Abstract
Introduction: Allosteric modulation of cannabinoid type-1 receptors (CB1) is a novel means through which signaling bias may be exerted. Org27569 remains the most-characterized CB1 allosteric modulator, yet there are conflicting reports regarding its effects on extracellular signal-regulated kinase (ERK) signaling. We conducted a systematic evaluation of Org27569's effects on cannabinoid agonists and ERK signaling.
Materials and Methods: HEK293 cells transfected with the human cannabinoid type-1 receptor (hCB1) were treated with Org27569 alone or in combination with the endocannabinoid 2-arachidonoylglycerol (2-AG), the synthetic cannabinoid CP55,940, or the phytocannabinoid delta-9-tetrahydrocannabinol (THC) and ERK activation was measured by western blot. Overnight treatment with pertussis toxin (PTX) was used to determine the role of Gi/o in Org27569's inverse agonist effects. HEK293 cells transfected with green fluorescent protein tagged rat CB1 receptor were used to assess effects of Org27569 on CP55,940-induced receptor internalization. Subcellular fractionation was used to determine effects of Org27569 on ERK phosphorylation in both nuclear and cytosolic compartments.
Results: We found that Org27569 is an antagonist of hCB1-mediated ERK signaling in HEK293 cells where it fully blocks CP55,940-but does not completely inhibit THC- and 2-AG-stimulated ERK1/2 activation following 5 min treatment. In rat CB1 HEK293 cells, CP55,940 (1 μM) treatment produced a significant increase in puncta at 20, 40, 60, and 120 min, consistent with receptor internalization. Org27569 (10 μM) co-treatment prevented internalization at each time point and alone had no effect. Org27569 reduced basal ERK phosphorylation in hCB1 HEK293 cells but not in untransfected cells following 20 min treatment. Overnight treatment with PTX abated this response. Following subcellular fractionation, Org27569 produced a significant decrease in ERK phosphorylation in the nuclear-enriched and cytosolic fractions.
Conclusions: These data are consistent with previous studies demonstrating that CB1-mediated ERK1/2 activation is Gi/o-dependent and that Org27569 is an inverse agonist of CB1 receptors. Abrogation of Org27569's ability to reduce basal ERK phosphorylation following treatment with PTX and lack of inverse agonist effects in untransfected HEK293 cells demonstrates that Org27569 acts via CB1-Gi/o to produce this effect. To our knowledge, this is the first reported demonstration of inverse agonism of ERK signaling by Org27569.
Keywords: allosteric, cannabinoid, CB1, ERK, signaling, Org27569
Introduction
The endocannabinoid system currently comprises two G-protein-coupled receptors (GPCR), cannabinoid type-1 (CB1)1 and type-2 (CB2),2 endogenous ligands (endocannabinoids), including N-arachidonoylethanolamine (anandamide; AEA)3 and 2-arachidonoylglycerol (2-AG),4 and the regulatory enzymes for the synthesis and degradation of endocannabinoids.5
While the phytocannabinoid delta-9-tetrahydrocannabinol (THC), the primary psychoactive constituent of cannabis,6 acts on the CB1 receptor to exert its abuse-related effects, the therapeutic effects of cannabinoids are also mediated, in part, by this receptor,5 and therefore, it is of much interest for the development of pharmacotherapeutics. The CB1 receptor couples to Gi/o-proteins, which on activation lead to (1) inhibition of adenylyl cyclase and L-, N-, and P/Q-type voltage-gated calcium channels7–9 and (2) activation of inwardly rectifying potassium channels10 and (3) mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK).11
The pleiotropic nature of GPCR signaling12 has led to the pursuit of compounds that can selectively activate specific signaling pathways to better study their contribution to the effects of cannabinoids and to develop pharmacotherapeutic strategies, which are efficacious yet lack the concomitant adverse effects typically observed with CB1 agonism. Classically, CB1 receptor signaling has been focused on using compounds that compete for an orthosteric binding site, thus offering a limited approach, in which a single compound activates a set response depending on its own intrinsic signaling biases. Agonists of the CB1 receptor exhibit bias/functional selectivity, in which certain signaling pathways can be preferentially activated.13
Allosteric modulation offers an additional layer of control over receptor signaling, in that binding to an allosteric site can further alter receptor conformation to affect agonist binding/efficacy and signaling specificity or impart signaling on its own in the absence of an agonist. Allosteric modulation of the CB1 receptor is a recent development in cannabinoid pharmacology with functionally negative14,15 and positive16–19 allosteric modulators having been reported only within the last decade.
The most characterized compound to date, Org27569, has been reported to act as an insurmountable antagonist/inverse agonist of CP55,940-stimulated [35S]GTPγS binding,14,20,21 while exhibiting positive binding cooperativity with [3H]CP55,940.14 Org27569 also attenuates cannabinoid agonists' ability to inhibit forskolin-stimulated cAMP production13,22 and acts as a CB1 inverse agonist, increasing cAMP levels over forskolin stimulation in a pertussis toxin (PTX)-sensitive manner.22 The effects of Org27569 on ERK1/2 signaling are unclear as Org27569 has been reported to act as either an allosteric agonist of ERK1/2 signaling via beta-arrestin1,23 or Gi/o,20 or as an allosteric antagonist.13
CB1 activation of ERK1/2 occurs through a number of mechanisms24 in a time-dependent manner, with peak effects occurring at ∼5 min when examined in CB1 HEK293 cells.25 Following activation, ERK1/2 translocates to the nucleus where it regulates gene expression,26 which impacts a number of functions, including those important for synaptic plasticity27 and the development of cannabinoid tolerance.28 Because there are separate pools of ERK1/2—cytoplasmic and nuclear—it is possible that measurement of ERK phosphorylation in total cell lysates could obscure differences in phosphorylation states that exist between these two compartments.
In this study, we therefore examined the effects of Org27569 alone on ERK1/2 phosphorylation in both cytoplasmic and nuclear compartments to ensure that changes in phosphorylation states in one compartment were not obscured by the other. ERK1/2 phosphorylation following Org27569 treatment was measured at 20 min to ensure we could observe a potential decrease, based on previous literature with other inverse agonists.29,30
To further investigate the aforementioned disparate findings of Org27569 effects on ERK signaling, we hypothesized that Org27569 would act as an antagonist/inverse agonist of ERK1/2 signaling, since previous literature indicates that Org27569 is a CB1 inverse agonist of [35S]GTPγS binding. Furthermore, since CB1-mediated ERK1/2 activation is Gi/o dependent11,31 and the CB1 antagonist/inverse agonist SR141716A elicits reductions in basal ERK1/2 phosphorylation29 that are PTX sensitive,30 we tested the PTX sensitivity of the ERK response elicited by Org27659.
Materials and Methods
Materials and reagents
CP55,940, delta-9-tetrahydrocannabinol (THC), SR141716A, 2-arachidonoylglycerol (2-AG), and Org27569 were provided by the National Institute on Drug Abuse (Bethesda, MD) and were dissolved in DMSO and diluted to a final working concentration of 0.1–0.2%. PTX (Calbiochem, San Diego, CA) was dissolved in MilliQ filtered water.
Cell cultures and transfection
Vendor authenticated human embryonic kidney (HEK293; American Type Culture Collection, Rockville, MD) cells were cultured in 5% fetal bovine serum (FBS) defined (Hyclone Labs, Logan, UT) in Dulbecco's modified Eagle's medium (DMEM; Corning Cellgro, Manassas, VA) at 37°C and 5% CO2. Cell lines were generated as previously described32 by transfection of the untagged hCB1 or N-terminally green fluorescent protein (GFP)-tagged rat CB1 receptor with Lipofectamine 2000 (Life Technologies, Gaithersburg, MD). Cells were maintained in a culture medium with geneticin (0.5 mg/mL; G418) and not used after 25 passages.
Cell treatment and phospho-ERK quantification
Cells were plated in poly-d-lysine (PDL)-coated 6-well plates or 100-mm dishes (for fractionation experiments) to a confluency of ∼70–80% and serum starved for 24 h before all treatments. For internalization experiments, cells were plated on PDL-coated cover-slips in a 24-well format. For experiments examining Gi/o protein mediation, cells were exposed to PTX (200 ng/mL) for 18 h before drug treatment when appropriate.
Cells were treated by application of vehicle or antagonist followed immediately by application of vehicle or agonist and incubated in serum-free DMEM at 37°C for times indicated, then washed once with ice-cold phosphate-buffered saline (PBS), and lysed with buffer containing HEPES (50 mM), NaCl (150 mM), EDTA (1 mM), EGTA (1 mM), glycerol (10%), Triton X-100 (1%), MgCl2 (10 μM), NaF (25 mM), Na3VO4 (1 mM), para-nitrophenyl phosphate (20 mM) and EDTA-free protease inhibitor. Lysates were incubated on ice for 30 min, then centrifuged at 16,000 g for 30 min at 4°C, and supernatants were collected.
Protein amount (20 μg) was determined by the Bradford method, separated by 10% SDS-PAGE, and transferred to nitrocellulose membranes. Membranes were blocked for 1 h with the Odyssey blocking buffer (1:1, PBS:Odyssey blocking buffer) and incubated at room temperature for 2 h or overnight at 4°C in primary antibodies directed at phospho-ERK1/2 (Sigma Aldrich M8159; 1:5000) and total ERK1/2 (Sigma Aldrich M5670; 1:8000).
Membranes were rinsed thrice in 0.1% Tween-20 in PBS before incubating with LiCor secondary antibodies (IRDye® 800CW Goat anti-Mouse; 1:6666; IRDye 680RD Goat anti-Rabbit; 1:10,000) at room temperature for 1 h. Blots were imaged using LiCor Odyssey imaging software, and phospho-ERK signal was normalized to total ERK and data expressed as percent of vehicle control. All data points are means of a minimum of three separate experiments run in duplicate or triplicate.
Receptor internalization
For internalization experiments, GFP-rat CB1 HEK293 cells were plated on PDL-coated cover-slips in the 24-well format and treated with vehicle (0.2% DMSO), CP55,940 (1 μM), Org27569 (10 μM), or both for 20–120 min. Cell images were taken blinded, converted to grayscale in Photoshop CS5, and then analyzed in ImageJ. Background was subtracted by using a “rolling ball” algorithm (rolling=3). Puncta within the range (size=3–400, circularity=0.01–1.00) were counted. These parameters were used based on image resolution and brightness. The same conditions were applied to all the images and image analyses.
Subcellular fractionation
Cells were grown and treated as described above on 100-mm dishes and washed once with ice-cold PBS. Cells were then lysed by addition of 300–500 μL of fractionation buffer (250 mM sucrose, 20 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, phosphatase and protease inhibitors). Lysate was passed through a 26-gauge syringe 10 times and then incubated on ice for 30 min. The nuclear-enriched fraction was pelleted following centrifugation at 700g for 5 min and rinsed twice in fractionation buffer then centrifuged again and reconstituted in nuclear buffer (fractionation buffer containing 10% glycerol and 0.1% SDS) and sonicated at a setting of two-continuous.
The initial S1 fraction was centrifuged at 10,000 g for 10 min and the resulting S2 fraction served as the cytosolic/membrane fraction. Protein was determined by the NanoDrop™ A280 assay (Thermo Scientific, Wilmington, DE). Nuclear fraction enrichment was established by immunoblotting for lamin A/C (1:2000; Cell Signaling Technologies).
Data analysis
Data were normalized as percent vehicle control and analyzed by one- or two-way ANOVA with Newman–Keuls and Bonferroni post hoc tests, and data were considered significant with p<0.05. For concentration–response studies, curve fitting was done using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA) nonlinear regression [log(inhibitor) vs. response]. Data reported are the mean±SEM of at least three independent experiments.
Results
Org27569 and SR141716A blocked CP55,940-induced ERK activation and receptor internalization
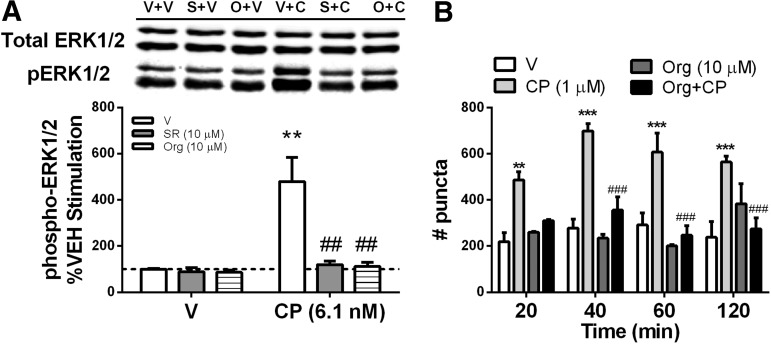
We first assessed the effects of Org27569 alone and in combination with the cannabinoid agonist CP55940 and included the selective CB1 antagonist SR141716A as a control (Fig. 1A). Following a 5-min incubation, Org27569 and SR141716A alone produced a small but nonsignificant reduction of ERK phosphorylation, whereas CP55,940 produced a robust increase in ERK phosphorylation (main effect: CP55,940 [F(1,12)=9.289, p<0.05]). However, both SR141716A and Org27569 prevented CP55,940-induced activation of ERK (main effect: [F(2,12)=6.593, p<0.05]; interaction effect: [F(2,12)=5.622, p<0.05]), suggesting that Org27569 can act as an antagonist of CB1-mediated ERK phosphorylation.
FIG. 1.
Org27569 and SR141716A blocked CP55,940-induced ERK activation and receptor internalization. (A) HEK293 cells stably transfected with the hCB1 receptor were exposed to the CB1 antagonist/inverse agonist SR141716A (10.0 μM) or the CB1 allosteric modulator Org27569 (10.0 μM) alone or in combination with CP55,940 (6.1 nM) for 5 min. (B) Org27569 prevented CP55,940-induced receptor internalization in HEK293 cells stably transfected with GFP-rat CB1 receptor. Protein was separated by SDS-PAGE and western blots for phospho-ERK normalized to total ERK. GFP-rat CB1 cells were seeded on PDL-coated cover-slips and treated with Org27569/CP55,940 or corresponding vehicle, imaged by a blinded observer, converted to grayscale and analyzed in ImageJ, which counted puncta. Data were analyzed by two-way ANOVA with Bonferroni post hoc tests. Data are the mean±SEM of at least three independent experiments. **p<0.01, ***p<0.001 compared to V+V control; ##p<0.01, ###p<0.001 compared to V+CP. CB1, cannabinoid type-1 receptor; CP, CP55,940; GFP, green fluorescent protein; hCB1, human CB1; ERK, extracellular signal-regulated kinase; PDL, poly-d-lysine; V, vehicle.
In addition, we sought to determine if Org27569 could prevent internalization of GFP-tagged rat CB1 receptors by the cannabinoid agonist CP55,940. We observed that CP55,940 (1 μM)-induced increases in puncta were prevented by Org27569 (10 μM) at every time point assessed (main effect: treatment [F(3,32)=44.8, p<0.0001]), suggesting that Org27569 prevented internalization of CB1 receptors (Fig. 1B).
Org27569 antagonizes cannabinoid agonist-induced ERK activation
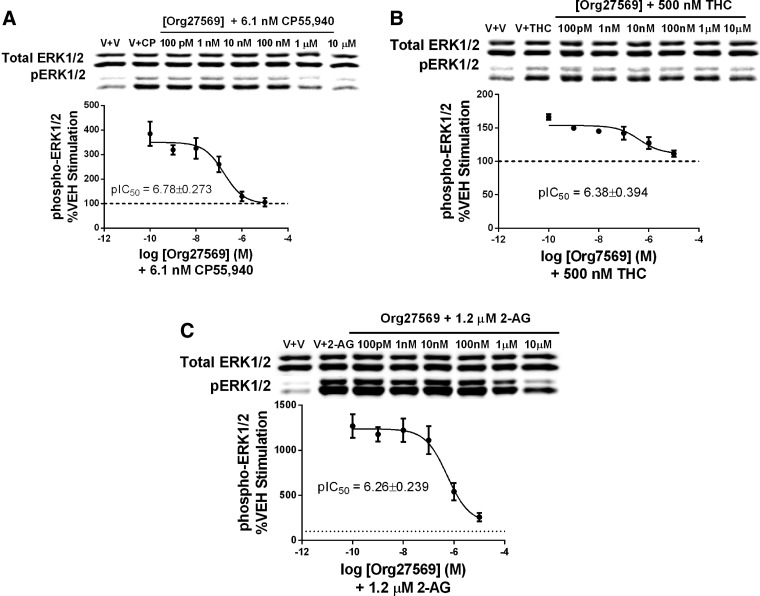
To determine the potency and extent of Org27569 ability to antagonize cannabinoid agonist-induced ERK activation, we first determined EC80 values for CP55,940 (EC80=6.1 nM), THC (EC80=500 nM), and 2-AG (EC80=1.2 μM). We then conducted full concentration–response curves for Org27569 in the presence of these agonists at their EC80 values (see Table 1 for summary of calculated IC50 values). Org27569 fully antagonized CP55,940-induced ERK activation (pIC50=6.78±0.273; Fig. 2A), while it did not completely attenuate THC-induced ERK activation (pIC50=6.38±0.394; Fig. 2B) or 2-AG-stimulated ERK activation (pIC50=6.26±0.238; Fig. 2C), with the bottom of the fit curves at 111.3%±7.72% and 196%±123.2%, respectively.
Table 1.
Org27569 IC50 Values Against Agonists and Alone on ERK Phosphorylation
| Probe agonist | IC50 (nM) | IC50 95% confidence interval (nM) | pIC50±SE | pIC50 95% confidence interval |
|---|---|---|---|---|
| CP55,940 (6.1 nM)a | 165 | 43–629 | 6.783±0.273 | 7.36–6.2 |
| THC (494 nM)a | 417 | 60–2800 | 6.38±0.394 | 7.22–5.54 |
| 2-AG (1.2 μM)a | 546 | 170–1760 | 6.26±0.239 | 6.77–5.76 |
| Noneb | 139 | 49–397 | 6.86±0.214 | 7.31–6.4 |
Cells treated for 5 min.
Cells treated for 20 min.
FIG. 2.
Org27569 antagonizes cannabinoid agonist-induced ERK activation. HEK293 cells stably transfected with the hCB1 receptor were exposed to specified agonists at their EC80 values and Org27569 for 5 min. Protein was separated by SDS-PAGE and western blots for phospho-ERK normalized to total ERK and curves were fitted using GraphPad Prism 5.0 to calculate IC50 values. (A) Org27569 concentration–response curve applied in combination with EC80 of CP55,940 (6.1 nM). (B) Org27569 concentration–response curve in the presence of EC80 THC (500 nM). (C) Org27569 concentration–response curve in the presence of EC80 2-AG (1.2 μM). Data are the mean ± SEM of three independent experiments. CP, CP55,940; Org, Org27569; V, vehicle.
Org27569 reduces basal ERK phosphorylation in both cytoplasmic and nuclear compartments via hCB1-Gi protein mechanism
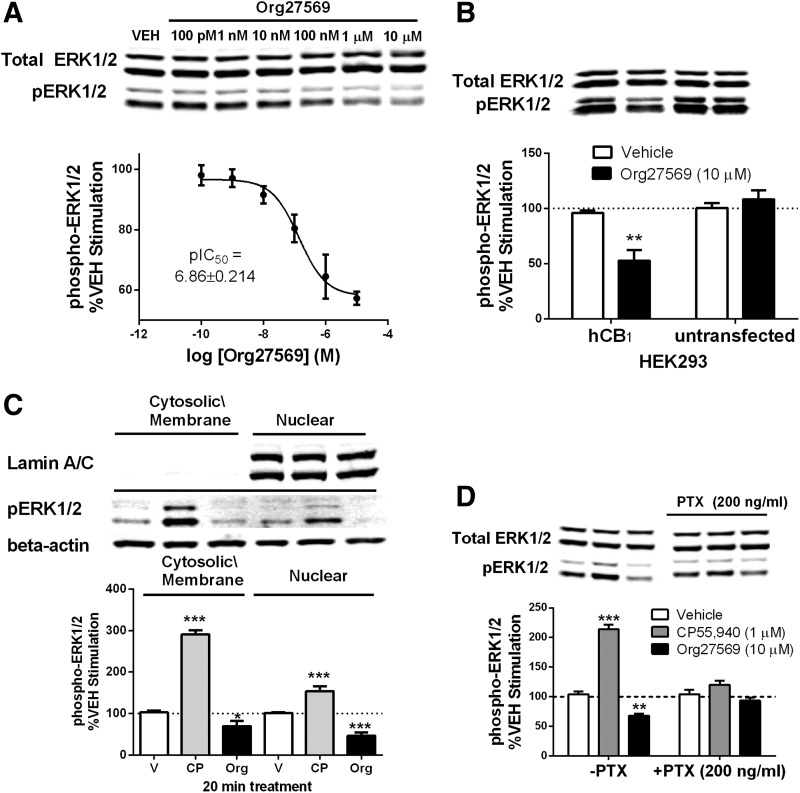
We observed small but nonsignificant reductions in basal ERK phosphorylation with Org27569 (10 μM) alone at 5 min (Fig. 1A). Previously, the CB1 inverse agonist SR141716A was shown to reduce basal ERK phosphorylation following a 10-min incubation,29,30 and since we found that internalization was complete at 20 min (Fig. 1B), we next tested Org27569 using a 20-min incubation time. We observed that Org27569 significantly reduced basal ERK phosphorylation following a 20-min exposure (pIC50=6.86±0.21; Fig. 3A).
FIG. 3.
Org27569 (10.0 μM) reduces basal ERK phosphorylation in both cytoplasmic and nuclear compartments via hCB1-Gi protein mechanism following 20-min treatment. (A) Concentration–response curve for Org27569 alone. (B) Org27569 (10.0 μM) reduces basal phospho-ERK in hCB1-transfected but not hCB1-untransfected HEK293 cells. (C) Org27569 (10.0 μM) resulted in significant decreases in cytosolic and nuclear phospho-ERK. (D) Org27569 (10.0 μM) reduced basal ERK phosphorylation, which was prevented by overnight treatment with PTX. HEK293 cells stably transfected with the hCB1 receptor were exposed to Org27569 for 20 min. PTX (200 ng/mL) treatment was done overnight. Protein was separated by SDS-PAGE and western blots for phospho-ERK normalized to total ERK (except in fractions where it was normalized to beta-actin). Separate gels containing the same samples were run for immunoblotting of phospho-ERK/beta-actin and lamin A/C. Data were analyzed by one- or two-way ANOVA with Newman–Keuls and Bonferroni post hoc tests, respectively. Data are the mean ± SEM of at least three independent experiments. *p<0.05, **p<0.01, ***p<0.001. CP, CP55,940; Org, Org27569; PTX, pertussis toxin; V, vehicle.
To determine receptor mediation, we tested Org27569 (10 μM) in hCB1-transfected and hCB1-untransfected HEK293 cells. We found that following a 20-min treatment, Org27569 reduced basal ERK phosphorylation in hCB1-transfected but not in hCB1-untransfected HEK293 cells (Fig. 3B) (main effect: Org27569 treatment [F(1,8)=6.765, p<0.05], cell type [F(1,8)=19.43, p<0.01] and significant interaction [F(1,8)=13.99, p<0.01]), suggesting that reductions in basal ERK phosphorylation occurred through the hCB1 receptor.
Org27569 has been reported to signal through beta-arrestin 1 to activate ERK.23 Beta-arrestin-mediated signaling through ERK has been suggested to occur through a large receptor–arrestin–ERK complex that cannot translocate into the nucleus, resulting in elevated cytosolic levels of phospho-ERK and decreased nuclear levels of phospho-ERK.33 A recent report showing that Org27569 alone did not activate ERK suggested that this may have been due to an inability to assess distinct pools of ERK (i.e., nuclear vs. cytosolic) at the subcellular level.13
To further delineate the effects of Org27569 at the subcellular level, fractionation experiments were conducted to determine if differences in ERK phosphorylation could be detected in these separate cellular compartments following Org27569 treatment. We observed that Org27569 (10 μM) significantly decreased cytosolic [F(2,9)=151.1, p<0.0001] and nuclear phospho-ERK levels [F(2,9)=41.27, p<0.0001] (Fig. 3C).
To further determine the mechanism of action of Org27569 effects, we examined the role of Gi/o proteins by preventing their activation with overnight treatment PTX (200 ng/mL). We observed that Org27569 produced a reduction in phospho-ERK levels in control cells but not in PTX-treated cells, suggesting a Gi/o-dependent effect (main effect: treatment [F(2,18)=105.5, p<0.0001], PTX [F(1,18)=20.22, p<0.001], and significant interaction [F(2,18)=52.34]) (Fig. 3D).
Discussion
Presently, Org27569 remains the most-studied and prototypical CB1 allosteric modulator, with newly synthesized compounds based on its structure being investigated for pharmacological properties. Org27569 and its structural analogs demonstrate positive binding cooperativity with the CB1/CB2 agonist CP55,940 and negative binding cooperativity with the selective CB1 antagonist/inverse agonist SR141716A. Functionally, Org27569 exhibits allosteric antagonism of CB1-mediated agonist-stimulated [35S]GTPγS binding and cAMP production.13,14,20,21 Furthermore, the Org27569 pharmacological profile is such that it decreases basal activity in both [35S]GTPγS20,21,34 and cAMP assays,22 suggesting that it is an inverse agonist of CB1-mediated G-protein signaling.
However, it has been reported that Org27569 can enhance or act as an allosteric agonist of ERK signaling via either Gi/o20 or beta-arrestin 1.23 In contrast, others have reported that Org27569 acts as a CB1 allosteric antagonist of ERK activation.13 In addition to this, Org27569 has also been reported to antagonize agonist-induced suppression of forskolin-stimulated cAMP production13,22 and act as an inverse agonist of CB1-mediated inhibition of cAMP production, which was prevented by treatment with PTX.22
In this study, we report that Org27569 serves as an antagonist of CB1-mediated ERK signaling by the cannabinoid agonists CP55,940, THC, and 2-AG and also that it acts as an inverse agonist of basal ERK phosphorylation. These findings are mostly congruent with those recently reported by Khajehali et al.13 as we observed full antagonism of CP55,940-induced ERK activation, whereas inhibition of 2-AG-induced ERK activation was not complete.
While we observed that Org27569 antagonized THC-induced ERK activation, Khajehali et al.13 reported no effect. There are some differences between our study and theirs. First, they used Chinese hamster ovary cells, whereas we used HEK293 cells. Furthermore, Khajehali et al.13 assessed ERK activation with the AlphaScreen SureFire kit, whereas we measured phospho-ERK levels by western analysis, so it may be that westerns are more sensitive for detecting these changes. Our calculated IC50 values for Org27569 (Table 1) when tested against the EC80 values of orthosteric cannabinoid agonists were not significantly different from one another, suggesting that Org27569 does not exhibit biased antagonism of THC, 2-AG or CP55,940 in terms of ERK signaling.
In consideration of the established data indicating that Org27569 reduces basal [35S]GTPγS binding consistent with an inverse agonist, and that the selective CB1 antagonist/inverse agonist SR141716A reduces basal ERK activation in a PTX-sensitive manner,30 we further hypothesized that Org27569 would act as an inverse agonist of ERK signaling in our cell line. Following a 20-min incubation, Org27569 did indeed reduce basal ERK phosphorylation in hCB1 HEK293 cells but not in untransfected cells, suggesting that Org27569 acts as an hCB1 inverse agonist of ERK signaling. We observed that overnight treatment with PTX prevented the reduction in phospho-ERK, suggesting that the Org27569 inverse agonist effects result from reduced basal activity of the receptor coupling with Gi/o.
Since HEK293 cells do not synthesize endocannabinoids and the cells were serum starved overnight before treatment, it is likely that the reduction in ERK phosphorylation following Org27569 treatment is due to inhibition of constitutive activity, consistent with an inverse agonist. ERK activation through CB1 by typical cannabinoid agonists has been shown to occur through Gi/o proteins,35 and therefore, these data are consistent with the known signaling properties of the CB1 receptor. Inverse agonism by Org27569 was not reported by Khajehali et al.13; this may be due to differences in CB1 expression between our cell lines since a higher expression level results in a greater basal activity making detection of inverse agonists easier.36
The lack of agonist effects of Org27569 alone on ERK signaling was previously suggested to be due to an inability to quantify pools of phospho-ERK, which may have a distinct subcellular localization.13 Indeed, ERK signaling by the angiotensin II type 1A (AT1a) receptor through a beta-arrestin mechanism was reported to result in an increase in cytosolic phosph-ERK with a concomitant reduction in nuclear phospho-ERK due to the inability of the receptor–arrestin–ERK complex to translocate into the nucleus.33
To test the hypothesis that Org27569 increases cytosolic phospho-ERK but decreases nuclear phospho-ERK, we performed subcellular fractionation of cells treated with Org27569 for 20 min to examine both nuclear and cytosolic phospho-ERK levels. We did not observe an increase in phospho-ERK levels following Org27569 in either the cytosolic or nuclear fractions, suggesting that Org27569 under these conditions does not serve as an agonist of ERK. We did observe decreases in phospho-ERK in the nuclear-enriched and cytosolic fractions, consistent with a reduction in basal ERK activation.
Previously, AT1a receptor signaling through beta-arrestin to activate ERK was observed following overexpression of beta-arrestins, which suggests that differences in levels of beta-arrestin expression between cell lines may explain the lack of observed biased agonist effects of Org27569 in our cell line. Activation of ERK by Org27569 was previously reported to be beta arrestin-1 dependent in HEK293 cells that were not overexpressing beta arrestin-1.23
The HEK293 cells used in our study were acquired from the American Type Culture Collection. These cells have been previously reported to express both beta arrestin-1 and beta arrestin-2,37 although this does not rule out the possibility of differences in expression level that may exist between our cell line and others. Nonetheless, these data are consistent with those one would expect from a CB1 receptor inverse agonist as the selective CB1 antagonist/inverse agonist SR141716A38 has been shown to serve as an inverse agonist at ERK in a PTX-sensitive manner.30 In addition, reported inverse agonist effects of Org27569 on cAMP production22 are also consistent with those of the CB1 inverse agonist SR141716A on adenylyl cyclase activity.39,40
We also tested whether Org27569 could prevent CP55,940-induced internalization of CB1 receptors. We found that CP55,940 resulted in an increase in puncta, consistent with receptor internalization, at each time point. Cotreatment with Org27569 prevented CP55,940-induced internalization at each time point. Org27569 alone did not significantly affect receptor internalization.
In summary, our data support previous studies that suggest Org27569 is an allosteric antagonist/inverse agonist of G-protein signaling.13,14,20,22 Consistent with the study by Khajehali et al.,13 we find that Org27569 acts as an antagonist of CB1-mediated ERK activation with the exception that we observed antagonism of THC-induced ERK1/2 phosphorylation, whereas Khajehali et al.13 did not. In contrast with studies by Ahn et al.23 and Baillie et al.,20 Org27569 did not serve as an agonist of ERK signaling as we observed no increases in phospho-ERK in either the cytosolic or nuclear-enriched subcellular fractions.
It is possible that levels of beta arrestin-1 in our cell line may be insufficient to allow biased coupling to this signaling pathway as reported by Ahn et al.23 Indeed, for the AT1a receptor, biased ERK signaling through beta arrestin was observed only following overexpression of beta arrestin1 and beta-arrestin 2.33 Thus, differences in beta-arrestin expression between cell lines may account for the differences observed in Org27569 effects when administered alone. In addition, we report that Org27569 exhibits inverse agonism of ERK signaling and that this is through the hCB1 receptor via Gi/o proteins. To our knowledge, this is the first report of inverse agonism of ERK signaling by Org27569; this adds to the reported inverse agonism of Org27569 on [35S]GTPγS binding14,20,21 and cAMP.22
Acknowledgment
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01DA23204, P01DA009158, T32DA007237, and P30DA013429].
Abbreviations Used
- AT1a
angiotensin II type 1A
- CB1
cannabinoid type-1 receptors
- CB2
cannabinoid type-2 receptors
- DMEM
Dulbecco's modified Eagle's medium
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- GPCR
G-protein-coupled receptors
- hCB1
human CB1
- PBS
phosphate-buffered saline
- PDL
poly-d-lysine
- PTX
pertussis toxin
Author Disclosure Statement
No competing financial interests exist.
Cite this article as: Gamage TF, Anderson JC, Abood ME (2016) CB1 allosteric modulator Org27569 is an antagonist/inverse agonist of ERK1/2 signaling, Cannabis and Cannabinoid Research 1:1, 272–280, DOI: 10.1089/can.2016.0028.
References
- 1. Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564 [DOI] [PubMed] [Google Scholar]
- 2. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65 [DOI] [PubMed] [Google Scholar]
- 3. Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949 [DOI] [PubMed] [Google Scholar]
- 4. Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylgylcerol: a possible endogenous cannabinoid receptor-ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97 [DOI] [PubMed] [Google Scholar]
- 5. Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009;60:77–84 [DOI] [PubMed] [Google Scholar]
- 6. Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647 [Google Scholar]
- 7. Caulfield MP, Brown DA. Cannabinoid receptor agonists inhibit Ca current in NG108-15 neuroblastoma cells via a pertussis toxin-sensitive mechanism. Br J Pharmacol. 1992;106:231–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci U S A. 1992;89:3825–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50 [DOI] [PubMed] [Google Scholar]
- 10. Guo J, Ikeda SR. Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol Pharmacol. 2004;65:665–674 [DOI] [PubMed] [Google Scholar]
- 11. Bouaboula M, Poinot-Chazel C, Bourrie B, et al. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312(Pt 2):637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kenakin T. Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther. 2011;336:296–302 [DOI] [PubMed] [Google Scholar]
- 13. Khajehali E, Malone DT, Glass M, et al. Biased agonism and biased allosteric modulation at the CB1 cannabinoid receptor. Mol Pharmacol. 2015;88:368–379 [DOI] [PubMed] [Google Scholar]
- 14. Price MR, Baillie GL, Thomas A, et al. Allosteric modulation of the cannabinoid CB1 receptor. Mol Pharmacol. 2005;68:1484–1495 [DOI] [PubMed] [Google Scholar]
- 15. Horswill JG, Bali U, Shaaban S, et al. PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br J Pharmacol. 2007;152:805–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pamplona FA, Ferreira J, de Lima OM, et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc Natl Acad Sci U S A. 2012;109:21134–21139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Navarro HA, Howard JL, Pollard GT, et al. Positive allosteric modulation of the human cannabinoid (CB) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br J Pharmacol. 2009;156:1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ignatowska-Jankowska BM, Baillie GL, Kinsey S, et al. A cannabinoid CB1 receptor-positive allosteric modulator reduces neuropathic pain in the mouse with no psychoactive effects. Neuropsychopharmacology. 2015;40:2948–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bauer M, Chicca A, Tamborrini M, et al. Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J Biol Chem. 2012;287:36944–36967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baillie GL, Horswill JG, Anavi-Goffer S, et al. CB(1) receptor allosteric modulators display both agonist and signaling pathway specificity. Mol Pharmacol. 2013;83:322–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shore DM, Baillie GL, Hurst DH, et al. Allosteric modulation of a cannabinoid G protein-coupled receptor: binding site elucidation and relationship to G protein signaling. J Biol Chem. 2014;289:5828–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cawston EE, Redmond WJ, Breen CM, et al. Real-time characterization of cannabinoid receptor 1 (CB1) allosteric modulators reveals novel mechanism of action. Br J Pharmacol. 2013;170:893–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahn KH, Mahmoud MM, Shim JY, et al. Distinct roles of beta-arrestin 1 and beta-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1). J Biol Chem. 2013;288:9790–9800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dalton GD, Howlett AC. Cannabinoid CB1 receptors transactivate multiple receptor tyrosine kinases and regulate serine/threonine kinases to activate ERK in neuronal cells. Br J Pharmacol. 2012;165:2497–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daigle TL, Kearn CS, Mackie K. Rapid CB 1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology. 2008;54:36–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roskoski R. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66:105–143 [DOI] [PubMed] [Google Scholar]
- 27. Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183 [DOI] [PubMed] [Google Scholar]
- 28. Rubino T, Forlani G, Vigano D, et al. Ras/ERK signalling in cannabinoid tolerance: from behaviour to cellular aspects. J Neurochem. 2005;93:984–991 [DOI] [PubMed] [Google Scholar]
- 29. Rinaldi-Carmona M, Le Duigou A, Oustric D, et al. Modulation of CB1 cannabinoid receptor functions after a long-term exposure to agonist or inverse agonist in the Chinese hamster ovary cell expression system. J Pharm Exp Ther. 1998;287:1038–1047 [PubMed] [Google Scholar]
- 30. Bouaboula M, Perrachon S, Milligan L, et al. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem. 1997;272:22330–22339 [DOI] [PubMed] [Google Scholar]
- 31. Chen X, Yang W, Fan Y, et al. Structural determinants in the second intracellular loop of the human cannabinoid CB1 receptor mediate selective coupling to Gs and Gi. Br J Pharmacol. 2010;161:1817–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tao Q, McAllister SD, Andreassi J, et al. Role of a conserved lysine residue in the peripheral cannabinoid receptor (CB2): evidence for subtype specificity. Mol Pharmacol. 1999;55:605–613 [PubMed] [Google Scholar]
- 33. Tohgo A, Pierce KL, Choy EW, et al. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–9436 [DOI] [PubMed] [Google Scholar]
- 34. Ahn KH, Mahmoud MM, Kendall DA. Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation. J Biol Chem. 2012;287:12070–12082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Korzh A, Keren O, Gafni M, et al. Modulation of extracellular signal-regulated kinase (ERK) by opioid and cannabinoid receptors that are expressed in the same cell. Brain Res. 2008;1189:23–32 [DOI] [PubMed] [Google Scholar]
- 36. Pertwee RG. Inverse agonism and neutral antagonism at cannabinoid CB 1 receptors. Life Sci. 2005;76:1307–1324 [DOI] [PubMed] [Google Scholar]
- 37. Atwood BK, Lopez J, Wager-Miller J, et al. Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics. 2011;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Landsman RS, Burkey TH, Consroe P, et al. SR141716A is an inverse agonist at the human cannabinoid CB 1 receptor. Eur J Pharmacol. 1997;334:R1–R2 [DOI] [PubMed] [Google Scholar]
- 39. Meschler JP, Kraichely DM, Wilken GH, et al. Inverse agonist properties of N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide HCl (SR141716A) and 1-(2-chlorophenyl)-4-cyano-5-(4-methoxyphenyl)-1H-pyrazole-3-carboxylic acid phenylamide (CP-272871) for the CB 1 cannabinoid receptor. Biochem Pharmacol. 2000;60:1315–1323 [DOI] [PubMed] [Google Scholar]
- 40. Mato S, Pazos A, Valdizan EM. Cannabinoid receptor antagonism and inverse agonism in response to SR141716A on cAMP production in human and rat brain. Eur J Pharmacol. 2002;443:43–46 [DOI] [PubMed] [Google Scholar]