Abstract
Background:
D-dimer has been widely used for the diagnosis and prognosis of ovarian cancer, but there is still controversy on its prediction value of ovarian cancer.
Objectives:
To explore the clinical significance of plasma D-dimer level on ovarian cancer systematically.
Methods:
Using PubMed, Cochrane Library, and Web of Science libraries, all the relevant studies for the diagnostic and prognostic value of plasma D-dimer for ovarian cancer and the relationship between elevated D-dimer level and venous thromboembolism (VTE) risk of ovarian cancer were searched till May 30, 2016. Standardized mean difference (SMD), odds ratio (OR), hazard ratio (HR), and 95% confidence interval (CI) were appropriately pooled.
Results:
A total of 15 eligible studies involving a total of 1437 cancer patients were included. No significant association was found between high D-dimer level and overall survival of patients with ovarian cancer (HR 1.32, 95% CI: 0.90–1.95, P = .044). However, subgroup analysis indicated that the sample sizes could explain the heterogeneity between studies. And elevated D-dimer could predict increased risk of mortality when the sample sizes were >100 (HR 1.800, 95% CI: 1.283–2.523, P = .845). Besides, plasma D-dimer level was significantly higher in malignant ovarian cancer patients compared with benign controls (SMD 0.774, 95% CI: 0.597–0.951, P = .39), higher in advanced ovarian cancer patients (International Federation of Gynecology and Obstetrics [FIGO] classification III and IV) than in early stage ovarian cancer patients (FIGO classification I and II, SMD 0.611, 95% CI: 0.373–0.849, P = .442). And high D-dimer level indicated high VTE risk (OR 4.068, 95% CI: 2.423–6.829, P = .629) of ovarian cancer patients.
Conclusion:
The plasma D-dimer level in ovarian cancer patients can predict the changes that correlated with disease progression and the VTE risk. But its predictive value for the prognosis of ovarian cancer was significantly dependent on the sample sizes. More well-designed studies with large sample sizes are needed to validate and update the findings of present study.
Keywords: D-dimer, meta-analysis, ovarian cancer, overall survival, venous thromboembolism
1. Introduction
Ovarian cancer is the leading cause of deaths of female with gynecological malignancies worldwide.[1] In 2012, an estimated 238,719 women were diagnosed with ovarian cancer and 151,900 of them died.[2] In the United States, about 87% of the serous ovarian cancer patients were diagnosed at advanced stage.[3] Lack of early predictive biomarkers is responsible for the high mortality rate. Until now, the most widely used biomarker for monitoring of ovarian cancer is carbohydrate antigen 125 (CA-125).[4] Approximately 83% of the patients at advanced stage have CA-125 levels >35 U/mL.[5] However, CA-125 is also elevated in a small proportion of people with endometriosis, pelvic inflammatory disease, pregnancy, hepatic cirrhosis, acute heart failure, tuberculosis, pancreatic cancer, lung cancer, liver cancer, and so on.[6,7] Therefore, it has a relatively low positive predictive value and is usually not considered as an independent predictor.[8] Besides, there is still controversy on its prognosis prediction value of ovarian cancer.[9–11] Thus, to find other reliable biomarkers or the biomarkers which could be used in combination with CA-125 for general population screening, effective evaluation and prognosis prediction of ovarian cancer is long-expected.
The development of cancer is often accompanied with several complicated changes of homeostatic system.[12] Patients with cancer especially in the advanced-stage often underlie a state of hypercoagulation and exaggerated fibrinolysis.[13] Tumor cells can activate the clotting-fibrinolytic system and release various of procoagulant fibrinolytic markers and hemostatic factors,[9] which in turn stimulate proliferation and differentiation of vascular endothelial cells to promote neoangiogenesis.[14] This stimulation further contributes to tumor growth, invasion, metastasis, and recidivism.[15–17] D-dimer, a signal of activated coagulation system and an end-product of fibrinogen,[18] has been accepted as an useful diagnosis and prognosis parameter for several malignancies.[19–21] Besides, D-dimer has been proved to be directly and positively correlated with CA-125,[12,22] and the combination of CA-125 and D-dimer to differentiate benign from malignant ovarian tumors was better than single detection of either CA-125 or D-dimer.[23] Studies have found that D-dimer level increased significantly in patients with ovarian cancer, but evidence was not sufficient as most studies included only small numbers of patients,[24–26] and the diagnostic and prognostic significance of plasma D-dimer in ovarian cancer has not yet been systematically analyzed. So we conducted this meta-analysis to explain the role of plasma D-dimer for diagnosis and survival prediction of ovarian cancer. In addition, we also evaluated the relationship between elevated D-dimer level and venous thromboembolism (VTE) risk.
2. Materials and methods
2.1. Protocol
Meta analysis integrates patients’ information from the articles that had been approved by either ethics committee or institutional review board, so our meta-analysis did not include the patient’ consent and ethical approval. We conducted this meta-analysis in accordance with the Preferred Reporting Items for Meta-Analyses (PRISMA) statement.
2.2. Search strategy
An electronic search was carried out from inception up to May 30, 2016, for the association between plasma D-dimer levels and ovarian cancer, from the databases PubMed (http://www.ncbi.nlm.nih.gov/pubmed); Cochrane Library (http://www.cochranelibrary.com/); and Web of Science (http://apps.webofknowledge.com/UA_GeneralSearch_input.do?product=UA&search_mode=GeneralSearch&SID=W1xTxGB1nHdk2AAHedn&preferencesSaved=).
Searches were limited to human studies using the MeSH terms combined with free words of “ovarian cancer” and “D-dimer,” without language restriction. Furthermore, hand searches were performed for unindexed study selection.
2.3. Study selection
Studies were included in this meta-analysis if they covered one of the following elements: comparisons of plasma D-dimer levels between groups of early and advanced ovarian cancers or ovarian cancers and benign controls, relationship between plasma D-dimer levels and VTE risk of ovarian cancers, prognostic effect of plasma D-dimer on overall survival (OS) or progression-free survival.
Inclusion criteria: prospective or retrospective studies on patients with ovarian cancer by pathological diagnosis, plasma D-dimer level was detected before operation or chemotherapy, and data of non-English papers can be extracted from the English abstract. Exclusion criteria: review, meta-analysis, repeated studies, and severe outliers.
2.4. Data extraction and quality assessment
All data were extracted by 2 independent reviewers. Discrepancies were resolved by discussion or via the involvement of a third reviewer when necessary. The quality of each selected article in our analysis was assessed by Newcastle–Ottawa scale (NOS). The following data were extracted: the first author's name, publication year, study location, detection method of D-dimer, group, sample sizes, cutoff point of D-dimer, follow-up period, International Federation of Gynecology and Obstetrics (FIGO) stage, and the other clinical parameters.
The D-dimer value was presented as mean ± standard deviation (mean ± SD). The data using median and range or interquartile range were converted to mean ± SD by using the method reported before.[27,28] Associations between D-dimer level and the outcome were presented as hazard ratios (HRs) and corresponding 95% confidence intervals (95% CIs). And the survival data presented as Kaplan–Meier curves or log-rank test were converted to HRs and 95% CIs using the method reported before.[29–31]
2.5. Statistical analysis
All analyses were completed with Stata V.12.0 (StataCorp, College Station, Texas). Heterogeneity between studies was calculated by Q and I2 test. If I2 ≤50%, then a fixed-effect model was used, otherwise, a random-effect model was adopted and a subgroup analysis was performed to find sources of the heterogeneity. Sensitivity analysis was carried out to exclude outliers. Publication bias was assessed by Begg funnel plots. P <.05 was considered statistically significant, except where otherwise specified. All statistical tests were 2-tailed.
3. Results
3.1. Literature search
The flowchart for the selection of literature is presented in Figure 1. In detail, a total of 171 records were returned (57 from PubMed, 4 from the Cochrane Library, 107 from Web of Science, and 3 from other sources). Eighty-three studies were excluded after the initial evaluation of titles and abstracts and 51 studies were excluded after the full-text reviewing. Besides, 15 studies were unable to extract the valid data and 4 duplicated studies were excluded. Finally, 18 studies with eligible data were included in this study. Six studies on the D-dimer level for the prognosis of ovarian cancer, 8 studies on the comparison of D-dimer levels between ovarian cancers (stages I–IV) and benign ovarian cysts, 6 studies on the comparison of D-dimer levels between early and advanced ovarian cancers, and 7 studies on the association between D-dimer level and VTE risk.
Figure 1.
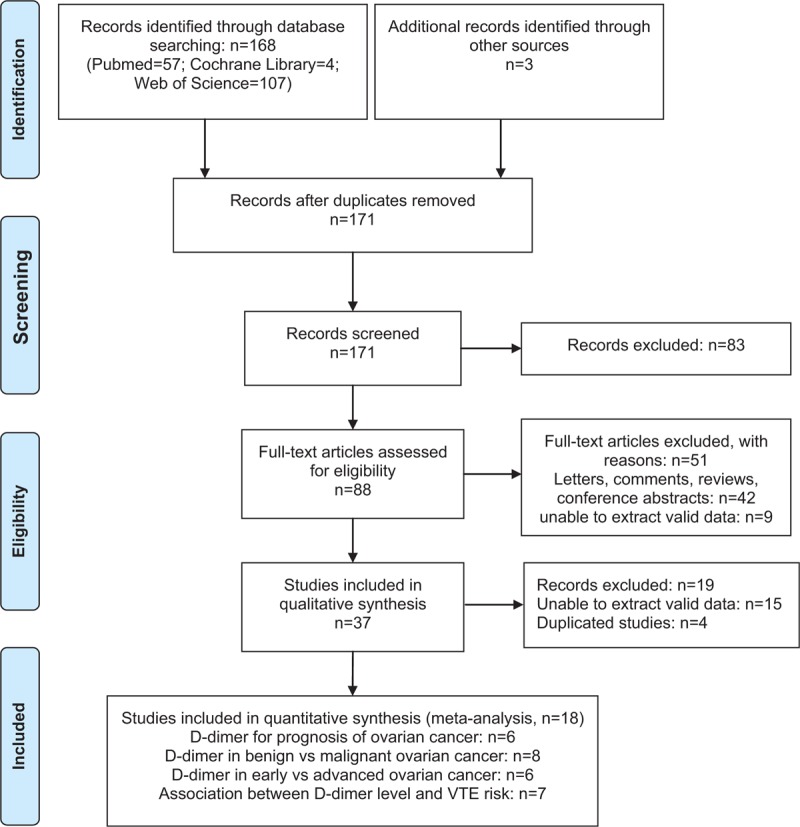
Flow chart of the study selection process.
3.2. Study characteristics
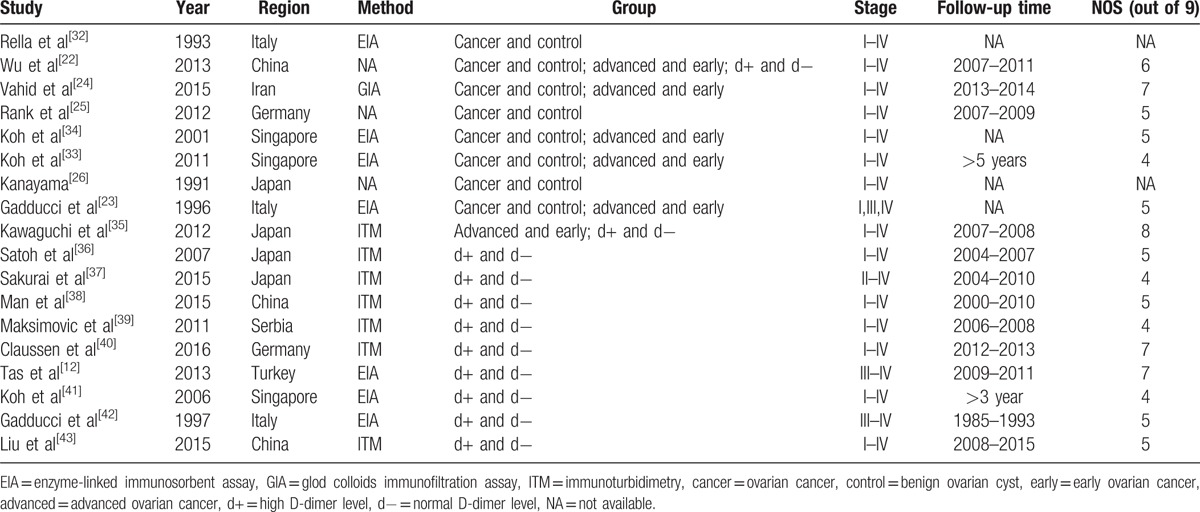
The main characteristics of the studies included are listed in Table 1. All eligible articles were published from 1991 to 2016, and the sample sizes ranged from 30 to 241. In prognosis analyses and VTE risk analyses, each contained 1 study whose D-dimer level was detected after surgery. Quality assessment performed with NOS score is shown in Table 1 and the median score was 5 (range 4–8).
Table 1.
Study characteristics.
3.3. Main analysis
3.3.1. D-dimer and prognosis
A total of 6 studies[12,37,38,41–43] were focused on the D-dimer level on OS of ovarian cancer patients. The random-effect model with HR and 95% CI on OS indicated that there was no significant association between D-dimer and OS of ovarian cancers (HR 1.323, 95% CI: 0.897–1.953, Fig. 2A). However, the heterogeneity was high (P = .044, I2 = 56.1%).
Figure 2.
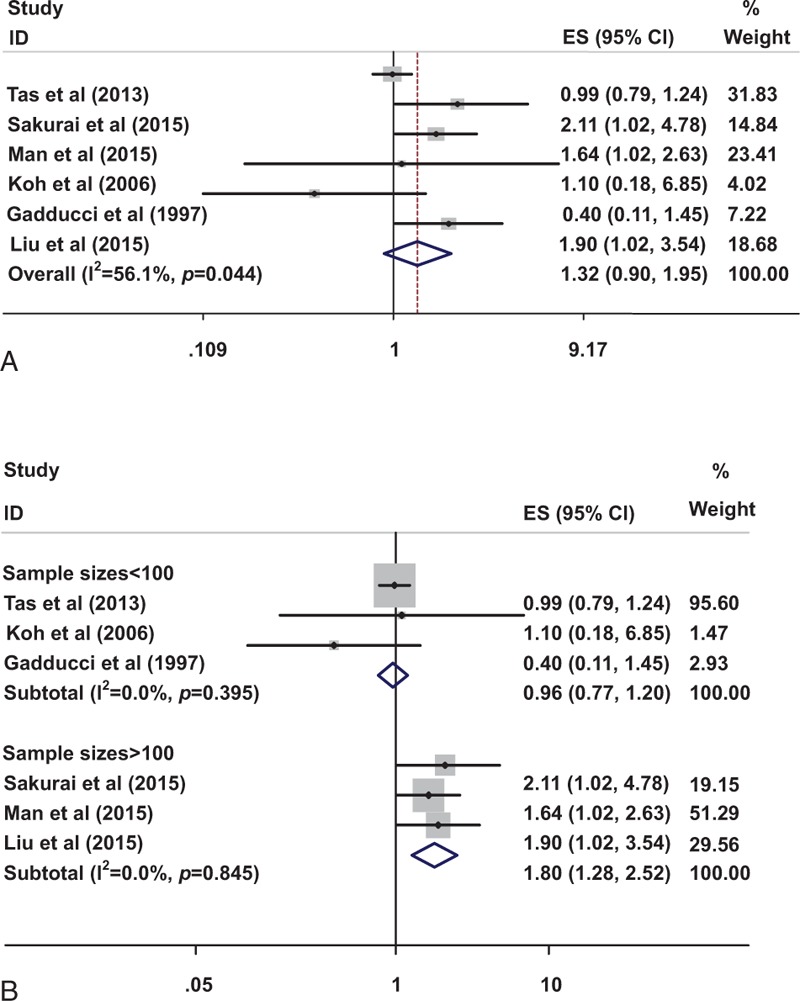
Impact of elevated D-dimer on OS (A) and subgroup analysis of different sample sizes on OS (B). OS = overall survival.
A subgroup analysis stratified by the sample sizes showed that patients with elevated D-dimer had a relatively reduced risk of mortality compared with normal D-dimer group when the sample sizes were >100 (HR 1.800, 95% CI: 1.283–2.523), without evidence of heterogeneity (P = .845, I2 = 0.0%, Fig. 2B). However, no significant difference in OS was found in these two groups (HR 0.963, 95% CI: 0.771–1.201) when the sample sizes were <100 (P = .395, I2 = 0.0%, Fig. 2B). These results indicated that the small sample size may be the main source of heterogeneity.
3.3.2. Comparison of the plasma D-dimer level in ovarian cancers (stages I–IV) and benign ovarian cysts
A total of 7 studies[22,24–26,32–34] involving 366 ovarian cancer patients and 233 benign controls investigated the association between D-dimer and ovarian cancer. Our results showed that patients with ovarian cancer (stages I–IV) showed a higher D-dimer level compared with benign controls (SMD = 0.774, 95% CI: 0.597–0.951), with low degree of heterogeneity (P = .390, I2 = 4.9%, Fig. 3A) after the study by Gadducci et al[23] was omitted (The sensitivity analysis showed that this paper lacked the data of FIGO stage II responsible for the heterogeneity (P = .000, I2 = 82.8%, data not shown).
Figure 3.
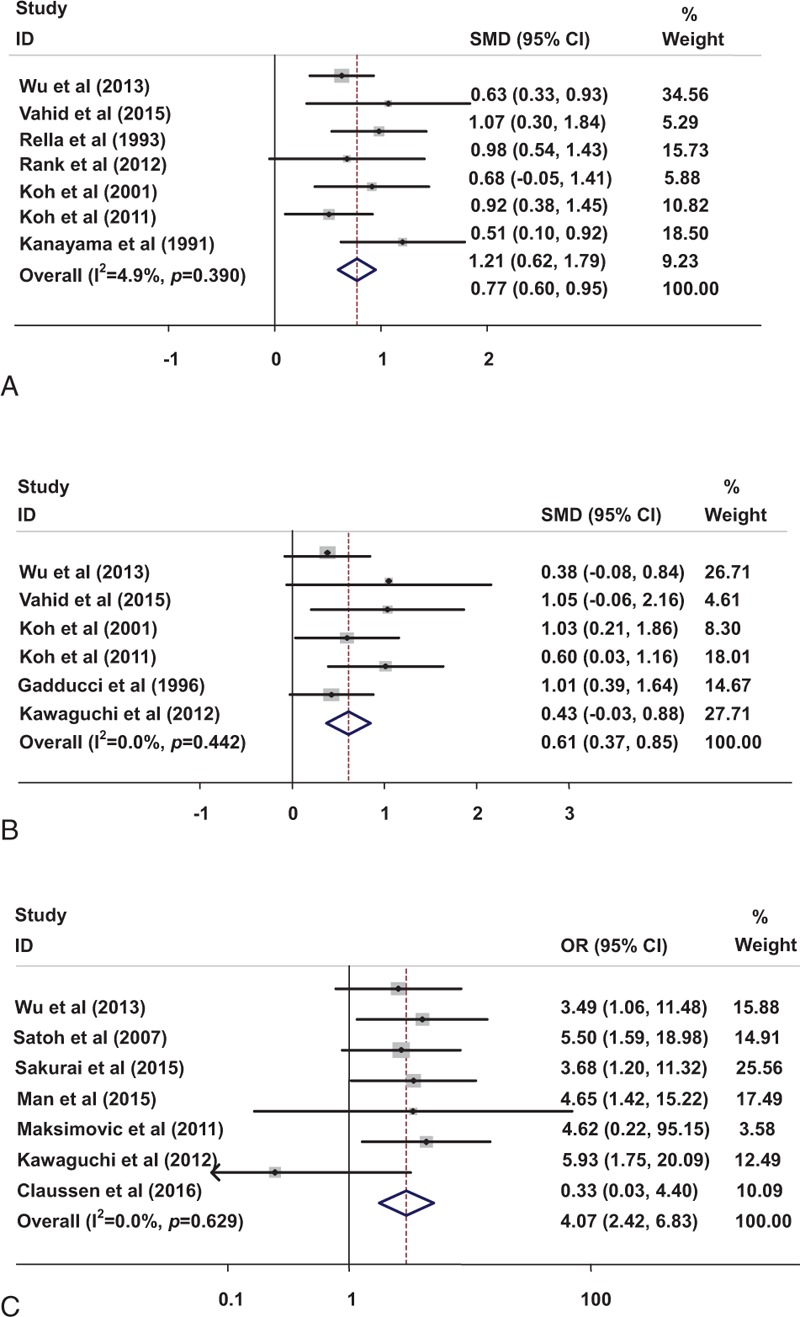
Forest plot for D-dimer in benign and malignant ovarian tumor (A), in early and advanced ovarian cancer (B). Risk of VTE associated with D-dimer level in ovarian cancer (C). VTE = venous thromboembolism.
3.3.3. Comparison of the plasma D-dimer level in early ovarian cancers (stages I–II) and advanced ovarian cancers (stages III–IV)
With no evidence of heterogeneity (P = .442, I2 = 0.0%), a fixed-effect model was used in 6 studies[22–24,33–35] to calculate the pooled SMD and 95% CI for the association of plasma D-dimer level with the FIGO stage of ovarian cancer. The results showed that the plasma D-dimer level was higher in advanced ovarian cancer patients (stages III–IV) than in the early stage ovarian cancer patients (stages I–II, SMD = 0.611, 95% CI: 0.373–0.849; Fig. 3B).
3.3.4. Risk of VTE in ovarian cancers with high D-dimer level and normal D-dimer level
The adjusted odds ratios (ORs) for 7 studies[22,35–40] including the association between D-dimer level and VTE risk of ovarian cancer patients are shown in Fig. 3C. The results showed that the high D-dimer level was significantly associated with high VTE risk of ovarian cancer patients (OR: 4.068, 95% CI: 2.423–6.829), with no evidence of heterogeneity (P = .629, I2 = 0.0%).
3.4. Publication bias
The funnel plots showed no signs of asymmetry. And the P-value of the Begg rank correlation test (Fig. 4A–C, P >.05) showed that no publication bias existed among the included studies.
Figure 4.
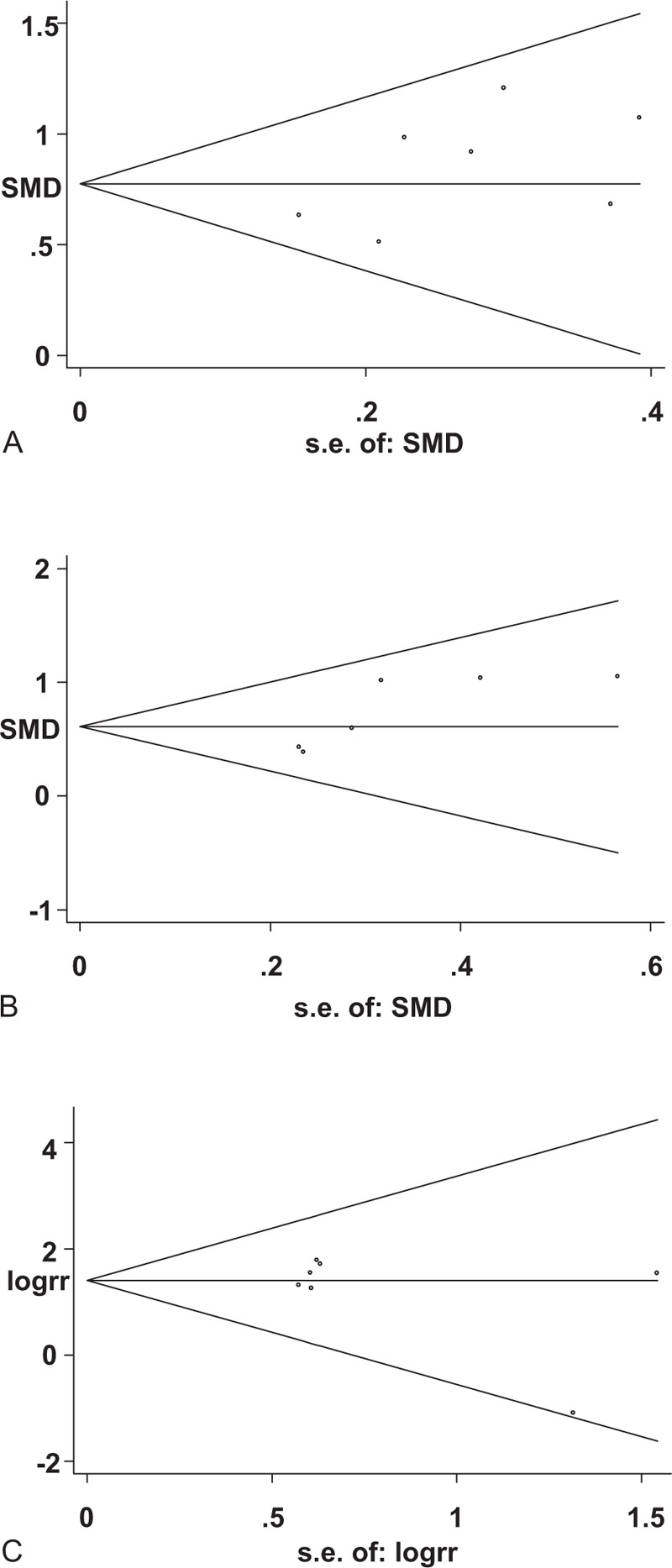
Begg's funnel plot for D-dimer in benign and malignant ovarian tumors (A), D-dimer in early and advanced ovarian cancers (B) and for VTE risk associated with D-dimer in ovarian cancer patients (C). VTE = venous thromboembolism.
4. Discussion
D-dimer is a stable final product of fibrin degradation.[44] Fibrinogen is converted into fibrin monomers by thrombin, and then the monomers polymerize to form fibrin clots during blood coagulation.[17] Fibrin degradation products such as fragments E and D are released into the bloodstream after fibrin clots being digested by plasmin.[14] Finally, D-dimers are formed steady with 2 covalently bound D-domains by factor XIII.[18] D-dimer has been used as a specific marker which reflects the enhanced secondary fibrinolysis in fibrinolytic process.[12] Its clinical value has been widely recognized, in particular for the exclusion of VTE.[45] The adequate extra cellular matrix for cancer cell migration, invasion, and metastasis is provided from fibrin formation and remodeling process embodied as constantly elevated plasma D-dimer level.[46] Studies[17,21,22] indicate that the D-dimer level is positively correlated with the clinical stage of malignant tumor, which is consistent with the above-mentioned view.
A growing body of research focused on the relationship between D-dimer and tumors. A study of 32 women with breast cancer and 43 healthy controls reported that a high D-dimer level was correlated with poor outcomes of breast cancer.[47] However, another study showed the opposite result.[48] Studies on colorectal cancer[49] and lung cancer[21,50] patients indicated the association of elevated D-dimer with a high tumor stage. Now what about the ovarian cancer?
Our meta-analysis showed that the plasma D-dimer level in ovarian cancer patients was higher than in benign controls. And it was positively correlated with the FIGO classification. However, the sample sizes of the studies involving the comparison of D-dimer in early ovarian cancer and benign controls were too small and their results were not exactly the same[22,23,33,34] or even opposite.[24] Thus, we could not draw any conclusion to identify the diagnosis value of D-dimer in early stage ovarian cancer. Further studies that focus more on this topic are suggested. D-dimer level
The association between VTE and malignancy was first recognized by Trousseau in 1865.[51] VTE includes deep vein thrombosis and pulmonary embolism and can significantly affect OS of patients.[52] Up to now, quite a lot of studies demonstrated the high incidence of VTE in cancer patients,[53–55] and they have confirmed that VTE was a common complication in malignant tumors.[56] Interestingly, the tumors with high risk of VTE are mostly abdominal tumors which suggest that the mechanical stress in the complex abdominal venous system is easy to cause venous embolism.[13,57] Ovarian cancer is one of the malignancies with the highest risk of VTE.[57]
Based on the formation mechanism, D-dimer has been used as a noninvasive screening index. The predictive value of D-dimer for VTE following ovarian cancer was still controversial.[38–40] Our results showed that ovarian cancer patients with elevated D-dimer had a significant higher risk of VTE compared with normal D-dimer group. In other words, plasma D-dimer test may help to select occult VTE at the high-risk group with ovarian cancer. However, previous studies indicated that D-dimer test was proposed for exclusion rather than for diagnosis.[35,36] The sensitivity of D-dimer for diagnosis and prognosis of ovarian is affected mainly by the sample sizes, the cutoff value, and the testing method.
This meta-analysis on prognostic value of D-dimer showed that OS was significantly reduced in patients with elevated D-dimer when the sample sizes were >100. However, no statistical difference were found between high and normal D-dimer level in terms of OS when sample sizes were <100. Besides, we also noticed that the cutoff point selection and the D-dimer detection method were also different between the large sample size group and small sample size group. Both cutoff point selection and D-dimer detection method are very essential for the evaluation of D-dimer level on OS. So we cannot conclude which part is the main source of heterogeneity. However, we consider that the cutoff point should be chosen according to the area under an ROC curve, but not just the median value. And the standard detection method should be recommended in the future.
As stated above, a lot of research has been done on this approach to detect tumor by using D-dimer as a marker, but its clinical use is limited possibly because of the following reasons. There are no unified testing methods of D-dimer and all standardization and harmonization attempts have not achieved satisfying results in daily practice.[58] Differences in antibody specificities of different assays result in inconsistent quantitative determination in plasma samples.[18] In conclusion, this meta-analysis indicates that plasma D-dimer can be used as a predictive marker in ovarian cancer and high D-dimer is related to subsequent VTE risk. Yet, there is insufficient evidence to support a significant association between elevated D-dimer and worse prognosis of ovarian cancer patients. Further researches are also required to confirm the value of D-dimer in the early diagnosis of ovarian cancer. Furthermore, multivariate models are needed to explore the independent effect of D-dimer on prognostic analysis.
Footnotes
Abbreviations: CA-125 = carbohydrate antigen 125, DVT = deep vein thrombosis, HR = hazard ratio, OR = odds ratio, OS = overall survival, PE = pulmonary embolism, PFS = progression-free survival, PRISMA = Preferred Reporting Items for Systematic reviews and Meta-Analyses, VTE = venous thromboembolism.
Author contributions: JW: protocol development, literature search and data extraction, data analysis, and manuscript writing; ZF: literature search and data extraction, data analysis, and manuscript writing; GL: data analysis. PX: literature search and data extraction; JX and XJ: protocol development and manuscript writing.
JW and ZF contributed equally to this work.
Funding: This study was funded by the National Natural Science Foundation of China (No. 81572556 & No. 81602285).
The authors have no conflict of interest to disclose.
References
- [1].Zhang WW, Liu KJ, Hu GL, et al. Preoperative platelet/lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumour Biol 2015;36:8831–7. [DOI] [PubMed] [Google Scholar]
- [2].Watrowski R, Zeillinger R. Simple laboratory score improves the preoperative diagnosis of adnexal mass. Tumour Biol 2016;37:4343–9. [DOI] [PubMed] [Google Scholar]
- [3].Narod S. Can advanced-stage ovarian cancer be cured? Nat Rev Clin Oncol 2016;13:255–61. [DOI] [PubMed] [Google Scholar]
- [4].Wei SU, Li H, Zhang B. The diagnostic value of serum HE4 and CA-125 and ROMA index in ovarian cancer. Biomed Rep 2016;5:41–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ogata Y, Heppelmann CJ, Charlesworth MC, et al. Elevated levels of phosphorylated fibrinogen-alpha-isoforms and differential expression of other post-translationally modified proteins in the plasma of ovarian cancer patients. J Proteome Res 2006;5:3318–25. [DOI] [PubMed] [Google Scholar]
- [6].Miralles C, Orea M, Espana P, et al. Cancer antigen 125 associated with multiple benign and malignant athologies. Ann Surg Oncol 2003;10:150–4. [DOI] [PubMed] [Google Scholar]
- [7].Shiau C-S, Chang M-Y, Chiang C-H, et al. Ovarian endometrioma associated with very high serum CA-125 levels. Chang Gung Med J 2003;26:695–9. [PubMed] [Google Scholar]
- [8].Tiwari RK, Saha K, Mukhopadhyay D, et al. Evaluation of preoperative serum levels of CA 125 and expression of p53 in ovarian neoplasms: a prospective clinicopathological study in a tertiary care hospital. J Obstetrics Gynaecol India 2016;66:107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gadducci A, Cosio S, Tana R, et al. Serum and tissue biomarkers as predictive and prognostic variables in epithelial ovarian cancer. Crit Rrev Oncol/Hematol 2009;69:12–27. [DOI] [PubMed] [Google Scholar]
- [10].Zhang YL, Chen JH, Lu W, et al. Efficacy of postoperative hormone replacement therapy on prognosis of patients with serous ovarian carcinoma. Chin Med J (Engl) 2016;129:1316–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Diaz-Gil D, Fintelmann FJ, Molaei S, et al. Prediction of 5-year survival in advanced-stage ovarian cancer patients based on computed tomography peritoneal carcinomatosis index. Abdom Radiol (NY) 2016;41:2196–202. [DOI] [PubMed] [Google Scholar]
- [12].Tas F, Kilic L, Bilgin E, et al. Clinical and prognostic significance of coagulation assays in advanced epithelial ovarian cancer. Int J Gynecol Cancer 2013;23:276–81. [DOI] [PubMed] [Google Scholar]
- [13].Bakhru A. Effect of ovarian tumor characteristics on venous thromboembolic risk. J Gynecologic Oncol 2013;24:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kolodziejczyk J, Ponczek MB. The role of fibrinogen, fibrin and fibrin(ogen) degradation products (FDPs) in tumor progression. Contemporary Oncol (Poznan, Poland) 2013;17:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang X, Liu ZQ, Zhang W, et al. A retrospective analysis of plasma D-dimer dynamic variation in terminal stage cancer patients: implications for disease progression. Int J Clin Exp Med 2014;7:2395–401. [PMC free article] [PubMed] [Google Scholar]
- [16].Blackwell K, Hurwitz H, Lieberman G, et al. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer 2004;101:77–82. [DOI] [PubMed] [Google Scholar]
- [17].Buccheri GR, Torchio P, Ferrigno D. Plasma levels of D-dimer in lung carcinoma-Clinical and prognostic significance. Cancer 2003;97:3044–52. [DOI] [PubMed] [Google Scholar]
- [18].Kogan AE, Mukharyamova KS, Bereznikova AV, et al. Monoclonal antibodies with equal specificity to D-dimer and high-molecular-weight fibrin degradation products. Blood Coagul Fibrinolysis 2015;27:542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ma X, Li Y, Zhang J, et al. Prognostic role of D-dimer in patients with lung cancer: a meta-analysis. Tumour Biol 2014;35:2103–9. [DOI] [PubMed] [Google Scholar]
- [20].Fregoni V, Regolo L, Da PG, et al. No correlation between plasma D-dimer levels and lymph node involvement in opreable breast cancer. Breast (Edinburgh, Scotland) 2012;21:220. [DOI] [PubMed] [Google Scholar]
- [21].Inal T, Anar C, Polat G, et al. The prognostic value of D-dimer in lung cancer. Clin Respir J 2015;9:305–13. [DOI] [PubMed] [Google Scholar]
- [22].Wu X, Xue X, Tang J, et al. Evaluation of risk factors for venous thromboembolism in Chinese women with epithelial ovarian cancer. Int J Gynecol Cancer 2013;23:65–72. [DOI] [PubMed] [Google Scholar]
- [23].Gadducci A, Baicchi U, Marrai R, et al. Preoperative evaluation of D-dimer and CA 125 levels in differentiating benign from malignant ovarian masses. Gynecol Oncol 1996;60:197–202. [DOI] [PubMed] [Google Scholar]
- [24].Vahid Dastjerdi M, Ahmari S, Alipour S, et al. The comparison of plasma D-dimer levels in benign and malignant tumors of cervix, ovary and uterus. Int J Hematol-Oncol Stem Cell Res 2015;9:107–11. [PMC free article] [PubMed] [Google Scholar]
- [25].Rank A, Liebhardt S, Zwirner J, et al. Circulating microparticles in patients with benign and malignant ovarian tumors. Anticancer Res 2012;32:2009–14. [PubMed] [Google Scholar]
- [26].Kanayama N. The role of D-dimer in diagnosis of ovarian cancer. Nihon Sanka Fujinka Gakkai zasshi 1991;43:485–8. [PubMed] [Google Scholar]
- [27].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Green S, Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, England: Wiley-Blackwell; 2008. [Google Scholar]
- [29].Higgins JP, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat Med 2008;27:6072–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Mthodol 2012;12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rella C, Coviello M, Defrenza N, et al. Plasma D-dimer measurement as a marker of gynecologic tumors—comparison with Ca-125. Tumori 1993;79:347–51. [DOI] [PubMed] [Google Scholar]
- [33].Koh SCL, Razvi K, Chan YH, et al. The association with age, human tissue kallikreins 6 and 10 and hemostatic markers for survival outcome from epithelial ovarian cancer. Arch Gynecol Obstet 2011;284:183–90. [DOI] [PubMed] [Google Scholar]
- [34].Koh SCL, Tham KF, Razvi K, et al. Hemostatic and fibrinolytic status in patients with ovarian cancer and benign ovarian cysts: could D-dimer and antithrombin III levels be included as prognostic markers for survival outcome? Clin Appl Thromb Hemost 2001;7:141–8. [DOI] [PubMed] [Google Scholar]
- [35].Kawaguchi R, Furukawa N, Kobayashi H. Cut-off value of D-dimer for prediction of deep venous thrombosis before treatment in ovarian cancer. J Gynecol Oncol 2012;23:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Satoh T, Oki A, Uno K, et al. High incidence of silent venous thromboembolism before treatment in ovarian cancer. Brit J Cancer 2007;97:1053–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sakurai M, Satoh T, Matsumoto K, et al. High pretreatment plasma D-dimer levels are associated with poor prognosis in patients with ovarian cancer independently of venous thromboembolism and tumor extension. Int J Gynecol Cancer 2015;25:593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Man YN, Wang YN, Hao J, et al. Pretreatment plasma D-dimer, fibrinogen, and platelet levels significantly impact prognosis in patients with epithelial ovarian cancer independently of venous thromboembolism. Int J Gynecol Cancer 2015;25:24–32. [DOI] [PubMed] [Google Scholar]
- [39].Maksimovic M, Maksimovic M, Gojnic M, et al. Surgical treatment of ovarian cancer and early detection of venous thromboembolism. Eur J Gynaecol Oncol 2011;32:415–8. [PubMed] [Google Scholar]
- [40].Claussen C, Rausch AV, Lezius S, et al. Microvesicle-associated tissue factor procoagulant activity for the preoperative diagnosis of ovarian cancer. Thromb Res 2016;141:39–48. [DOI] [PubMed] [Google Scholar]
- [41].Koh SCL, Khalil R, Lim FK, et al. The association between fibrinogen, von Willebrand Factor, antithrombin III, and D-dimer levels and survival outcome by 36 months from ovarian cancer. Clin Appl Thromb Hemost 2006;12:3–8. [DOI] [PubMed] [Google Scholar]
- [42].Gadducci A, Marrai R, Baicchi U, et al. Preoperative D-Dimer plasma assay is not a predictor of clinical outcome for patients with advanced ovarian cancer. Gynecol Oncol 1997;66:85–8. [DOI] [PubMed] [Google Scholar]
- [43].Liu P, Wang Y, Tong L, et al. Elevated preoperative plasma D-dimer level is a useful predictor of chemoresistance and poor disease outcome for serous ovarian cancer patients. Cancer Chemother Pharmacol 2015;76:1163–71. [DOI] [PubMed] [Google Scholar]
- [44].Oya M, Akiyama Y, Okuyama T, et al. High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jap J Clin Oncol 2001;31:388–94. [DOI] [PubMed] [Google Scholar]
- [45].Michiels JJ, Moosdorff W, Maasland H, et al. Duplex ultrasound, clinical score, thrombotic risk, and D-dimer testing for evidence based diagnosis and management of deep vein thrombosis and alternative diagnoses in the primary care setting and outpatient ward. Int Angiol 2014;33:1–9. [PubMed] [Google Scholar]
- [46].Suega K, Bakta IM. Correlation between clinical stage of solid tumor and D dimer as a marker of coagulation activation. Acta Medica Indonesiana 2011;43:162–7. [PubMed] [Google Scholar]
- [47].Batschauer AP, Figueiredo CP, Bueno EC, et al. D-dimer as a possible prognostic marker of operable hormone receptor-negative breast cancer. Ann Oncol 2010;21:1267–72. [DOI] [PubMed] [Google Scholar]
- [48].Yigit E, Gönüllü G, Yücel İ, et al. Relation between hemostatic parameters and prognostic/predictive factors in breast cancer. Eur J Int Med 2008;19:602–7. [DOI] [PubMed] [Google Scholar]
- [49].Kilic L, Yildiz I, Sen FK, et al. D-dimer and international normalized ratio (INR) are correlated with tumor markers and disease stage in colorectal cancer patients. Cancer Biomark 2015;15:405–11. [DOI] [PubMed] [Google Scholar]
- [50].Chen Y, Yu H, Wu C, et al. Prognostic value of plasma D-dimer levels in patients with small-cell lung cancer. Biomed Pharmacother 2016;81:210–7. [DOI] [PubMed] [Google Scholar]
- [51].Echrish H, Madden LA, Greenman J, et al. The hemostasis apparatus in pancreatic cancer and its importance beyond thrombosis. Cancers (Basel) 2011;3:267–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Diaz ES, Walts AE, Karlan BY, et al. Venous thromboembolism during primary treatment of ovarian clear cell carcinoma is associated with decreased survival. Gynecol Oncol 2013;131:541–5. [DOI] [PubMed] [Google Scholar]
- [53].Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Int Med 2006;166:458–64. [DOI] [PubMed] [Google Scholar]
- [54].Bleau N, Patenaude V, Abenhaim HA. Risk of venous thrombo-embolic events in pregnant patients with cancer. J Matern Fetal Neonatal Med 2016;29:380–4. [DOI] [PubMed] [Google Scholar]
- [55].Duska LR, Garrett L, Henretta M, et al. When ’never-events’ occur despite adherence to clinical guidelines: the case of venous thromboembolism in clear cell cancer of the ovary compared with other epithelial histologic subtypes. Gynecol Oncol 2010;116:374–7. [DOI] [PubMed] [Google Scholar]
- [56].Timp JF, Braekkan SK, Versteeg HH, et al. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712–23. [DOI] [PubMed] [Google Scholar]
- [57].Abu Saadeh F, Norris L, O’Toole S, et al. Tumour expresion of tissue factor and tissue factor pathway inhibitor in ovarian cancer- relationship with venous thrombosis risk. Thromb Res 2013;132:627–34. [DOI] [PubMed] [Google Scholar]
- [58].Perisanidis C, Psyrri A, Cohen EE, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev 2015;41:960–70. [DOI] [PubMed] [Google Scholar]


