Abstract
Background:
Ankylosing spondylitis (AS) is a chronic immune-mediated disease affecting the sacroiliac joints and the spine, manifesting with new bone formation and osteopenia. Five tumor necrosis factor-alpha (TNF-α) inhibitors (infliximab, etanercept, adalimumab, certolizumab, and golimumab) are available for the treatment of AS, however, the results for the safety of TNF-α inhibitors in the treatment of AS are not consistent.
Methods:
In this study, we conducted a meta-analysis to determine the safety of TNF-α inhibitors compared with placebo in reducing pain, swelling, and inflammation of AS patients. Eight relevant articles including 2049 patients were included for this meta-analysis study. We observed that the incidence of adverse events (RR = 1.22, 95% CI: 1.12–1.33; P = .501, I2 = 0%) and injection-site reaction (RR = 2.93, 95% CI: 2.02–4.23; P = .691, I2 = 0%) in AS patients’ treatment with TNF-α inhibitors was significantly higher than that with placebo.
Results:
However, there was no significant difference in the incidence of serious adverse event, infection, serious infection, and discontinuations due to adverse event. TNF-α inhibitors may be a promising treatment for AS, but carries an increased incidence rate of adverse events and injection-site reaction.
Conclusion:
Due to the existence of the unstable factors, further studies need to be done to verify the result of this study.
Keywords: ankylosing spondylitis, meta-analysis, randomized controlled trials, tumor necrosis factor-alpha inhibitors
1. Introduction
As a chronic inflammatory disease, ankylosing spondylitis (AS) affects the axial skeleton and also the peripheral joints and nonarticular structures to a varying degree. AS is a prototype of an interrelated group of disorders called spondyloarthropathies (SpAs). AS is more common in men than women, with a ratio of approximately 2–3:1. The common features of AS are: restrictions in spine movements, chronic inflammatory back pain, spondylitis, and sacroiliitis; early symptoms of AS are recognized in teenagers or in young adults. The prevalence of AS is 0.52% to 0.55% in the USA and 0.3% in China.[1–3]
AS is progressive inflammatory disease, leading to a large number of people with functional limit and impact on the daily activities of patients.[4] The goals of treatment of AS are to alleviate symptoms (stiffness, pain, and joint swelling), improve body function, and delay or avoid structural damage, resulting in physical damage and deformity. AS is currently managed through a multidisciplinary approach that involves exercise, physiotherapy, and drug therapy.[5,6] Nonsteroidal anti-inflammatory drugs (NSAIDs) are the mainstay of AS therapy, reducing the stiffness and pain of inflammation. However, at least one-third of the patients were less responsive to NSAID treatment or severe side effects, and therefore need disease control drugs, in addition to improving symptoms treatment.[7,8]
The drug's safety and effectiveness must meet the requirements of US Food and Drug Administration (FDA) that has determined that a drug produces the benefits it is supposed to without causing side effects that would outweigh the benefits.[9] When analyzing the safety of a drug, it is essential to determine how to inform adverse events (AEs) and so the safety profile known. The approval of a drug as a treatment by the drugs regulatory agencies, such as the FDA and European Medicines Agency (EMA), is usually based on the results of clinical trials.[10] An alternative approach to analyzing the safety profile is meta-analyses, which combine the results of clinical trials in order to analyze a large number of patients exposed to the biological agent.
Tumor necrosis factor-alpha (TNF-α) is a multifunctional cytokine in the course of disease as previous studies found abundant levels of TNF-α in the sacroiliac joint of AS patients.[11,12] TNF-α inhibitors, adalimumab, etanercept, certolizumab, golimumab, and infliximab have proved to be effective treatment options for patients with AS.[13–15] According to the meta-analysis, adalimumab, etanercept, and infliximab showed similar effects on reducing signs and symptoms of AS.[16] However, the results for the safety of TNF-α inhibitors in the treatment of AS were not consistent. Therefore, the safety of TNF-α inhibitors for the treatment of AS should be systematically evaluated. Here in this study, we performed a meta-analysis of eligible studies to assess the safety of TNF-α inhibitors (adalimumab, infliximab, etanercept, certolizumab, and golimumab) in patients with AS.
2. Materials and methods
As this study is a meta-analysis of data in the literatures, the ethical approval was waived.
2.1. Search strategy
To perform this meta-analysis, we conducted a structured search in PubMed (ncbi.nlm.nih.gov/pubmed) and EMBASE (http://www.embase.com) databases up to November 2015 using the following search terms: “adalimumab” or “infliximab” or “etanercep” or “certolizumab” or “golimumab” or “TNF-α inhibitors”, and “ankylosing spondylitis”. References from the articles that met the eligibility criteria were also examined and evaluated and were selected for this meta-analysis if they also met the criteria.
2.2. Selection criteria
The inclusion criteria included: 1) eligibility is limited to randomized controlled trials (RCT) in patients with AS; 2) study compared the safety of TNF-α inhibitors in treatment of AS. We excluded clinical cases, literature reviews, commentaries, letters to the editor, and experimental studies.
2.3. Data extraction
All the available data were extracted from each study by 2 investigators independently according to the inclusion criteria listed above. The safety outcomes included: AEs; serious adverse events (SAEs); injection site reactions; discontinuations due to AEs; infections; and serious infections.
2.4. Statistical analysis
All results were summarized using STATA Software (version 12, StataCorp, College Station, TX). We calculated the mean difference (MD) and 95% confidence intervals (CI) for the continuous data, and calculate the risk ratio (RR) and 95% CI for dichotomous data. Statistical heterogeneity between the studies was assessed using χ2 test and I2, which assumes the presence of heterogeneity at P <. 10 and/or I2 > 50%. Preliminary analysis was done using a fixed effect model (Mantel-Haenszel method), if there was study heterogeneity (P < .1), it was done using a random effects model. Relative influence of each study on the pooled estimate was assessed by omitting 1 study at a time for sensitivity analysis. Begger's funnel plot and Egger's test were performed to assess the publication bias of the eligible studies (P < .05 was considered statistically significant).
3. Results
3.1. Characteristics of the studies
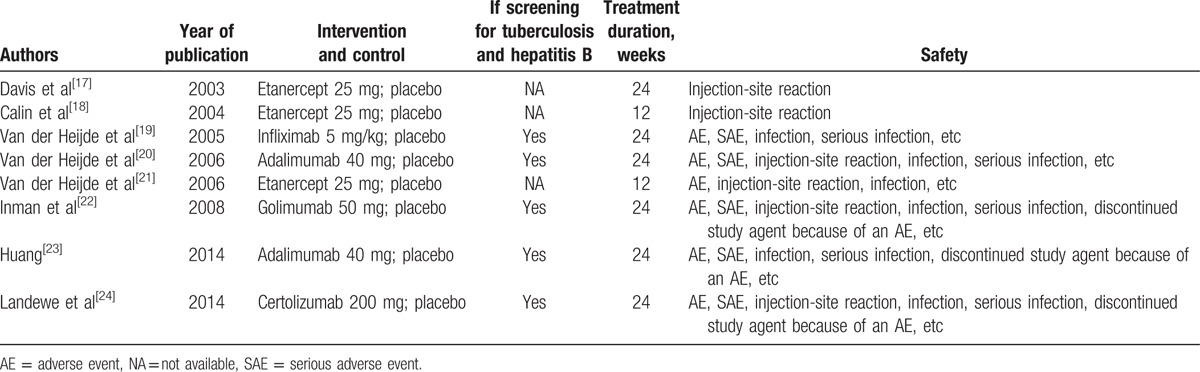
The present search strategy identified 227 articles, 191 of which were excluded after the title and abstract were reviewed. For the remaining 36 articles, 28 articles were excluded due to letters, reviews, meta-analysis (n = 9), not human studies (n = 5), not focusing on TNF-α inhibitors (n = 8), without control (n = 3), and not present the usable data (n = 3). Finally, 8 articles were included in the present meta-analysis. Of these, 2 trials studied adalimumab, 1 trial studied certolizumab, 3 trials studied etanercept, 1 trial studied golimumab, and 1 trial studied infliximab. In all included studies, the screening for tuberculosis (TB) and hepatitis B was performed in 5 studies.[19,20,22–24] A flow diagram of the selection process for the inclusion of studies in the present meta-analysis is shown in Fig. 1. The characteristics of the 8 trials are presented in Table 1.
Figure 1.
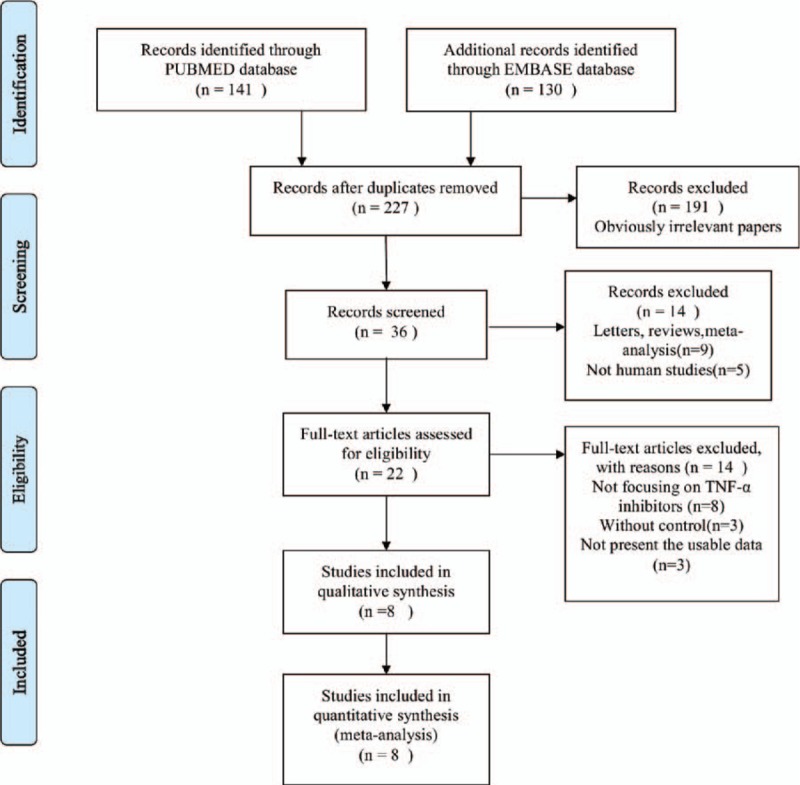
Flow diagram of studies identification.
Table 1.
Characteristics of randomized controlled trials included in this meta-analysis.
3.2. Quantitative synthesis
The 8 studies were included in the meta-analysis about the safety of TNF-α inhibitors in the treatment of AS.
3.2.1. Adverse events
This outcome was reported in 6 trials, all comparing TNF-α inhibitors to placebo. There was no heterogeneity between the studies (P = .501, I2 = 0%); the fixed effect model was used. There was a significant increase in the incidence of AEs in patients who received TNF-α inhibitors compared with those who received placebo (RR = 1.22, 95% CI: 1.12–1.33), as shown in Fig. 2A.
Figure 2.
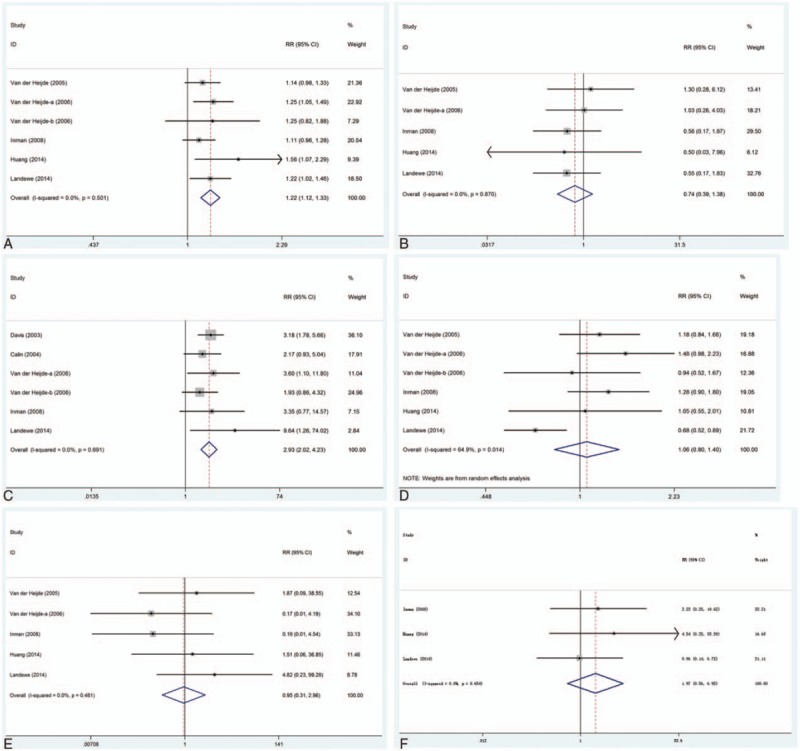
Safety outcomes of TNF-α inhibitors in the treatment of AS. (A) AEs; (B) SAEs; (C) injection-site reaction; (D) infection; (E) serious infection; (F) discontinuations due to AEs. AEs = adverse events, AS = ankylosing spondylitis, SAEs = serious adverse events, TNF-α = tumor necrosis factor-alpha.
3.2.2. Serious adverse events
This outcome was reported in 5 trials, all comparing TNF-α inhibitors to placebo. There was no heterogeneity between the studies (P = .870, I2 = 0%); the fixed effect model was used. There was no significant difference in the incidence of SAEs (RR = 0.74, 95% CI: 0.39–1.38), as shown in Fig. 2B.
3.2.3. Injection-site reaction
This outcome was reported in 6 trials, all comparing TNF-α inhibitors to placebo. There was no heterogeneity between the studies (P = .691, I2 = 0%); the fixed effect model was used. There was a significant increase in the incidence of injection-site reaction in patients who received TNF-α inhibitors compared with those who received placebo (RR = 2.93, 95% CI: 2.02–4.23), as shown in Fig. 2C.
3.2.4. Infection
This outcome was reported in 6 trials, all comparing TNF-α inhibitors to placebo. There was significant heterogeneity between the studies (P = .014, I2 = 64.9%); the random effect model was used. There was no significant difference in the incidence of infection (RR = 1.06, 95% CI: 0.80–1.40), as shown in Fig. 2D.
3.2.5. Serious infection
This outcome was reported in 5 trials, all comparing TNF-α inhibitors to placebo. There was no heterogeneity between the studies (P = .481, I2 = 0%); the fixed-effect model was used. There was no significant difference in the incidence of serious infection (RR = 0.95, 95% CI: 0.31–2.96), as shown in Fig. 2E.
3.2.6. Discontinuations due to AEs
This outcome was reported in 3 trials, all comparing TNF-α inhibitors to placebo. There was no heterogeneity between the studies (P = .654, I2 = 0%); the fixed-effect model was used. There was no significant difference in the incidence of discontinuations due to AEs (RR = 1.97, 95% CI: 0.56–6.93), as shown in Fig. 2F.
3.3. Sensitive analysis
Sensitivity analyses were performed to assess the influence of individual dataset on the pooled RRs by sequentially removing each eligible study. As seen in Fig. 3, any single study was omitted, while the overall statistical significance does not change, indicating that our results are statistically robust.
Figure 3.
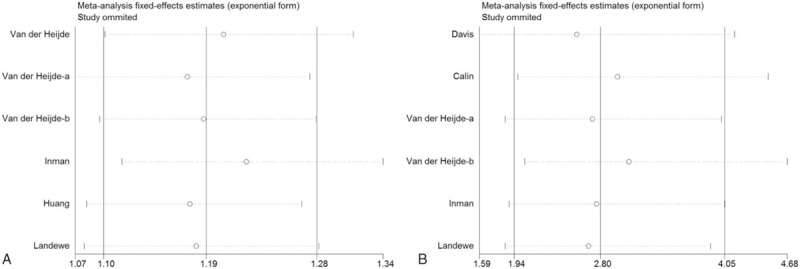
Sensitivity analysis: examining the influence of individual studies to pooled RR. (A) Incidence of AEs; (B) incidence of injection-site reaction. AEs = adverse events, RR = relative risk.
3.4. Publication bias
Begg funnel plot and Egger test were performed to assess publication bias among the literatures. As shown in Fig. 4, there was no evidence of publication bias for incidence of AEs (Begg test P = .260; Egger test P = .092) and incidence of injection-site reaction (Begg test P = .452; Egger test P = .330).
Figure 4.
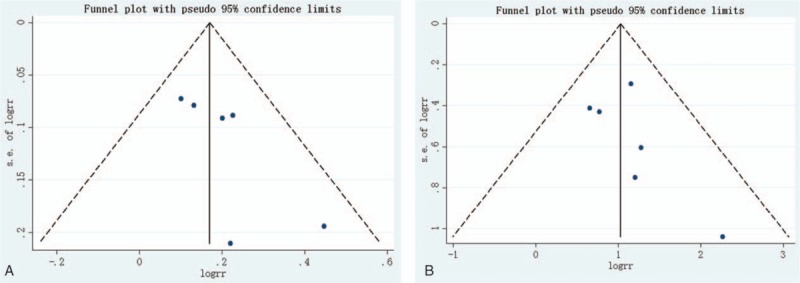
Begg funnel plot for publication bias test. Each point represents a separate study for the indicated association. (A) Incidence of AEs; (B) incidence of injection-site reaction. AEs = adverse events.
4. Discussion
AS is a progressive inflammatory disease of uncertain etiology that primarily affects the spine column, which is characterized by excessive bone formation in the form of syndesmophytes and ankylosis.[25] Evidences indicated that TNF-α seems to be a crucial effector cytokine involved in the key downstream effector pathways.[26,27] Activation of Wnt/beta-catenin signaling upregulates the TNF-α expression, and thus TNF-α may, through the Wnt signaling pathway, regulate new bone formation.[28] A previous study found that compared to AS patients without syndesmophytes, patients with syndesmophyte formation show lower serum levels of Dickkopf-1 (DKK1).[29] In addition, the association between Del1 polymorphisms and AS susceptibility in Chinese Han population was reported.[30] DKK1 and Del1 both were potent inhibitors of the Wnt signaling pathway. These evidences supported a potential involvement of this pathway in the etiology of AS.
TNF-α promotes inflammation and subsequent pain, tenderness, swelling, and fever in several inflammatory conditions, including AS. Five TNF-α inhibitors including adalimumab (ADA, Humira, Abbvie Inc., Chicago, IL), etanercept (ETN, Enbrel, Immunex, Thousand Oaks, CA), golimumab (GOL, Simponi, Janssen Biotech Inc., Malvern, PA), certolizumab (CZP, Cimzia, UCB Pharma, Brussels, Belgium), and infliximab (IFX, Remicade, Janssen Biotech Inc., Malvern, PA) have been developed to target TNF-α and alleviate joint swelling, pain, and inflammation in AS patients.[31] Through different mechanisms 5 drugs affect the function of TNF-α. Adalimumab is a recombinant human immunoglobulin (Ig)G1 monoclonal antibody (mAb) specific for human TNF-α,[32] while golimumab is a human mAb binding to both soluble and transmembrane bioactive forms of human TNF.[33] Etanercept is a human TNF receptor fusion protein that binds specifically to TNF-α receptors.[34] Certolizumab is a PEGylated Fab’ fragment of a humanized monoclonal antibody that binds and neutralizes human TNF-α.[35] Infliximab is a chimeric (mouse/human) IgG1κ monoclonal antibody that binds specifically to TNF-α with a high affinity.[36] All 5 agents are able to prevent TNF-α from promoting inflammatory response, leading to its use as an effective treatment for AS patients.
Major drawbacks are infectious complications with the use of anti-TNF-α antibodies due to the “shutdown” of the immune system. Additionally, Davis et al[17] reported that an injection-site reaction, upper respiratory tract infection, and accidental injury were the only undesirable occurrence that appeared more frequently (P <.05) in a group treated with etanercept. Similarly, Calin et al[18] noted a SAE was acute myocardial infarction when underwent angioplasty in an etanercept-treated patient. Moreover, in a study by Van der Heijde et al,[19] 7 patients (3.5%) treated with infliximab had SAEs, such as cholecystitis, dizziness, pneumonia, arthritis, and leukocytosis. Inman et al[22] revealed that, in a combined golimumab group, headache, injection-site erythema, nasopharyngitis, fatigue, upper respiratory tract infections, diarrhea, and increases in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels were more prevalent compared to the placebo group. Huang[23] reported that 4 SAEs were concussion, contusion, and skin laceration; elective abortion; peritoneal TB, pulmonary TB; and TB pleurisy and viral hepatitis. Landewé et al[24] noted that the most common infectious AEs were nasopharyngitis (8.8% CZP vs 6.5% placebo) and upper respiratory tract infection (4.0% CZP vs 2.8% placebo). In this study, we conducted a meta-analysis to determine the safety of TNF-α inhibitors (adalimumab, infliximab, etanercept, certolizumab, and golimumab) compared with placebo in reducing pain, swelling, and inflammation of AS patients. Eight relevant articles including 2049 patients were included for this meta-analysis study. We observed that the incidence of AEs (RR = 1.22, 95% CI: 1.12–1.33; P = .501, I2 = 0%) and injection-site reaction (RR = 2.93, 95% CI: 2.02–4.23; P = .691, I2 = 0%) in AS patients treatment with TNF-α inhibitors was significantly higher than that with placebo. However, there was no significant difference in the incidence of SAE, infection, serious infection, and discontinuations due to AE.
The new infection susceptibility or the reactivation of concurrent or incident infections is increased with TNF-α inhibitors. Thus, before treatment, screening for TB and certain viral infections (such as hepatitis B virus, herpes virus, and cytomegalovirus) is recommended.[37] In this meta-analysis, the screening for TB and hepatitis B was performed in 5 studies.[19,20,22–24] Moreover, patients with AS using anti-TNF treatments experience TB reactivation and hepatitis B virus infection reactivation. However, compared to other anti-TNF drugs, etanercept is not as likely to the reactivate TB. In fact, it is proposed that etanercept is less immunogenic, particularly for AS patients.[38]
The present meta-analysis has several limitations. First of all, among the RCTs, only 8 had a bias of risk that was considered low, and the number of subjects included in this meta-analysis was restricted. Some safety data are not available in the articles and thus were not used in our meta-analysis. Secondly, some of the studies included had sample sizes that were small, which could contribute to a lower statistical power. Thirdly, the findings described here were based on an unadjusted assessment of the RRs, and this might have some influence on the results. Thus, these results should be interpreted with caution, given the limitations described above.
In conclusion, compared to placebo, TNF-α inhibitors treatment significantly increased the incidence of AEs and injection-site reaction in AS patients; however, there was no difference in the incidence of SAE, infection, serious infection, and discontinuations due to AE. TNF-α inhibitors may be a promising treatment for AS, but carries an increased incidence rate of AEs and injection-site reaction. However, due to the existence of the unstable factors, further studies need to be done to verify the result of this study.
Footnotes
Abbreviations: AEs = adverse events, AS = ankylosing spondylitis, MD = mean difference, NSAIDs = nonsteroidal anti-inflammatory drugs, RCT = randomized controlled trials, RR = risk ratio, SAEs = serious adverse events, SpAs = spondyloarthropathies, TNF-α = tumor necrosis factor-alpha.
The authors have no conflicts of interest to disclose.
References
- [1].Zhen W, Lin Z, Wei Q, et al. Clinical features of ankylosing spondylitis may correlate with HLA-B27 polymorphism. Rheumatol Int 2009;29:389–92. [DOI] [PubMed] [Google Scholar]
- [2].Reveille JD, Weisman MH. The epidemiology of back pain, axial spondyloarthritis and HLA-B27 in the United States. Am J Med Sci 2013;345:431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Braun J, Sieper J. Ankylosing spondylitis. Lancet 2007;369:1379–90. [DOI] [PubMed] [Google Scholar]
- [4].El Tecle NE, Abode-Iyamah KO, Hitchon PW, et al. Management of spinal fractures in patients with ankylosing spondylitis. Clin Neurol Neurosur 2015;139:177–82. [DOI] [PubMed] [Google Scholar]
- [5].Wendling D. An overview of investigational new drugs for treating ankylosing spondylitis. Expert Opin Inv Drug 2015;25:95–104. [DOI] [PubMed] [Google Scholar]
- [6].Sari İ, Öztürk MA, Akkoç N. Treatment of ankylosing spondylitis. Turk J Med Sci 2015;45:416–30. [DOI] [PubMed] [Google Scholar]
- [7].Wanders A, Heijde DVD, Landewé R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheumatol 2005;52:1756–65. [DOI] [PubMed] [Google Scholar]
- [8].Chen J, Liu C. Sulfasalazine for ankylosing spondylitis—art. no. CD004800.pub2. Cochrane Db Syst Rev 2005;11:CD004800–14800. [DOI] [PubMed] [Google Scholar]
- [9].Görög S. Drug safety, drug quality, drug analysis. J Pharm Biomed Anal 2008;48:247–53. [DOI] [PubMed] [Google Scholar]
- [10].Hernández MV, Sanmartí R, Cañete JD. The safety of tumor necrosis factor-alpha inhibitors in the treatment of rheumatoid arthritis. Exp Opin Drug Safety 2016;15:613–24. [DOI] [PubMed] [Google Scholar]
- [11].JÜRgen Braun MD, Matthias Bollow MD, Neure L, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheumatol 1995;38:499–505. [DOI] [PubMed] [Google Scholar]
- [12].François RJ, Neure L, Sieper J, et al. Immunohistological examination of open sacroiliac biopsies of patients with ankylosing spondylitis: detection of tumour necrosis factor alpha in two patients with early disease and transforming growth factor beta in three more advanced cases. Ann Rheum Dis 2006;65:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Braun J, Brandt J, Listing J, et al. Treatment of active ankylosing spondylitis with infliximab: a randomised controlled multicentre trial. Lancet 2002;359:1187–93. [DOI] [PubMed] [Google Scholar]
- [14].Higuchi M, Aggarwal BB. Treatment of ankylosing spondylitis via tumor necrosis factor-alpha inhibition. New Engl J Med 2002;346:1349–56. [DOI] [PubMed] [Google Scholar]
- [15].Lambert RGW, Salonen D, Rahman P, et al. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheumatol 2007;56:4005–14. [DOI] [PubMed] [Google Scholar]
- [16].Machado MADÁ, Barbosa MM, Almeida AM, et al. Treatment of ankylosing spondylitis with TNF blockers: a meta-analysis. Rheumatol Int 2013;33:2199–213. [DOI] [PubMed] [Google Scholar]
- [17].Davis JC, Heijde DVD, Braun J, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: A randomized, controlled trial. Arthritis Rheumatol 2003;48:3230–6. [DOI] [PubMed] [Google Scholar]
- [18].Calin A, Dijkmans BAC, Emery P, et al. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis 2004;63:1594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Désirée van der Heijde, Ben Dijkmans, Piet Geusens, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheumatol 2005;52:582–91. [DOI] [PubMed] [Google Scholar]
- [20].van der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 2006;54:2136–46. [DOI] [PubMed] [Google Scholar]
- [21].Heijde DVD, Silva JCD, Dougados M, et al. Etanercept 50 mg once weekly is as effective as 25 mg twice weekly in patients with ankylosing spondylitis. Ann Rheum Dis 2006;65:1572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Inman RD, Davis JC, Jr, Heijde Dv, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheumatol 2008;58:3402–12. [DOI] [PubMed] [Google Scholar]
- [23].Huang F. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: results of a randomised, controlled trial. Ann Rheum Dis 2014;73:587–94. [DOI] [PubMed] [Google Scholar]
- [24].Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis 2014;73:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sieper J, Braun J, Rudwaleit M, et al. Ankylosing spondylitis: an overview. Ann Rheum Dis 2002;61suppl 3:iii8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Braun J, Bollow M, Neure L, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum 1995;38:499–505. [DOI] [PubMed] [Google Scholar]
- [27].Keffer J, Probert L, Cazlaris H, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. Embo j 1991;10:4025–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hiyama A, Yokoyama K, Nukaga T, et al. A complex interaction between Wnt signaling and TNF-alpha in nucleus pulposus cells. Arthritis Res Ther 2013;15:R189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Heiland GR, Appel H, Poddubnyy D, et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann Rheum Dis 2012;71:572–4. [DOI] [PubMed] [Google Scholar]
- [30].Lin Z, Bei JX, Shen M, et al. A genome-wide association study in Han Chinese identifies new susceptibility loci for ankylosing spondylitis. Nat Genet 2012;44:73–7. [DOI] [PubMed] [Google Scholar]
- [31].Osman MS, Maksymowych WP. An update on the use of tumor necrosis factor alpha inhibitors in the treatment of ankylosing spondylitis. Expert Rev Clin Immunol 2016;10:1–7. [DOI] [PubMed] [Google Scholar]
- [32].Weinblatt ME, Keystone EC, Furst DE, et al. Adalimumab, a fully human anti–tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: The ARMADA trial. Arthritis Rheum 2003;48:35–45. [DOI] [PubMed] [Google Scholar]
- [33].Keystone EC, Genovese ML, Golimumab, et al. A human antibody to tumour necrosis factor α given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: The GO-FORWARD Study. Ann Rheum Dis 2009;68:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. New Engl J Med 1999;340:253–9. [DOI] [PubMed] [Google Scholar]
- [35].Nesbitt A, Fossati G, Bergin M, et al. Mechanism of action of certolizumab pegol (CDP870): In vitro comparison with other anti-tumor necrosis factor α agents. Inflamm Bowel Dis 2007;13:1323–32. [DOI] [PubMed] [Google Scholar]
- [36].Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999;354:1932–9. [DOI] [PubMed] [Google Scholar]
- [37].Nathan DM, Angus PW, Gibson PR. Hepatitis B and C virus infections and anti-tumor necrosis factor-alpha therapy: guidelines for clinical approach. J Gastroenterol Hepatol 2006;21:1366–71. [DOI] [PubMed] [Google Scholar]
- [38].Senabre-Gallego JM, Santos-Ramírez C, Santos-Soler G, et al. Long-term safety and efficacy of etanercept in the treatment of ankylosing spondylitis. Patient Preference Adherence 2013;7:961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


