DC activation, moDCs were obtained by culturing isolated human monocytes in the presence of GM-CSF and IL-4 for 6 days, and subsequently exposed to each vaccine for 18h. TIV-09 upregulated the surface expression of hallmark DC maturation markers CD40, CD80, CD83, CD86, HLA-DR, PD-L1, PD-L2 and CCR7 (Fig. 1A, n=7). Compared to TIV-09, MIV-09 induced significantly (p<0.01) lower expression of these markers. moDC activation was further assessed by measuring the release of cytokines at different time points following vaccine challenge. IFN-β reached its peak level at 12h and decreased to near baseline values after 48h, suggesting its consumption. MIP-1β reached a peak at 12h and slightly decreased thereafter. Other cytokines such as IFN-α, IL-29, IL-6 and IP-10 attained peak levels by 12–24h and remained stable at 48h. Conversely, MIV-09 did not induce cytokine secretion, with the exception of MIP-1β (Fig. 1B, n=10). Because TIV (45 μg) contains three times more viral protein than MIV-09 (15 μg), a dose-dependent analysis was conducted. Even at high concentration (>4-fold), MIV-09 failed to induced the secretion of IFN-β and IP-10 by moDCs, suggesting that protein concentration cannot explain the observed differences (Fig. S1). Staining with propidium iodide and Annexin V at 4, 8, 12 and 24h also revealed that the lack of cytokines observed with H1N1 was not due to vaccine-induced cell death (Fig. 1C, n=6). We further assessed IP-10 production by moDCs in the presence of other inactivated viral vaccines such as Human Papilloma Virus (Merk), Hepatitis A, Hepatitis B (GSK) and Rabies (Sanofi Pasteur) (Table S1). Only TIV-09 induced secretion of IP-10 by moDCs (Fig. S2).
Figure 1. TIV-09, but not MIV-09, activates moDCs.
(A) moDCs were stimulated with TIV-09 (6 μL/mL), MIV-09 (6 μL/mL) or media for 18h. Surface expression of moDC activation markers was assessed by flow cytometry (N=7). (B) moDCs were cultured in the presence of TIV-09 or MIV-09 (6 μL/mL) for 0, 6, 12, 24 and 48h. Supernatants were harvested for quantification of cytokines by Luminex (N=10). Statistics shown represent the comparison between TIV-09 and MIV-09 at each time point. (C) moDCs were stained with Annexin V and PI to examine cell death at 4h, 8h, 12h and 24h post-vaccine treatment (N =6). (D) Transcriptional analysis of IFNs and other cytokines in moDCs cultured with TIV-09 or MIV-09 for 6h. Data were normalized to media control (N =6). (Mann-Whitney test; *: p<0.05; **: p<0.01; ***: p<0.001).
Gene expression profiling was performed by microarray on moDCs from six healthy individuals cultured for 6h with TIV-09 or MIV-09. TIV-09 upregulated all known IFN-α transcripts, IFN-β, IFN-ω and IL-29 (Fig. 1D, n=6). Consistent with the protein data, MIV-09 failed to increase the transcription of type I and III IFN genes. In addition, TIV-09 upregulated the expression of other pro-inflammatory and regulatory cytokines including TNF, IL-6, IL-1β and IL-10, which were also slightly upregulated in response to MIV-09. Taken together, these results indicate that the seasonal Influenza vaccine TIV-09, but not MIV-09, potently activates moDCs including induction of type I and III IFNs.
Cell-intrinsic type I IFN production contributes to moDC activation
Upon activation, several cell types first transcribe the IFN-β gene, which results in the prompt secretion of IFN-β. IFN-β then binds to the IFN receptor (IFNAR), consequently initiating IFN-α production (30, 31). To determine whether the early secretion of IFN-β by moDCs might contribute to their further activation, moDCs were exposed to TIV-09 in the presence of a blocking anti-IFN-α/βR2 monoclonal antibody. Activation was assayed by measuring the secretion of the IFN-inducible cytokine IP-10. Addition of anti-IFN-α/βR2 antibody abolished IP-10 production and strongly reduced production of IFN-α and IL-29 without affecting that of IFN-β (Fig. 2A, n=4). In addition, IFNAR blockade resulted in the upregulation of IL-1β, TNF, and IL-10, suggesting an autocrine inhibitory loop (32). The addition of anti-IFN-α/βR2 antibody also partially inhibited the upregulation of CD80, CD83, CD86, CD70, and CD40 (Fig. 2B, n=4), suggesting that moDC maturation is dependent on type I IFN secretion. These data indicate that the early burst of IFN-β production contributes to TIV-09-induced activation of moDCs.
Figure 2. TIV-09 is a strong inducer of type I IFN.
(A) moDCs were treated with increasing doses of TIV-09 with or without anti-IFNAR antibody (10 μg/mL) for 8h. Supernatants were assessed by Luminex for the presence of type I IFN and inflammatory cytokines. Statistics shown represent the comparison between TIV-09 and TIV-09 with IFNα/β R2 antibody at each concentration. (Mann-Whitney test; *: p<0.05; **: p<0.01; ***: p<0.001). (B) moDCs were stimulated with TIV-09 (6 μL/mL) with or without anti-IFNAR antibody for 18h. Surface expression of DC markers was assessed by flow cytometry. The figure is representative of experiments in four independent donors.
MIV-09 inhibits the IFN response in moDC
We next analyzed the effects of adding the H1N1 strain A/California/07/2009 X-179A in vaccines by testing several Fluzone® batches prepared over different years on moDCs. Fluzone® vaccines from years 2006–2007, 2008–2009, and 2009–2010, composed of three closely related influenza strains (see Methods) induced moDCs to secrete similar levels of IP-10 (Fig. 3A, n=7). However, Fluzone® 2010–2011, which contains the H1N1 strain A/California/07/2009 X-179A, failed to induce IP-10 secretion by moDCs.
Figure 3. MIV-09 inhibits type I IFN.
(A) moDCs were treated with increasing doses of Influenza vaccines used during the 2006–2007, 2008–2009, 2009–2010 and 2010–2011 seasons. The graph summarizes data from seven donors. (B) moDCs were treated with 25 μL/mL TIV-09 combined with increasing doses of MIV-09 for 8h. Supernatants were harvested for ELISA. The graph summarizes data from seven donors. (C) moDCs were activated with TIV-09 (6 μL/mL), LPS (50 ng/mL), CD40L (1 μg/mL), R848 (3 μg/mL), or CL097 (5 μg/mL) alone or with MIV-09 (10 μL/mL) with or without recombinant IFN-α (2000 U/mL). Supernatant cytokine concentrations were assessed by Luminex. The graph summarizes data from seven donors. (D) moDCs were stimulated with TIV-09, LPS, CD40L, R848 or CL097 with or without MIV-09. The surface expression of moDC activation markers was examined by flow cytometry. The figure is representative of experiments in four independent donors. (E) Ingenuity Pathway Analysis of the transcripts unique to each vaccine and most highly expressed after vaccine exposure. The top three canonical pathways enriched in the transcripts induced by each vaccine are shown (N=3). (Mann-Whitney test; *: p<0.05; **: p<0.01; ***: p<0.001).
To determine whether MIV-09 could inhibit moDC activation, we activated moDCs with TIV-09 or selected TLR agonists, including LPS (TLR4 ligand), R848 (TLR7/8 ligand), CL097 (TLR7/8 ligand) and Poly(I:C) (TLR3 and RIG-I/MDA5 ligand), with or without MIV-09. At a concentration of 25 μg/mL, MIV-09 not only completely abrogated the secretion of IP-10 induced by TIV-09, but also the secretion of other IFN-related cytokines including IFN-β, IL-29 and IL-6 (Fig. 3B, 3C, n=7). MIV-09 also inhibited the secretion of cytokines by moDCs activated by CD40L and all TLR agonists tested. As MIV-09 could inhibit the early IFN-β response, we wondered whether addition of exogenous recombinant IFN-α might reverse the inhibitory effects of MIV-09 on the secretion of other cytokines, which was not the case (Fig. 3C, n=7). MIV-09 also prevented the expression of CD80, CD83, CD86, CD70 and CD40 induced by TIV-09 and all tested DC-activating stimuli (Fig. 3D, n=4). These results indicate that MIV-09 acts as a potent inhibitor of cytokine secretion and maturation in activated moDC.
To further understand the molecular alterations induced by both vaccines at the pathway level, we conducted Ingenuity Pathway Analysis (IPA) on the transcriptional fingerprints of vaccine treated-cells (Fig 3E). The top pathways associated with TIV-09 exposure included IFN signaling (yellow, blue), protein ubiquitination (blue), interferon regulatory factor (IRF) signaling (yellow) and retinoic acid-mediated signaling (yellow). These pathways were suppressed when moDCs were exposed to MIV-09. On the other hand, MIV-09 enhanced the ERK/MAPK signaling pathway (purple), which can inhibit IFN responses (33). These results suggest that MIV-09 inhibits IFN signals and moDC maturation by skewing the transcriptional cascade of vaccine-exposed moDC towards ERK-mediated pro-inflammation.
TIV-09 elicits a type I IFN signature in blood cDCs
As moDCs and blood cDCs differ phenotypically and functionally, we next assessed the effects of TIV-09 and MIV-09 on sorted ex vivo blood cDCs. As observed in moDCs, the surface expression of CD80, CD83, CD86 and HLA-DR was increased on cDCs exposed for 18h to TIV-09. Exposure to MIV-09 was associated with lower induction of CD80, CD83 and CD86 (Fig. 4A, n=4). While cDCs express significantly lower levels of type I IFN than pDC (34), cDCs exposed to TIV-09, but not to MIV-09, displayed upregulation of both IFN-inducible transcripts and IP-10 secretion (Fig. 4B, n=3 and 4C, n=6). As observed with moDCs, MIV-09 prevented the TIV-09-dependent secretion of IP-10 by cDCs (Fig. 4D, n=6) and downregulated TIV-09-induced surface expression of CD83, CD86 and HLA-DR (Fig. 4E, n=4). These data suggest that MIV-09 also blocks IFN signals in ex vivo cDCs.
Figure 4. TIV-09 activates cDCs.
(A) Sorted blood cDCs were stimulated in vitro for 18h with media alone, TIV-09 or MIV-09. Surface expression of activation markers was examined by flow cytometry. The figure is representative of experiments in four independent donors. (B) Transcriptional analysis of IFNs in cDCs stimulated with TIV-09 or MIV-09 for 6h. Data were normalized to medium control (N=3) (C) cDCs were activated in vitro with media, TIV-09 or MIV-09 for 0, 6, 24 and 48h. IP-10 levels were measured by ELISA. The graph summarizes data from six donors (D) cDCs were treated with TIV-09 (6 μL/mL), combined with different doses of MIV-09 for 8h. Supernatants were harvested and IP-10 was measured by ELISA. The graph summarizes data from six donors (E) cDCs were stimulated with TIV-09 in the presence or absence of MIV. Surface expression of CD80, CD86, CD83 and HLA-DR was measured by flow cytometry. The figure is representative of a result from experiments on four independent donors. (Mann-Whitney test; *: p<0.05; **: p<0.01; ***: p<0.001).
Both vaccines elicit a type I IFN signature in blood pDCs
Plasmacytoid DCs (pDCs) promptly secrete large amounts of type I IFN in response to a number of stimuli including viruses (35). Therefore, we exposed pDCs to TIV-09 or MIV-09 for 18h and assessed the expression of activation markers by FACS and IFN transcription by microarray. Both vaccines upregulated CD80, CD86 and HLA-DR expression with TIV-09 being consistently more potent than MIV-09 (Fig. 5A, n=4). Transcriptional analysis revealed that both vaccines upregulated type I IFN transcripts (Fig. 5B, n=3). MIV-09 was less efficient than TIV-09 at inducing the secretion of IP-10 and IFN-β (Fig. 5C, n=6, 5D, n=6). When the two vaccines were combined, MIV-09 suppressed TIV-09-induced IP-10 production by about 70% (Fig. 5E, n=6) and downregulated TIV-09-induced surface expression of CD83 (Fig. 5F, n=4).
Figure 5. TIV-09 activates pDCs.
(A) Sorted pDCs were activated in vitro with TIV-09 or MIV-09 or control media for 18h and surface expression of activation markers was measured by flow cytometry. The figure is representative of experiments in four independent donors (B) Transcriptional analysis of IFNs in pDCs stimulated with TIV-09 or MIV-09 for 6h. Data were normalized to medium control (N=3) (C, D) pDCs were activated in vitro with media, TIV-09 or MIV-09 for 0, 6, 24 and 48h. IP-10 and IFN-β levels were measured by ELISA. (E) pDCs were treated with TIV-09 (6 μL/mL), combined with different doses of MIV-09 for 8h. Supernatants were harvested and IP-10 levels were measured by ELISA. Figs 5 C, D and E summarize data from six donors. (F) pDC were stimulated with TIV-09 in the presence or absence of MIV surface expression of surface expression of CD80, CD86, CD83 and HLA-DR, measured by flow cytometry. The figure is representative of a result from experiments on four independent donors. (Mann-Whitney test; *: p<0.05; **: p<0.01; ***: p<0.001).
MIV-09 fails to induce a type I IFN signature in vivo
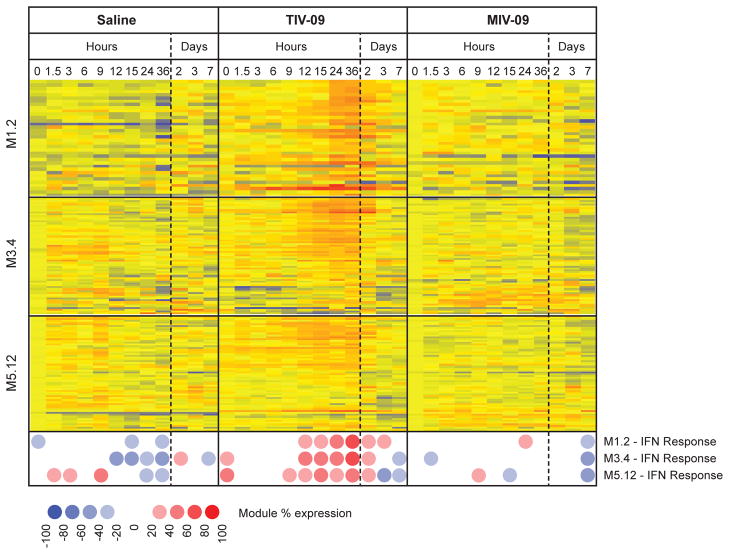
To better understand the early immune response to influenza vaccination in vivo, we vaccinated healthy individuals with TIV-09, MIV-09 or saline control and assessed the transcriptional profiles of whole blood at 0h, 1.5h, 3h, 6h, 9h, 12h, 15h, 24h, 36h, 2d, 3d and 7d post-vaccination by microarray. Data were analyzed both at the single transcript level and using a previously described framework of modules of co-expressed transcripts, focusing on three IFN modules (36). Consistent with previous studies from our group (18) as well as others (19–23), TIV-09 induced a transient type I IFN signature 24–48h post vaccination. This signature was however not induced in response to MIV-09 (Fig. 6). These results indicate that MIV-09 fails to induce an IFN response in vivo.
Figure 6. TIV-09 but not MIV-09 induces an IFN signature in the blood of vaccinated individuals at day 1.
Healthy individuals were vaccinated at t=0h with saline, TIV-09 or MIV-09 (N=3 per group), and blood was drawn at 0h, 1.5h, 3h, 6h, 9h, 12h, 15h, 24h, 36h, 2d, 3d and 7d. Data were normalized to the median of the 0h samples across donors. Transcripts from three IFN blood modules (M1.2, M3.4 and M5.12) (36) were selected for analysis. Both transcript-level and module-level analyses are displayed. Module expression is calculated as the percentage of transcripts from a specific module that are over or underexpressed in a specific condition as compared to the 0h reference control.
DISCUSSION
This study focuses on how non-adjuvanted influenza vaccines that display different effectiveness affect the biological functions of human DC subsets. We first show that the Fluzone® vaccines that do not contain the [A/California/07/2009 X-179A (H1N1)] H1N1 Influenza strain activate moDCs and blood cDCs as revealed by secretion of type I IFN, IL-6, IL-29 and MIP-1b and increased surface expression of several DC activation markers. Blocking IFN signaling with an anti-IFNAR antibody further revealed the partial dependence of this activation on an early wave of IFN-β production by DCs. TIV-09 also induced pDCs to transcribe and secrete type I IFNs. We then show that the monovalent non-adjuvanted MIV-09, which displayed limited clinical efficacy, i) did not induce an IFN response and maturation of moDCs and cDCs; ii) blocked the IFN-mediated activating effects of TIV-09 in vitro, and IFN signaling in vivo. These results suggest that in the absence of adjuvant, certain vaccines but not others retain immunogenic properties through IFN-mediated DC activation.
These findings might partly explain why in vivo administration of TIV-09 results in the short-term expression of an IFN signature in circulating blood cells. Type I IFNs play a pivotal role in the induction of adaptive immune responses by promoting the expansion of CD4+ and CD8+ T cells and the maturation of B cells into plasmablasts (24, 26). High vaccine responses correlate with increased early expression of interferon signaling and antigen processing and presentation (21). Furthermore, type I IFNs act as powerful adjuvants when administered with influenza vaccine to mice (37). Thus, one could speculate that the antibody response elicited against TIV-09 might at least in part be due to this early IFN response.
MIV-09, on the other hand, was not able to induce moDC and blood cDC activation as measured by secretion of cytokines including type I IFN and induction of activation markers. Moreover, MIV-09 acted as a powerful inhibitor of the effects of TIV-09 on the production of cytokines, including IFN and induction of activation markers by moDCs and cDCs. Instead, exposure to MIV-09 was associated with a unique transcriptional signature in moDCs that was enriched for ERK/MAPK signaling. Studies with murine macrophages indicate that IFN-β production is inhibited through ERK activation (33), thereby providing a plausible mechanism for the IFN-inhibitory activity of MIV-09 in moDCs and blood cDC. The inability of MIV-09 to completely shut down the IFN machinery in pDCs may be due to constitutive expression of IRF7 in these cells (38). Moreover, the combination of MIV-09 with TIV-09 results in production levels of IP-10 that are lower than those induced by TIV-09. These results suggest that MIV-09 has the potential to activate the IFN transcription cascade but this action is blocked by the presence of an inhibitory element in this vaccine.
Comparative studies between TIV-09 and the 23-valent pneumococcal vaccine Pneumovax®23 demonstrated that the two vaccines induce protective antibody responses but differ in the blood transcriptional profiles they elicit (18). When assessing IP-10 production by moDCs in the presence of other inactivated viral vaccines including Human Papilloma Virus, Hepatitis A, Hepatitis B and Rabies (Table S1), only TIV-09 induced secretion of IP-10 by moDCs (Fig. S2). Most seasonal influenza vaccines have been shown to exhibit early IFN signatures (18, 21–23), suggesting that type I IFN plays an important role in shaping protective immunity against influenza. Our study shows that unlike seasonal influenza vaccines, MIV-09 fails to exhibit the classic type I signature in vitro as well as in vivo.
Higher effectiveness has been observed in MIV-09 supplemented with squalene-based adjuvants MF59 and AS03 as compared with its non-adjuvanted form (3, 4). Several observations support a direct effect of MF59 on DCs. MF59 rapidly localizes to intracellular DEC-205+ DC compartments after injection in mice (39), promotes recruitment of human myeloid cells to the injection site, enhances DC maturation and antigen uptake and facilitates DC migration to draining lymph nodes (40). A recent study in mice also demonstrated that MF59 promotes the retention of unprocessed antigen within the lymph nodes, thereby facilitating antigen encounters with follicular DCs (41). Whether these two adjuvants can induce the IFN response in DCs is still unknown.
Due to the presence of broadly cross-reactive antibody responses as a result of prior vaccination or infection (42, 43), it will be challenging to determine whether the lack of in vitro and in vivo activation of type I IFN signature reflects the reduced immunogenicity of MIV-09 as compared to TIV-09. Vaccines against Pneumococcus or Rabies are fully immunogenic in vivo despite lacking interferogenic properties in DC in vitro (29). These observations suggest the existence of IFN-dependent and IFN-independent mechanisms for mounting immune responses against specific pathogens.
Our study is limited by its in vitro nature. Our model of vaccine-challenged monocyte-derived and sorted blood DC does not fully represent molecular and cellular events occurring in situ after vaccination. In particular, it does not account for DC activation mechanisms that can be triggered indirectly through the recruitment of other inflammatory cell populations. The phenotype of the DC populations studied may also not accurately reflect the phenotype of DCs found in skin and muscle, especially that of Langerhans cells. Finally, these studies are complicated by the evolving nature of the influenza vaccine and the scarcity of vaccines from earlier years.
Altogether, this study sheds light on the mechanisms of DC activation by influenza vaccines. Furthermore, it highlights the differences between non-adjuvanted influenza vaccines in inducing IFN and pro-inflammatory responses in vitro and in vivo, further unraveling the mechanisms of action of vaccines at the systemic level and the role of early inflammatory responses in the process of vaccination.
METHODS
Subjects and study design
This study was approved by the Baylor Institute for Immunology Research Institutional Review Board (BIIR IRB 009-282). Written informed consent was obtained for all subjects. For in vitro studies, cells were obtained from apheresis of 10 healthy individuals (6 females, 4 males) aged 27 to 59 years. For the vaccination study, healthy adults, aged 18 to 64 years, were enrolled to receive a single intra-muscular dose of TIV-09 [A/Brisbane/59/2007 IVR 148 (H1N1), A/Uruguay/716/2007 NYMC X-175C (H3N2), B/Brisbane/60/2008], MIV-09 [A/California/07/2009 X-179A (H1N1)] or placebo (saline) (n=3 per group). Exclusion criteria included pregnancy, active allergy, or vaccinations within the previous two months. Blood was drawn by finger prick at 0h, 1.5h, 3h, 6h, 9h, 12h, 15h, 24h, 36h, 2d, 3d and 7d. Power analysis was conducted to estimate sample size for moDC experiments based on preliminary results from microarray, luminex and FACS analyses. With N=6, the study was adequately powered (>0.8) to detect a 3-fold difference when as few as 2000 genes are differentially expressed (microarray), a 2-fold difference in secreted proteins (Luminex) and a change ≥7.5% of positive cells for the surface markers considered (FACS).
In vitro DC culture and stimulation
Monocyte-derived DCs (moDCs) were generated from monocytes isolated from healthy donor apheresis fractionated by elutriation. Monocytes were enriched by negative selection using EasySep Human Monocyte Enrichment without CD16 Depletion Kit (Stemcell Technologies) and cultured in CellGro DC media (CellGenix) with 1% penicillin/streptomycin. Cells were cultured in sterile 50 mL tissue culture bags (AFC) at an initial density of 1×106 cells/mL along with GM-CSF at 10 ng/mL and IL-4 (R&D systems) at 50 ng/mL for 6 days. Cells were supplemented with additional cytokine doses on days 2 and 4. On day 6, DCs were harvested and further cultured in 200 μL of RPMI 1640 supplemented with (1% glutamine, 1% penicillin/streptomycin, 1% HEPES, 1% non-amino-acids, 1% sodium pyruvate and 0.1% β-mercaptoethanol) (Invitrogen) and 10% fetal bovine serum (FBS) at a density of 1×105 cells/mL in 96-well plates. TIV-09 (45 μg viral protein, 6 μL/mL) and/or MIV-09 (15 μg viral protein, 6 μL/mL) (Sanofi Pasteur) was added, and cells were incubated at 37°C for 0, 6, 12, 24 and 48h. Supernatants were collected and stored at −80°C.
Another set of experiments was performed with Fluzone® from different vaccination seasons. moDCs were exposed for 8h to one of the following vaccines, all from Sanofi Pasteur: i) Fluzone®2006–2007 [A/New Caledonia/20/99 (H1N1), A/Wisconsin/67/05 (H3N2), B/Malaysia/250604], ii) Fluzone®2008–2009 [A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2), B/Florida/04/2006], or iii) Fluzone®2010–2011 ([A/California/07/2009 X-179A (H1N1), A/Victoria/210/2009 X-187 (H3N2), B/Brisbane/60/2008]. Supernatants were collected and stored at −80°C.
Separate experiment was performed to assessed IP-10 production by moDCs in the presence of other inactivated viral vaccines such as Human Papilloma Virus (Merk), Hepatitis A, Hepatitis B (GSK) and Rabies (Sanofi Pasteur). moDcs were treated with vaccines for 18h. Supernatants were sored at −80°C.
DC phenotype and cytokine assay
DCs (1×105) were stimulated with selected vaccines for 18h, and labeled with fluorochrome-conjugated anti-CD83, anti-CD80, anti-CD86, anti-HLA DR, anti-PD-L1, anti-PD-L2, anti-CCR7, anti-CXCR4 and anti-CD40 antibodies (BD Biosciences). Cell death was assessed by Annexin V and PI staining (BD Biosciences). Labeled cells were analyzed using a FACS Canto (BD Biosciences). IP-10 (BD Biosciences) and IFN-β (R&D Systems) levels were measured by ELISA, and other cytokines were assessed using bead-based cytokine multiplex analysis (Luminex, Bio-Rad).
Purification and activation of pDCs and mDCs
Buffy coats obtained from healthy donors (Carters Blood Care) were fractionated on a Ficoll gradient. Peripheral blood mononuclear cells (PBMCs) were further enriched for DCs through negative selection using the Pan DC kit (Stemcell Technologies). The enriched cells were sorted on a FACS Aria (BD Biosciences) for LIN−/HLA-DR+/CD123+ and CD11c− pDCs and LIN−/HLA DR+/CD123− and CD11c+ mDCs (Fig. S3). Purity was routinely >98%. DCs were stimulated at a density of 5×104 cells per well with vaccines (6 μL/mL) in 200 μL complete medium in 96-well plates for 0, 6, 16, 24 and 48h. For microarray analysis, 0.5×106 cells were cultured with (6 μL/mL) vaccine in 0.5 mL complete medium for 6h, and processed for RNA analysis.
RNA processing and transcriptional analysis
After vaccine stimulation, cells were lysed in RLT buffer (RNeasy Plus Mini Kit, Qiagen), 1% β-mercaptoethanol and stored at −80°C. Total RNA was isolated from cell lysates according to the manufacturer’s instructions. RNA from samples passing quality control was amplified and labeled with Illumina TotalPrep RNA amplification kit (Illumina). RNA integrity was assessed using an Agilent 2100 Analyzer (Agilent Technologies). Biotinylated complementary RNA (cRNA) was hybridized to Illumina Human-6 Beadchip Array version 2 and scanned in Illumina Beadstation 500. Fluorescent hybridization signals were assessed with Beadstudio software (Illumina). Statistical analysis and hierarchical clustering were performed using GeneSpring 7.3.1 software (Agilent Technologies). For the vaccinated healthy cohort, data were normalized to the median of the t=0h samples across donors.
Statistical analysis
Data from cell culture experiments were expressed as mean of replicate experiments. Statistical significance was determined by non-parametric test such as Mann Whitney test. p<0.05 was considered statistically significant. For microarray analysis, unless otherwise specified, we conducted Welch’s unequal variances t-test (p<0.01) adjusting the FDR with the Benjamini-Hochberg procedure. For all figures, horizontal lines or bars represent the mean, and whiskers the standard deviation.
Supplementary Material
Figure S1: Dose-dependent induction of IFN-β and IP-10 by TIV-09 and MIV-09 in moDCs.
Figure S2: IP-10 production by moDCs challenged with various inactivated viral vaccines.
Figure S3: mDC and pDC sort gating strategy
Table S1: Vaccines used for comparative studies in moDCs.
Acknowledgments
We wish to thank Nicole Baldwin for her help with the manuscript. We thank Gerard and Sandra Zurawski for their help with vaccine fractionation.
Funding: The project was supported by NIH U19 AI057234, NIH U01AI124297 (to J.B.) and U19 AI089987 (to K.P. and V.P.).
Footnotes
For all experiments where n < 20, please include the source/primary data (see Checklist) as a separate Excel file; this is easily done with the data organized by Figures on separate tabs
Author contributions: S.A. and L.T.S. performed culture experiments and wrote the manuscript. R.B. and Y.W. analyzed microarray data and R.B. wrote the manuscript. K.P. and V.P. contributed to the overall design and writing of the manuscript. J.B. designed and oversaw the study and wrote the manuscript.
Competing interests: The authors declare no competing interest.
Data and materials availability: The datasets presented in this manuscript are deposited in the NCBI Gene Expression Omnibus under GEO Series accession numbers GSE81692.
References
- 1.Lambert LC, Fauci AS. Influenza vaccines for the future. N Engl J Med. 2010;363:2036–2044. doi: 10.1056/NEJMra1002842. [DOI] [PubMed] [Google Scholar]
- 2.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21:1776–1779. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 3.Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011;3:85ra48. doi: 10.1126/scitranslmed.3002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rumke HC, Richardus JH, Rombo L, Pauksens K, Plassmann G, Durand C, Devaster JM, Dewe W, Oostvogels L. Selection of an adjuvant for seasonal influenza vaccine in elderly people: modelling immunogenicity from a randomized trial. BMC Infect Dis. 2013;13:348. doi: 10.1186/1471-2334-13-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 6.Baras B, de Waal L, Stittelaar KJ, Jacob V, Giannini S, Kroeze EJ, van den Brand JM, van Amerongen G, Simon JH, Hanon E, Mossman SP, Osterhaus AD. Pandemic H1N1 vaccine requires the use of an adjuvant to protect against challenge in naive ferrets. Vaccine. 2011;29:2120–2126. doi: 10.1016/j.vaccine.2010.12.125. [DOI] [PubMed] [Google Scholar]
- 7.Widgren K, Magnusson M, Hagstam P, Widerstrom M, Ortqvist A, Einemo IM, Follin P, Lindblom A, Makitalo S, Wik O, Osterlund A, Grunewald M, Uhnoo I, Linde A. Prevailing effectiveness of the 2009 influenza A(H1N1)pdm09 vaccine during the 2010/11 season in Sweden. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2013;18:20447. [PubMed] [Google Scholar]
- 8.Skowronski DM, Janjua NZ, De Serres G, Hottes TS, Dickinson JA, Crowcroft N, Kwindt TL, Tang P, Charest H, Fonseca K, Gubbay JB, Bastien N, Li Y, Petric M. Effectiveness of AS03 adjuvanted pandemic H1N1 vaccine: case-control evaluation based on sentinel surveillance system in Canada, autumn 2009. BMJ. 2011;342:c7297. doi: 10.1136/bmj.c7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fielding JE, Grant KA, Garcia K, Kelly HA. Effectiveness of seasonal influenza vaccine against pandemic (H1N1) 2009 virus, Australia, 2010. Emerg Infect Dis. 2011;17:1181–1187. doi: 10.3201/eid1707.101959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borse RH, Shrestha SS, Fiore AE, Atkins CY, Singleton JA, Furlow C, Meltzer MI. Effects of vaccine program against pandemic influenza A(H1N1) virus, United States, 2009–2010. Emerg Infect Dis. 2013;19:439–448. doi: 10.3201/eid1903.120394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin MR, Monto AS, Belongia EA, Treanor JJ, Chen Q, Chen J, Talbot HK, Ohmit SE, Coleman LA, Lofthus G, Petrie JG, Meece JK, Hall CB, Williams JV, Gargiullo P, Berman L, Shay DK, Network USFV. Effectiveness of non-adjuvanted pandemic influenza A vaccines for preventing pandemic influenza acute respiratory illness visits in 4 U.S. communities. PLoS One. 2011;6:e23085. doi: 10.1371/journal.pone.0023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long CB, Ramos I, Rastogi D, Manwani D, Janow G, Del Rio M, Mayers M, Herold BC, Fernandez-Sesma A, Madan RP. Humoral and cell-mediated immune responses to monovalent 2009 influenza A/H1N1 and seasonal trivalent influenza vaccines in high-risk children. J Pediatr. 2012;160:74–81. doi: 10.1016/j.jpeds.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pebody R, Hardelid P, Fleming D, McMenamin J, Andrews N, Robertson C, Thomas D, Sebastianpillai P, Ellis J, Carman W, Wreghitt T, Zambon M, Watson J. Effectiveness of seasonal 2010/11 and pandemic influenza A(H1N1)2009 vaccines in preventing influenza infection in the United Kingdom: mid-season analysis 2010/11. Euro surveillance: bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2011;16 [PubMed] [Google Scholar]
- 14.Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- 15.McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC. T cell responses are better correlates of vaccine protection in the elderly. Journal of immunology. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 16.Saurwein-Teissl M, Zisterer K, Schmitt TL, Gluck R, Cryz S, Grubeck-Loebenstein B. Whole virus influenza vaccine activates dendritic cells (DC) and stimulates cytokine production by peripheral blood mononuclear cells (PBMC) while subunit vaccines support T cell proliferation. Clin Exp Immunol. 1998;114:271–276. doi: 10.1046/j.1365-2249.1998.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CruzCo MD, Orphin L, Cruz J, Pazoles P, Green KM, Potts J, Leporati AM, Babon JA, Evans JE, Ennis FA, Terajima M. In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses. Vaccine. 2009;27:319–327. doi: 10.1016/j.vaccine.2008.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obermoser G, Presnell S, Domico K, Xu H, Wang Y, Anguiano E, Thompson-Snipes L, Ranganathan R, Zeitner B, Bjork A, Anderson D, Speake C, Ruchaud E, Skinner J, Alsina L, Sharma M, Dutartre H, Cepika A, Israelsson E, Nguyen P, Nguyen QA, Harrod AC, Zurawski SM, Pascual V, Ueno H, Nepom GT, Quinn C, Blankenship D, Palucka K, Banchereau J, Chaussabel D. Systems scale interactive exploration reveals quantitative and qualitative differences in response to influenza and pneumococcal vaccines. Immunity. 2013;38:831–844. doi: 10.1016/j.immuni.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsang JS, Schwartzberg PL, Kotliarov Y, Biancotto A, Xie Z, Germain RN, Wang E, Olnes MJ, Narayanan M, Golding H, Moir S, Dickler HB, Perl S, Cheung F, Baylor HC Consortium CHI. Global analyses of human immune variation reveal baseline predictors of postvaccination responses. Cell. 2014;157:499–513. doi: 10.1016/j.cell.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Rouphael N, Duraisingham S, Romero-Steiner S, Presnell S, Davis C, Schmidt DS, Johnson SE, Milton A, Rajam G, Kasturi S, Carlone GM, Quinn C, Chaussabel D, Palucka AK, Mulligan MJ, Ahmed R, Stephens DS, Nakaya HI, Pulendran B. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nature immunology. 2014;15:195–204. doi: 10.1038/ni.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bucasas KL, Franco LM, Shaw CA, Bray MS, Wells JM, Nino D, Arden N, Quarles JM, Couch RB, Belmont JW. Early patterns of gene expression correlate with the humoral immune response to influenza vaccination in humans. The Journal of infectious diseases. 2011;203:921–929. doi: 10.1093/infdis/jiq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz-Mitoma F, Alvarez-Maya I, Dabrowski A, Jaffey J, Frost R, Aucoin S, Kryworuchko M, Lapner M, Tadesse H, Giulivi A. Transcriptional analysis of human peripheral blood mononuclear cells after influenza immunization. J Clin Virol. 2004;31:100–112. doi: 10.1016/j.jcv.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, Means AR, Kasturi SP, Khan N, Li GM, McCausland M, Kanchan V, Kokko KE, Li S, Elbein R, Mehta AK, Aderem A, Subbarao K, Ahmed R, Pulendran B. Systems biology of vaccination for seasonal influenza in humans. Nature immunology. 2011;12:786–795. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 26.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 27.Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, Pamer EG, Littman DR, Lang RA. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 29.Banchereau R, Baldwin N, Cepika AM, Athale S, Xue Y, Yu CI, Metang P, Cheruku A, Berthier I, Gayet I, Wang Y, Ohouo M, Snipes L, Xu H, Obermoser G, Blankenship D, Oh S, Ramilo O, Chaussabel D, Banchereau J, Palucka K, Pascual V. Transcriptional specialization of human dendritic cell subsets in response to microbial vaccines. Nat Commun. 2014;5:5283. doi: 10.1038/ncomms6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conzelmann KK. Transcriptional activation of alpha/beta interferon genes: interference by nonsegmented negative-strand RNA viruses. Journal of virology. 2005;79:5241–5248. doi: 10.1128/JVI.79.9.5241-5248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 32.Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, Derrick SC, Shi R, Kumar NP, Wei W, Yuan X, Zhang G, Cai Y, Babu S, Catalfamo M, Salazar AM, Via LE, Barry CE, 3rd, Sher A. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511:99–103. doi: 10.1038/nature13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang HT, Wang Y, Zhao X, Demissie E, Papoutsopoulou S, Mambole A, O’Garra A, Tomczak MF, Erdman SE, Fox JG, Ley SC, Horwitz BH. NF-kappaB1 inhibits TLR-induced IFN-beta production in macrophages through TPL-2-dependent ERK activation. Journal of immunology. 2011;186:1989–1996. doi: 10.4049/jimmunol.1001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzgerald-Bocarsly P, Feng D. The role of type I interferon production by dendritic cells in host defense. Biochimie. 2007;89:843–855. doi: 10.1016/j.biochi.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 36.Chaussabel D, Baldwin N. Democratizing systems immunology with modular transcriptional repertoire analyses. Nat Rev Immunol. 2014;14:271–280. doi: 10.1038/nri3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proietti E, Bracci L, Puzelli S, Di Pucchio T, Sestili P, De Vincenzi E, Venditti M, Capone I, Seif I, De Maeyer E, Tough D, Donatelli I, Belardelli F. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. Journal of immunology. 2002;169:375–383. doi: 10.4049/jimmunol.169.1.375. [DOI] [PubMed] [Google Scholar]
- 38.Izaguirre A, Barnes BJ, Amrute S, Yeow WS, Megjugorac N, Dai J, Feng D, Chung E, Pitha PM, Fitzgerald-Bocarsly P. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J Leukoc Biol. 2003;74:1125–1138. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- 39.Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, van Nest G, Ott G, McDonald DM. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cellular immunology. 1998;186:18–27. doi: 10.1006/cimm.1998.1283. [DOI] [PubMed] [Google Scholar]
- 40.Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. Journal of immunology. 2008;180:5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- 41.Cantisani R, Pezzicoli A, Cioncada R, Malzone C, De Gregorio E, D’Oro U, Piccioli D. Vaccine adjuvant MF59 promotes retention of unprocessed antigen in lymph node macrophage compartments and follicular dendritic cells. Journal of immunology. 2015;194:1717–1725. doi: 10.4049/jimmunol.1400623. [DOI] [PubMed] [Google Scholar]
- 42.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O’Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. The Journal of experimental medicine. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, Mulligan M, Das SR, Yewdell JW, Mehta AK, Wilson PC, Ahmed R. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109:9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Dose-dependent induction of IFN-β and IP-10 by TIV-09 and MIV-09 in moDCs.
Figure S2: IP-10 production by moDCs challenged with various inactivated viral vaccines.
Figure S3: mDC and pDC sort gating strategy
Table S1: Vaccines used for comparative studies in moDCs.