Abstract
Purpose
To evaluate fall-relevant gait features in older glaucoma patients.
Methods
The GAITRite Electronic Walkway was used to define fall-related gait parameters in 239 patients with suspected or manifest glaucoma under normal usual-pace walking conditions and while carrying a cup or tray. Multiple linear regression models assessed the association between gait parameters and integrated visual field (IVF) sensitivity after controlling for age, race, sex, medications, and comorbid illness.
Results
Under normal walking conditions, worse IVF sensitivity was associated with a wider base of support (β = 0.60 cm/5 dB IVF sensitivity decrement, 95% confidence interval [CI] = 0.12–1.09, P = 0.016). Worse IVF sensitivity was not associated with slower gait speed, shorter step or stride length, or greater left–right drift under normal walking conditions (P > 0.05 for all), but was during cup and/or tray carrying conditions (P < 0.05 for all). Worse IVF sensitivity was positively associated with greater stride-to-stride variability in step length, stride length, and stride velocity (P < 0.005 for all). Inferior and superior IVF sensitivity demonstrated associations with each of the above gait parameters as well, though these associations were consistently similar to, or weaker than, the associations noted for overall IVF sensitivity.
Conclusion
Glaucoma severity was associated with several gait parameters predictive of higher fall risk in prior studies, particularly measures of stride-to-stride variability. Gait may be useful in identifying glaucoma patients at higher risk of falls, and in designing and testing interventions to prevent falls in this high-risk group.
Translational Relevance
These findings could serve to inform the development of the interventions for falls prevention in glaucoma patients.
Keywords: gait, glaucoma, visual field, mobility
Introduction
Falls are the most common reason for accidental death in the elderly, and are also a frequent reason for hospitalization.1 Additionally, falls impart a high economic toll on society, accounting for nearly $20 billion in direct costs annually in the United States alone.2 Poor vision is widely recognized as a risk factor for falls, with visual field (VF) damage having a particularly strong association with fall rates.3–5 Up to one-half of glaucoma patients fell over the course of a year in prior studies, and up to one-third demonstrated an injurious fall.6–8
High fall rates in glaucoma may be caused by, or reflected in, alterations in gait that result from VF damage. Previous research has identified numerous gait parameters, such as slower walking speed and greater stride-to-stride variability, which are associated with a higher rates of falling in older populations.9–11 Some prior studies have also linked VF damage in the general population or patients with glaucoma to slower walking speeds and shorter stride lengths, which may reflect attempts by visually impaired individuals to maintain stability and adopt a safer walking style.12–15 However, most studies assessing the relationship between the VF damage and gait have had small sample sizes, were not focused specifically on glaucoma patients, and employed a limited gait assessment, predominantly evaluating gait speed, and not factors such as stride-to-stride variability, which may play an important role in falls. Prior work on gait during dual-task conditions in persons with VF damage is also lacking, though research has shown that changes in gait during cognitive (i.e., counting backwards) or manual (i.e., carrying a cup of water while walking) dual-task performance are associated with a greater risk of falls in elderly.16–19
Here, we assess gait in a large cohort of glaucoma patients using the GAITRite Electronic Walkway, allowing, for the first time, detailed spatiotemporal characterization of gait parameters identified as relevant to falls in previous research.11,20–22 We hypothesize that severity of VF damage is associated with slower gait speed, shorter stride length, and greater base of support, and also investigate if other fall-relevant gait characteristics such as measures of stride-to-stride variability are associated with severity of VF damage. If so, measures of gait might serve as markers for glaucoma patients at risk for falls, and guide interventions designed to prevent falls in this high-risk group.
Methods
Study Design and Study Population
Patients were enrolled and tested as part of the baseline assessment from the Falls in Glaucoma Study (FIGS), an observational prospective cohort of patients with glaucoma or suspect glaucoma conducted at the Wilmer Eye Institute at Johns Hopkins. Inclusion criteria for participants were: (1) age 60 and older (or turning age 60 over the course of the study), (2) glaucoma suspect or diagnosis of primary open angle glaucoma, primary angle closure glaucoma, pseudoexfoliation glaucoma, or pigmentary glaucoma, (3) residence within a 60-mile radius from the Wilmer Eye Institute, and (4) ability to perform VF testing. Exclusion criteria for participants were: (1) presence of visually significant concurrent eye disease, (2) any ocular surgery in the past 2 months, (3) any hospitalization in the past month, and (4) confinement to a bed or wheelchair, (5) history of stroke or other neurological disorders causing VF damage.23
Recruitment
Patients were recruited during glaucoma clinic visits to the Wilmer Eye Institute and, if agreeable, provided written informed consent for all study procedures. A total of 245 participants were recruited from September 2013 through March 2015 and completed an in-clinic baseline visit at the Wilmer Eye Institute. All study procedures were approved by the Johns Hopkins institutional review board.
Gait Evaluation
Gait data were collected using the GAITRite Electronic Walkway (CIR System Inc., Franklin, NJ).24–27 The GAITRite system measures temporal and spatial gait parameters via an electronic walkway that contains eight sensor pads encapsulated in a roll-up carpet to produce an active area 61-cm wide and 488-cm long. The active area contains a grid of 48 × 384 sensors. Participants' gait measurements were first collected barefoot during their normal usual-pace walking and subsequently under two dual task performance conditions simulating real world scenarios: carrying a cup and carrying a tray. For each condition, participants wore their normal distance spectacles and walked four lengths of the GAITRite Electronic Walkway (back and forth 2 times, with a short pause in between each walk) at their natural pace. During the cup carrying trial, participants carried an empty coffee mug in their right hand and were instructed to hold it as steady as possible while walking to mimic the daily activity of a cup carrying with a beverage. Participants received similar instructions during the tray carrying trial, in which they were asked to carry a breakfast tray holding it with both hands as steady as possible.
Gait Outcome Measures
The following gait parameters were chosen as the primary outcome variables based on their ability to be captured using the GAITRite walkway, as well as prior association with fall risk in previous studies.11,20–22 All five parameters (Fig.) were averaged over the four walks performed.
Figure.
Graphical depiction of the gait parameters analyzed in the manuscript. AB, line of progression; CD, base of support; AE, step length; CF, stride length; CG, drift.
Base of support (CD) – distance (in cm) between the heel center of the dominant foot (based on patient report) and the line of progression created by the prior and subsequent heel strikes of the nondominant leg.
Step length (AE) – distance along the forward-backward axis (in cm) between the heel center of the nondominant leg to the following heel center of the dominant leg.
Stride length (CF) – distance (in cm) between the heel centers of two consecutive footprints of the dominant leg.
Stride velocity – stride length (in cm) of the dominant leg divided by stride time (in seconds).
Gait speed – distance walked by the patient (in cm) divided by the time (in seconds) it took to walk the distance.
Additional variables were also generated to capture the stride-to-stride variability across the four length-of-mat walks given prior data showing the relevance of gait variability to falls.9,10,28 Specifically, coefficients of variation (ratio of the standard deviation [SD] to the mean multiplied by 100) were calculated for each of the measures above. Coefficients were expressed as percentage, with higher percentages reflecting greater variability. While prior studies only examined variability in the forward direction, we speculated that glaucoma patients may also demonstrate more variability in the left–right axis. There, we examined drift values, defined as the range (in cm) of dominant leg heel center positions along the left-right axis of the GAITRite Walkway (CG – Fig.) during the fourth (and final) walk, which was assumed to be most representative given greater familiarity with the task.
Visual Assessment
Humphrey 24-2 SITA standard VF tests were obtained at the study visit or a recent clinic visit (median time = 2.5 months) and all VFs were screened for reliability by a glaucoma specialist (PR) based on their reliability parameters, the absence of artifacts (i.e., rim or lid defects), and consistency with prior test results (i.e., excluding tests with unusually dramatic changes inconsistent with the patient's clinical course). Sensitivities of spatially corresponding points from left and right eye 24-2 VF tests obtained from a HFA-2 perimeter (Carl Zeiss Meditec, Dublin, CA) were merged to calculate mean sensitivity in the integrated VF (IVF). Briefly, the maximum sensitivity between the right and left eye was taken as the sensitivity for each pair of spatially corresponding points between the two eyes. Decibel sensitivity values in the IVF were then converted to raw sensitivity values, averaged over all points in the full, superior, or inferior VF, and then transformed back into decibel values to generate total, superior, and inferior mean sensitivity values. Visual acuity was assessed using a back-lit Early Treatment Diabetic Retinopathy Study (ETDRS) chart placed at 4-m distance with patients wearing their habitual correction. Letters read were converted to logarithm of the minimum angle of resolution (logMAR) values for analysis. Contrast sensitivity was evaluated using the MARS chart (Mars Perceptrix, Chappaqua, NY) with participants wearing their usual corrective lenses. Letters read were converted to log units (logCS).
Evaluation of Covariates
Age, sex, and race were gathered using standard questionnaires. Noneye drop medication lists were generated by direct observation of pill containers when possible, or otherwise by patient report, and classified as polypharmacy if five or more prescription medications were used.29 Patients were questioned about 15 comorbid medical conditions known to affect physical activity (arthritis, broken or fractured hip, back problems, history of heart attack, history of angina/chest pain, congestive heart failure, peripheral vascular disease, high blood pressure, diabetes, emphysema, asthma, stroke, Parkinson's disease, cancer other than the skin cancer, and history of vertigo or Meniere's disease) using a standardized questionnaire, and comorbidity was quantified as the total number of comorbid conditions.30 The small number of participants with more than five comorbidities (n = 9) were reclassified to have five comorbidities. Above mentioned covariates were included in the models based on their prior association with glaucoma and/or gait parameters.31–34
Statistical Analysis
Outcome variables were treated as continuous after being confirmed to be normally distributed. LOWESS plots were used to confirm linear relationships between VF damage and each gait parameter over the range of observed IVF sensitivities. Multiple linear regression models were then used in which the gait parameters (or variability of these gait parameters) was the dependent variable, sensitivity in either the full IVF, superior IVF, or inferior IVF was the main exposure, and age, sex, race, polypharmacy, and number of medical comorbidities were included as covariates. All analyses were conducted using STATA version 14.0/IC (StataCorp LP, College Station, TX).35
Results
Description of Study Population
All visual and gait assessments were completed by 239 of 245 subjects enrolled (97.6%). Roughly one-quarter (22.5%) of study participants were glaucoma suspects, just under one-half were female (49%), just under one-third were African American (29%), and average participant age was 70.6 years (Table 1). More than one-half of participants (64%) had at least one comorbid illness, and 33% of the participants used five or more noneye drop prescription medications. Median IVF sensitivity of the population was 28.0 dB (interquartile range [IQR] = 26.09–29.67 dB; normal value in absence of VF damage = 31 dB), while median mean deviation (MD) of the better eye was −2.56 (IQR = −5.41 to −0.68 dB) and median MD of the worse eye was −5.33 (IQR = −12.39 to −2.46 dB). Median better-eye acuity (logMAR) was 0.06 (IQR = −0.01 to 0.16) and median binocular logCS was 1.72 (IQR = 1.64–1.76).
Table 1.
Falls in Glaucoma Study Population Characteristics

Examination of Gait Parameters Associated with IVF Sensitivity
In multivariable models evaluating normal usual-pace walking, worse IVF sensitivity was associated with a broader base of support (0.60 cm/5-dB decrement; 95% CI = 0.12–1.09; P = 0.016; mean value = 10.2 cm). IVF sensitivity was not associated with shorter step or stride length, slower stride velocity, or greater drift (P > 0.2 for all) during normal usual-pace walking, but was associated with each of these gait parameters during cup and/or tray carrying conditions (Table 2). A similar pattern was also observed for overall walking speed, which was 2.76-cm/s slower for every 5-dB decrement in the IVF sensitivity during the tray carrying condition (95% confidence interval [CI] = −5.36 to −0.15; P = 0.038; mean value = 101.8 cm/s), but not significantly associated with walking speed during normal usual-pace walking or walking while carrying a cup (P > 0.13 for both).
Table 2.
Multiple Linear Regressions Assessing the Association between Different Gait Characteristics and Integrated Visual Field Sensitivity under Three Different Walking Conditions
In no case did IVF sensitivity in the inferior or superior hemifields show an association with any of the gait parameters except when an association was also noted for total IVF sensitivity. Several gait parameters were also noted to be associated with the total number of comorbid conditions, polypharmacy, age, sex, and race (Table 2).
Examination of IVF Sensitivity as a Predictor of Gait Variability
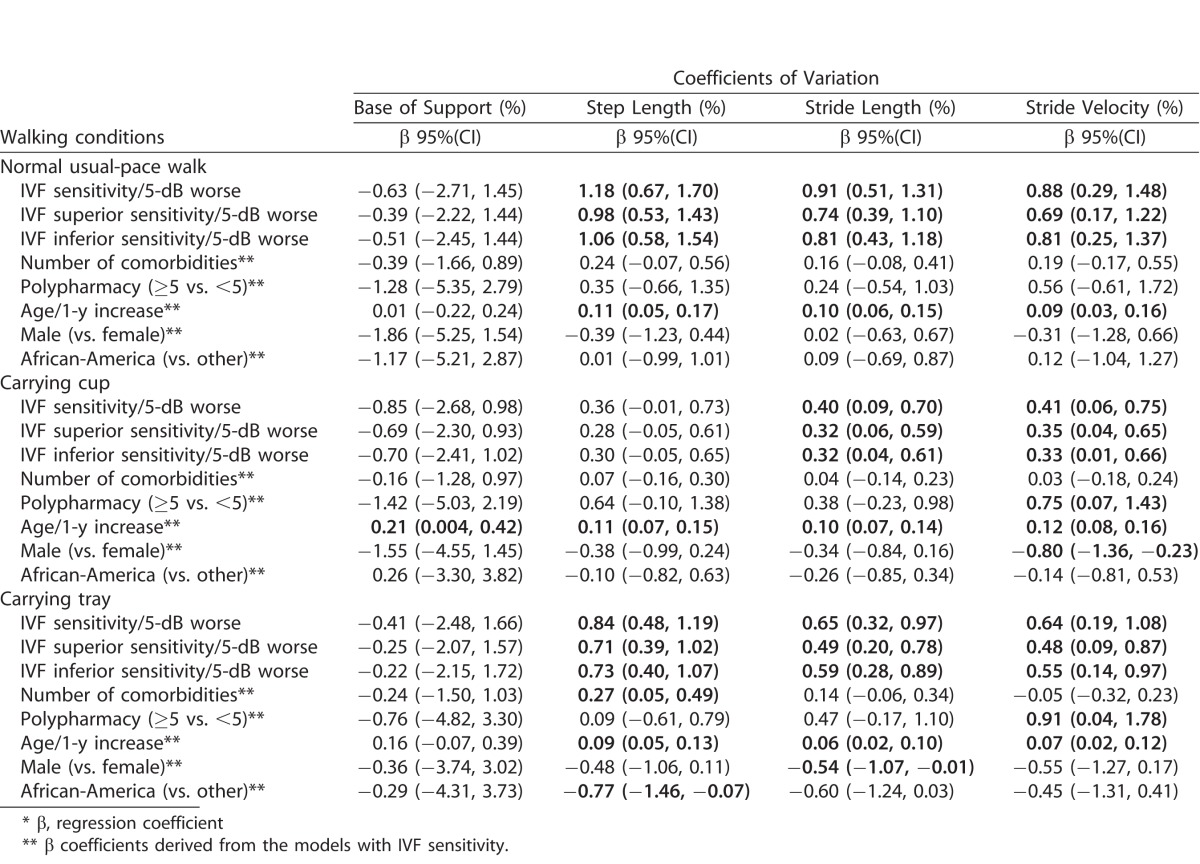
To determine if gait parameters varied more from stride-to-stride in patients with more severe disease, additional multivariable models were constructed to examine the association between IVF sensitivity and the stride-to-stride coefficient of variation (CV) in gait parameters (Table 3). During normal usual-pace walking, worse IVF sensitivity was associated with a greater coefficient of variation in step length (β = 1.18%/5-dB decrement; 95% CI = 0.67–1.70%, P < 0.001; mean value = 5.95%), stride length (β = 0.91%/5-dB decrement; 95% CI = 0.51–1.31%, P < 0.001; mean value = 4.75%), and stride velocity (β = 0.88%/5-dB decrement; 95% CI = 0.29–1.48%, P = 0.004; mean value = 7.06%). No association was noted between IVF sensitivity and variability in the base of support (P = 0.55).
Table 3.
Multiple Linear Regressions Assessing the Association between Coefficients of Variation and Integrated Visual Field Sensitivity under Three Different Walking Conditions
In each case where overall IVF sensitivity demonstrated an association with one of the gait variability measures, associations were also noted between superior and inferior IVF sensitivity and the same parameter, though in each case the strength of association was weaker. Additionally, IVF sensitivity was associated with variability in step length, stride length, and stride velocity during either cup carrying or tray carrying conditions (Table 3), but in no case was the strength of these associations stronger than that observed for the same parameter during normal usual-pace walking trial. Variability in several gait parameters were also noted to be associated with the total number of comorbid conditions, polypharmacy, age, sex, and race (Table 3). Given that the frequency of diabetes, but no other specific comorbid conditions, increased with the severity of VF damage, all significant associations were confirmed to persist in sensitivity models including diabetes as an additional covariate.
Discussion
Several gait characteristics associated with a higher risk of falling, including a number of measures evaluating stride-to-stride variability, were positively associated with glaucoma severity, as judged by VF sensitivity. These factors included a broader base of support and greater variability in step length, stride length, and stride velocity. To our knowledge, this is the first study to examine the impact of the severity of VF damage in glaucoma patients using detailed spatial and temporal characteristics of gait.
In this study, we found no association between IVF sensitivity and gait speed during normal usual-pace walking, but a significant decrease in speed during the tray-carrying trial. Prior studies showed a decrease in gait speed in visually impaired individuals and those with glaucoma during challenging conditions such as walking through an obstacle course.36–38 A few studies also reported lower walking speeds in visually impaired persons as compared with normally sighted individuals on straight, unobstructed routes, though these studies evaluated persons with more severe visual impairment than those evaluated in the current study.12–14 In the longitudinal Beaver Dam study in which most visual impairment was mild or moderate, Klein et al.39 reported no association between visual measures and changes in walking speed on an unobstructed 3-m walking route. Together, these findings suggest that moderate VF damage (like in our study population, median IVF sensitivity = 28.0 dB) might not be strongly associated with slower gait speed during the simple walking, but does play a role during more complex walking situations, such as obstacle courses or dual-task conditions.
Persons with greater IVF damage had a wider base of support, and this broader base of support may represent an adaptation to walking with their vision loss. A broad base of support is found in several neurological conditions (e.g., Parkinson's Disease, Huntington's Disease), and may be a nonspecific adaptation to poor balance.40,41 Prior work has demonstrated that older adults with a broader base of support have a greater fear of falling and a higher risk of falls.21,42 Interestingly, a training program designed to increase base of support in older individuals was noted to improve dynamic stability, suggesting that a broader base of support may serve as a marker for a higher fall risk, but nonetheless be an effective method for lowering fall risk.43
VF damage was also associated with greater stride-to-stride variability in velocity and step/stride length. These findings suggest that glaucoma patients are more irregular in their walking, perhaps in an effort to maintain a normal walking speed despite their visual limitations. Prior research in community-dwelling adults showed that greater variability in stride time, stride length, stride velocity, and double support time were associated with a great risk of falls.9,10,28 Moreover, prior research has shown that stride-to-stride variability of gait measures is more strongly associated with fall risk, while individual gait parameters (i.e., stride length and stride velocity) are more strongly associated with fear of falling.10 Our data suggest that glaucoma patients with more severe disease may continue to walk at the same speed, but become less able to control the regularity of their gait. In fact, greater VF damage was also associated with left–right drift while walking under distracted conditions, suggesting that in addition to maintaining a constant speed and stride length, glaucoma patients with more severe loss also have difficulty maintaining a straight line while walking.
Gait alterations may be elicited under distracted conditions, as these conditions challenge the cognitive effort required for maintaining a proper and regular gait.16–19,44 Indeed, several parameters including step and stride length, stride velocity, gait speed, and left-to-right drift were only associated with IVF sensitivity under dual task conditions, but not during normal walking. These data suggest that persons with VF damage may place greater cognitive effort toward maintaining a normal gait, but cannot maintain this normal gait when some of this cognitive effort must be reallocated toward a second task. At the same time, these changes in the gait under dual task conditions may reflect patients' attempt to adopt a safer gait in order to prevent falls.
We found that sensitivity in specific IVF regions had a similar impact on gait features as overall IVF sensitivity, suggesting that little additional information can be understood about gait by considering the location of VF damage. Our findings are somewhat opposed to prior work, which has found that inferior VF damage is more strongly associated with falls and balance.6,45 However, while related, falls, balance, and gait may have different relationships with VF damage, explaining the differences in findings between our study and previous work.
Gait may be an important predictor of falls given that it captures balance in motion. Indeed, 64% to 98% of the variance in gait parameters can be explained by measures of postural stability.46,47 Gait alterations are likely to lead to falls by placing patients into an unbalanced position from which they cannot recover. Individuals at a greater risk for falls demonstrate the lower capacity to recover from unexpected perturbations that could occur while walking. Elderly fallers have their center of mass closer to the boundary of their base of support (an area defined by all parts of the foot/feet touching the ground), suggesting that they keep their weight less centered over their base of support while walking.48 Additionally, older individuals move their center of mass closer to the edge of their base of support in response to a gait perturbation (experienced as an unexpected transition to a soft walking surface), suggesting that they are less able to “stay centered” in response to a perturbation.49 Further work is required to confirm which specific measures of gait relate to falls in glaucoma patients, and whether the gait measures associated with fall risk in this population are more likely to increase fall risk in patients with more advanced disease (indicating an interaction between gait parameters and severity of VF loss with regards to falls).
This study has a few limitations. First, there may have been selection bias in the assembly of our cohort, thought the direction of this potential bias is unclear. One possibility is that persons more predisposed to falling were more likely to participate, while an alternate possibility is that patients with greater mobility difficulties were less likely to participate due to difficulty attending the required study visits. Additionally, gait was tested while walking on the flat surface in a well-lit room, which does not capture the spectrum of walking conditions encountered in real life, such as the need to make turns or deal with steps or uneven terrain. Our ability to assess medications as a covariate was limited as we did not obtain all the requisite data for a more detailed assessment of medication burden, that is, the dosing and frequency of administration for each medication used, and interactions between medications used. Finally, our study population was found to be representative of the patient population being followed at the glaucoma clinic of the Wilmer Eye Institute from which we recruited our participants, but may not be representative of the overall glaucoma population in the United States. Strengths of the study include the large sample size compared with prior studies of gait and vision, the characterization of numerous previously unstudied gait parameters in this population, and an extensive characterization of parameters, which might confound the association between IVF sensitivity and gait.
In summary, numerous fall-relevant gait features were observed to change with severity of VF damage. Further research is needed to understand if these specific gait measures increase fall risk in glaucoma patients, and to determine whether the observed gait changes are adaptive (help prevent falls) or maladaptive (lead to falls). Given the multifactorial nature of falls, further work will need to integrate gait data and other risk factors to help identify glaucoma patients at higher risk of falling, and help guide the content of fall prevention programs in this high-risk group.
Acknowledgments
Supported by National Institutes of Health Grant EY022976 and the Research to Prevent Blindness Robert and Helen Schaub Special Scholar Award.
Disclosure: A. Mihailovic, None; B.K. Swenor, None; D.S. Friedman, None; S.K. West, None; L.N. Gitlin, None; P.Y. Ramulu, None
References
- 1. Centers for Disease Control and Prevention (CDC). Fatalities and injuries from falls among older adults - United States, 1993-2003 and 2001-2005. MMWR Morb Mortal Wkly Rep. 2006; 23: 479–481. [PubMed] [Google Scholar]
- 2. Stevens JA,, Corso PS,, Finkelstein EA,, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev. 2006; 12: 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coleman AL,, Cummings SR,, Yu F,, et al. Binocular visual-field loss increases the risk of future falls in older white women. J Am Geriatr Soc. 2007; 55: 357–364. [DOI] [PubMed] [Google Scholar]
- 4. Dhital A,, Pey T,, Stanford MR. Visual loss and falls: a review. Eye (Lond). 2010; 24: 1437–1446. [DOI] [PubMed] [Google Scholar]
- 5. Freeman EE,, Munoz B,, Rubin G,, West SK. Visual field loss increases the risk of falls in older adults: the Salisbury eye evaluation. Invest Ophthalmol Vis Sci. 2007; 48: 4445–4450. [DOI] [PubMed] [Google Scholar]
- 6. Black AA,, Wood JM,, Lovie-Kitchin JE. Inferior field loss increases rate of falls in older adults with glaucoma. Optom Vis Sci. 2011; 88: 1275–1282. [DOI] [PubMed] [Google Scholar]
- 7. Tanabe S,, Yuki K,, Ozeki N,, Shiba D,, Tsubota K. The association between primary open-angle glaucoma and fall: an observational study. Clin Ophthalmol. 2012; 6: 327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuki K,, Asaoka R,, Tsubota K. Investigating the influence of visual function and systemic risk factors on falls and injurious falls in glaucoma using the structural equation modeling. PLoS One. 2015; 10: e0129316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hausdorff JM,, Rios DA,, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001; 82: 1050–1056. [DOI] [PubMed] [Google Scholar]
- 10. Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997; 45: 313–320. [DOI] [PubMed] [Google Scholar]
- 11. Shimada H,, Kim H,, Yoshida H,, et al. Relationship between age-associated changes of gait and falls and life-space in elderly people. J Phys Ther Sci. 2010; 22: 419–424. [Google Scholar]
- 12. Hallemans A,, Ortibus E,, Truijen S,, Meire F. Development of independent locomotion in children with a severe visual impairment. Res Dev Disabil. 2011; 32: 2069–2074. [DOI] [PubMed] [Google Scholar]
- 13. Nakamura T. Quantitative analysis of gait in the visually impaired. Disabil Rehabil. 1997; 19: 194–197. [DOI] [PubMed] [Google Scholar]
- 14. Turano KA,, Rubin GS,, Quigley HA. Mobility performance in glaucoma. Invest Ophthalmol Vis Sci. 1999; 40: 2803–2089. [PubMed] [Google Scholar]
- 15. Tomomitsu MS,, Alonso AC,, Morimoto E,, Bobbio TG,, Greve JM. Static and dynamic postural control in low-vision and normal-vision adults. Clinics (Sao Paulo). 2013; 68: 517–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kressig RW,, Herrmann FR,, Grandjean R,, Michel JP,, Beauchet O. Gait variability while dual-tasking: fall predictor in older inpatients? Aging Clin Exp Res. 2008; 20: 123–130. [DOI] [PubMed] [Google Scholar]
- 17. Lundin-Olsson L,, Nyberg L,, Gustafson Y. “Stops walking when talking” as a predictor of falls in elderly people. Lancet. 1997; 349: 617. [DOI] [PubMed] [Google Scholar]
- 18. Lundin-Olsson L,, Nyberg L,, Gustafson Y. Attention, frailty, and falls: the effect of a manual task on basic mobility. J Am Geriatr Soc. 1998; 46: 758–761. [DOI] [PubMed] [Google Scholar]
- 19. Toulotte C,, Thevenon A,, Watelain E,, Fabre C. Identification of healthy elderly fallers and non-fallers by gait analysis under dual-task conditions. Clin Rehabil. 2006; 20: 269–276. [DOI] [PubMed] [Google Scholar]
- 20. Guimaraes RM,, Isaacs B. Characteristics of the gait in old people who fall. Int Rehabil Med. 1980; 2: 177–180. [DOI] [PubMed] [Google Scholar]
- 21. Gehlsen GM,, Whaley MH. Falls in the elderly: part I, gait. Arch Phys Med Rehabil. 1990; 71: 735–738. [PubMed] [Google Scholar]
- 22. Wolfson L,, Whipple R,, Amerman P,, Tobin JN. Gait assessment in the elderly: a gait abnormality rating scale and its relation to falls. J Gerontol. 1990; 45: M12–M19. [DOI] [PubMed] [Google Scholar]
- 23. Odden JL,, Mihailovic A,, Boland MV,, Friedman DS,, West SK,, Ramulu PY. Evaluation of central and peripheral visual field concordance in glaucoma. Invest Ophthalmol Vis Sci. 2016; 57: 2797–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bilney B,, Morris M,, Webster K. Concurrent related validity of the GAITrite® walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003; 17: 68–74. [DOI] [PubMed] [Google Scholar]
- 25. Cutlip RG,, Mancinelli C,, Huber F,, DiPasquale J. Evaluation of an instrumented walkway for measurement of kinematic parameters of gait. Gait Posture. 2000; 12: 134–138. [DOI] [PubMed] [Google Scholar]
- 26. McDonough AL,, Batavia M,, Chen FC,, Kwon S,, Ziai J. The validity and reliability of the GAITRite system's measurements: a preliminary evaluation. Arch Phys Med Rehabil. 2001; 82: 419–425. [DOI] [PubMed] [Google Scholar]
- 27. Webster KE,, Wittwer JE,, Feller JA. Validity of the GAITRite walkway system for the measurement of averaged and individual step parameters of gait. Gait Posture. 2005; 22: 317–321. [DOI] [PubMed] [Google Scholar]
- 28. Hausdorff JM,, Edelberg HK,, Mitchell SL,, Goldberger AL,, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997; 78: 278–283. [DOI] [PubMed] [Google Scholar]
- 29. Gnjidic D,, Hilmer SN,, Blyth FM,, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012; 65: 989–995. [DOI] [PubMed] [Google Scholar]
- 30. Ramulu PY,, Maul E,, Hochberg C,, Chan ES,, Ferrucci L,, Friedman DS. Real-world assessment of physical activity in glaucoma using an accelerometer. Ophthalmology. 2012; 119: 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tibaek S,, Holmestad-Bechmann N,, Pedersen T,, Bramming S,, Friis A. Reference values of maximum walking speed among independent community-dwelling Danish adults aged 60 to 79 years: a cross-sectional study. Physiotherapy. 2015; 101: 135–140. [DOI] [PubMed] [Google Scholar]
- 32. Pirker W,, Katzenschlager R. Gait disorders in adults and the elderly: a clinical guide. Wien Klin Wochenschr. 2017; 129: 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kirkness C,, Ren J. Race differences: use of walking speed to identify community-dwelling women at risk for poor health outcomes-osteoarthritis initiative study. Phys Ther. 2015; 95: 955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gohdes D,, Balamurugan A,, Larsen B,, Maylahn C. Age-related eye diseases: an emerging challenge for public health professionals. Prev Chronic Dis. 2005; 2: A17. [PMC free article] [PubMed] [Google Scholar]
- 35. StataCorp LP. Stata data analysis and statistical software: release 14.0. College Station, TX: Stata Corporation; 2015. [Google Scholar]
- 36. Turano KA,, Broman AT,, Bandeen-Roche K,, et al. Association of visual field loss and mobility performance in older adults: Salisbury Eye Evaluation Study. Optom Vis Sci. 2004; 81: 298–307. [DOI] [PubMed] [Google Scholar]
- 37. Friedman DS,, Freeman E,, Munoz B,, Jampel HD,, West SK. Glaucoma and mobility performance: the Salisbury Eye Evaluation Project. Ophthalmology 2007. December; 114: 2232–2237. [DOI] [PubMed] [Google Scholar]
- 38. Patel I,, Turano KA,, Broman AT,, Bandeen-Roche K,, Munoz B,, West SK. Measures of visual function and percentage of preferred walking speed in older adults: the Salisbury Eye Evaluation Project. Invest Ophthalmol Vis Sci. 2006; 47: 65–71. [DOI] [PubMed] [Google Scholar]
- 39. Klein BE,, Moss SE,, Klein R,, Lee KE,, Cruickshanks KJ. Associations of visual function with physical outcomes and limitations 5 years later in an older population. Ophthalmology 2003; 110: 644–650. [DOI] [PubMed] [Google Scholar]
- 40. Rao AK,, Quinn L,, Marder KS. Reliability of spatiotemporal gait outcome measures in Huntington's disease. Mov Disord. 2005; 20: 1033–1037. [DOI] [PubMed] [Google Scholar]
- 41. Hass CJ,, Malczak P,, Nocera J,, et al. Quantitative normative gait data in a large cohort of ambulatory persons with Parkinson's disease. PLoS One. 2012; 7: e42337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chamberlin ME,, Fulwider BD,, Sanders SL,, Medeiros JM. Does fear of falling influence spatial and temporal gait parameters in elderly persons beyond changes associated with normal aging? J Gerontol A Biol Sci Med Sci. 2005; 60: 1163–1167. [DOI] [PubMed] [Google Scholar]
- 43. Bierbaum S,, Peper A,, Arampatzis A. Exercise of mechanisms of dynamic stability improves the stability state after an unexpected gait perturbation in elderly. Age (Dordr). 2013; 35: 1905–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beauchet O,, Dubost V,, Gonthier R,, Kressig RW. Dual-task-related gait changes in transitionally frail older adults: the type of the walking-associated cognitive task matters. Gerontology. 2005; 51: 48–52. [DOI] [PubMed] [Google Scholar]
- 45. Black AA,, Wood JM,, Lovie-Kitchin JE,, Newman BM. Visual impairment and postural sway among older adults with glaucoma. Optom Vis Sci. 2008; 85: 489–497. [DOI] [PubMed] [Google Scholar]
- 46. Petrofsky JS,, Cuneo M. Correlation between gait, balance, and age when people are standing and walking in normal, subdued, and no light conditions. Phys Occup Ther Geriatr. 2008; 26: 23–40. [Google Scholar]
- 47. Heitmann DK,, Gossman MR,, Shaddeau SA,, Jackson JR. Balance performance and step width in noninstitutionalized, elderly, female fallers and nonfallers. Phys Ther. 1989; 69: 923–931. [DOI] [PubMed] [Google Scholar]
- 48. Lugade V,, Lin V,, Chou LS. Center of mass and base of support interaction during gait. Gait Posture. 2011; 33: 406–411. [DOI] [PubMed] [Google Scholar]
- 49. Bierbaum S,, Peper A,, Karamanidis K,, Arampatzis A. Adaptational responses in dynamic stability during disturbed walking in the elderly. J Biomech. 2010; 43: 2362–68. [DOI] [PubMed] [Google Scholar]