Abstract
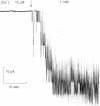
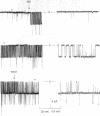
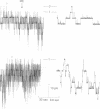
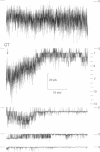
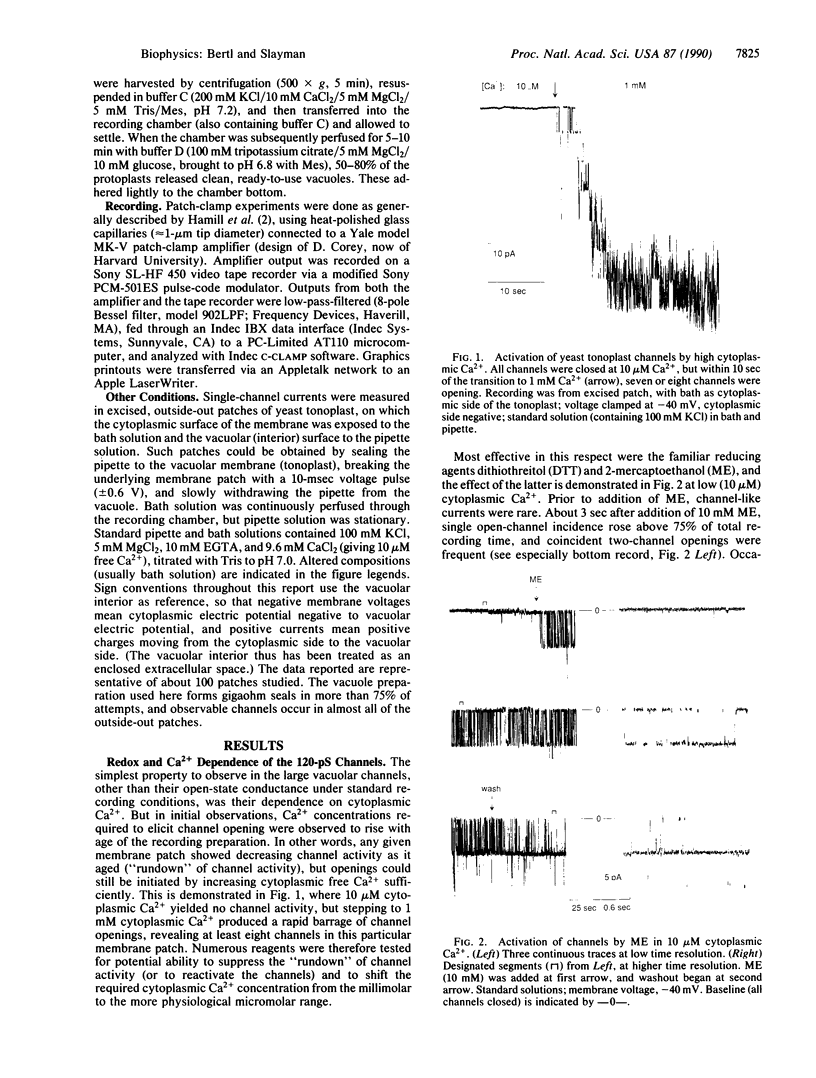
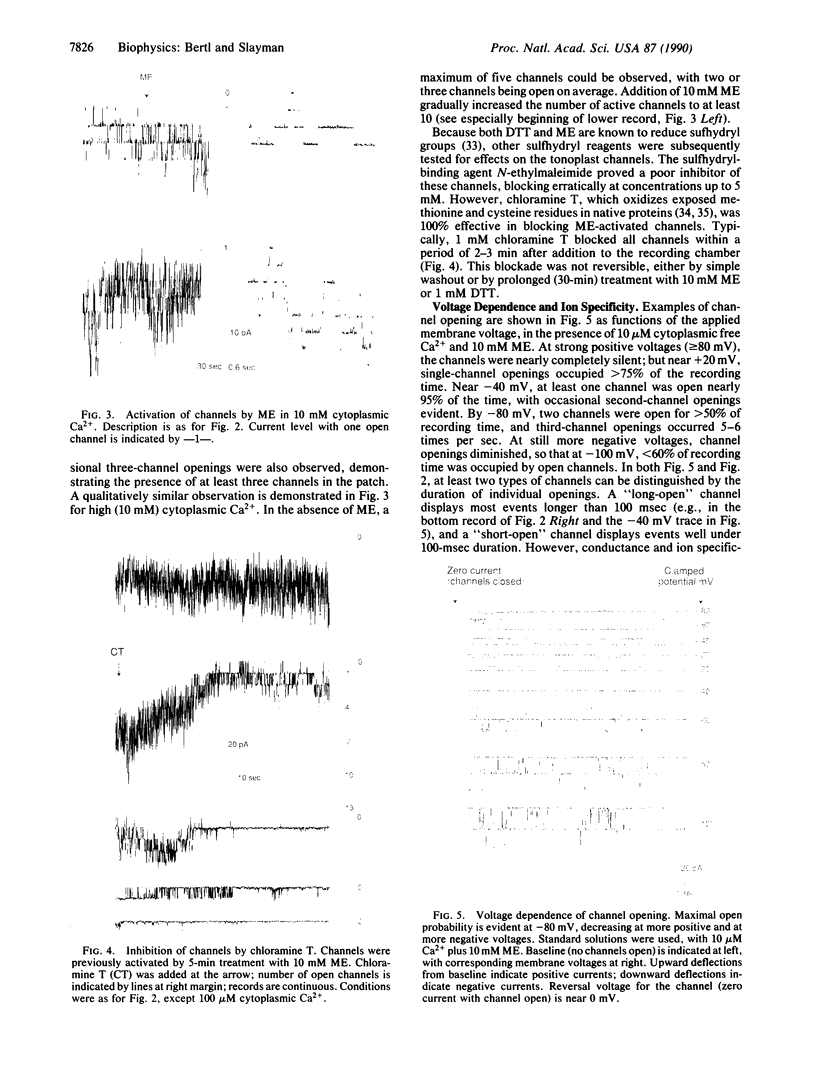
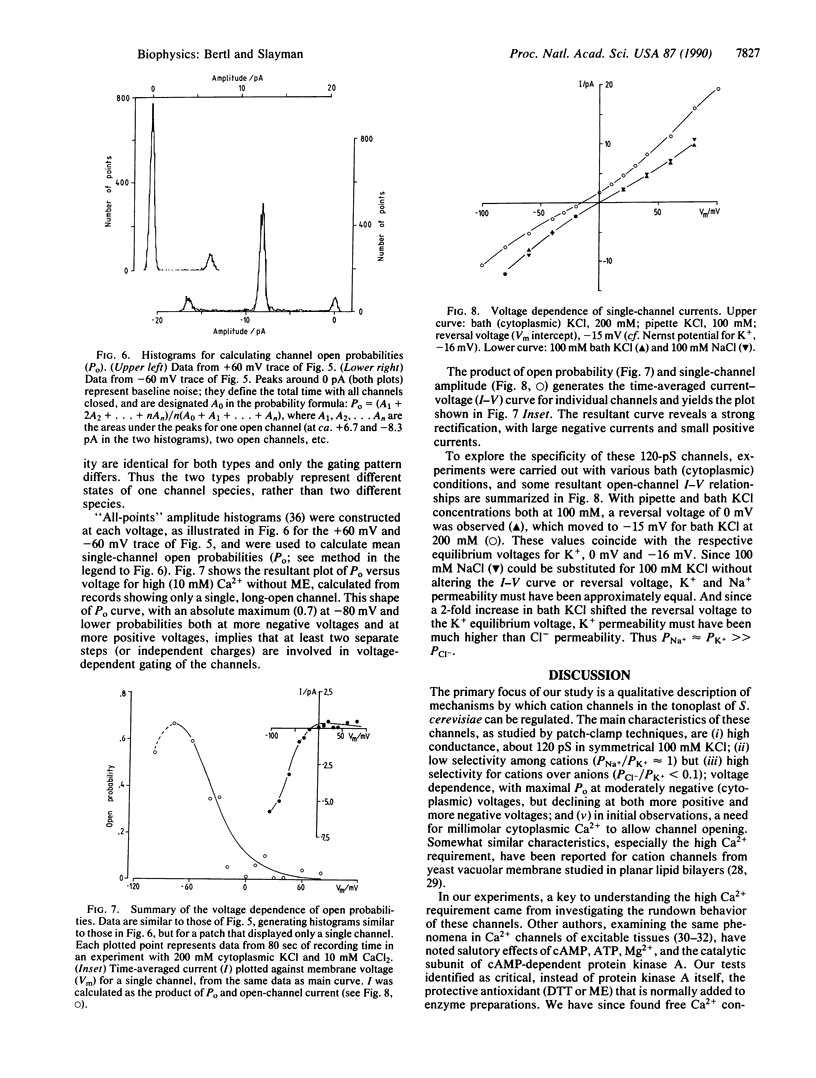
The vacuolar membrane of the yeast Saccharomyces cerevisiae, which is proposed as a system for functional expression of membrane proteins, was examined by patch-clamp techniques. Its most conspicuous feature, in the absence of energizing substrates, is a cation channel with a characteristic conductance of approximately 120 pS for symmetric 100 mM KCl solutions and with little selectivity between K+ and Na+ (PNa+/PK+ approximately 1) but strong selectivity for cations over anions (PCl-/PK+ less than 0.1). Channel gating is voltage-dependent; open probability, Po, reaches maximum (approximately 0.7) at a transmembrane voltage of -80 mV (cytoplasmic surface negative) and declines at both more negative and more positive voltages (i.e., to 0 around +80 mV). The time-averaged current-voltage curve shows strong rectification, with negative currents (positive charges flowing from vacuolar side to cytoplasmic side) much larger than positive currents. The open probability also depends strongly on cytoplasmic Ca2+ concentration but, for ordinary recording conditions, is high only at unphysiologically high (greater than or equal to 1 mM) Ca2+. However, reducing agents such as dithiothreitol and 2-mercaptoethanol poise the channels so that they can be activated by micromolar cytoplasmic Ca2+. The channels are blocked irreversibly by chloramine T, which is known to oxidize exposed methionine and cysteine residues specifically.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballarin-Denti A., Den Hollander J. A., Sanders D., Slayman C. W., Slayman C. L. Kinetics and pH-dependence of glycine-proton symport in Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Nov 21;778(1):1–16. doi: 10.1016/0005-2736(84)90442-5. [DOI] [PubMed] [Google Scholar]
- Belles B., Malécot C. O., Hescheler J., Trautwein W. "Run-down" of the Ca current during long whole-cell recordings in guinea pig heart cells: role of phosphorylation and intracellular calcium. Pflugers Arch. 1988 Apr;411(4):353–360. doi: 10.1007/BF00587713. [DOI] [PubMed] [Google Scholar]
- Blatt M. R., Rodriguez-Navarro A., Slayman C. L. Potassium-proton symport in Neurospora: kinetic control by pH and membrane potential. J Membr Biol. 1987;98(2):169–189. doi: 10.1007/BF01872129. [DOI] [PubMed] [Google Scholar]
- Byerly L., Yazejian B. Intracellular factors for the maintenance of calcium currents in perfused neurones from the snail, Lymnaea stagnalis. J Physiol. 1986 Jan;370:631–650. doi: 10.1113/jphysiol.1986.sp015955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compagno C., Coraggio I., Ranzi B. M., Alberghina L., Viotti A., Martegani E. Translational regulation of the expression of zein cloned in yeast under an inducible GAL promoter. Biochem Biophys Res Commun. 1987 Jul 31;146(2):809–814. doi: 10.1016/0006-291x(87)90602-4. [DOI] [PubMed] [Google Scholar]
- Felle H., Porter J. S., Slayman C. L., Kaback H. R. Quantitative measurements of membrane potential in Escherichia coli. Biochemistry. 1980 Jul 22;19(15):3585–3590. doi: 10.1021/bi00556a026. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Gradmann D. Electrical properties of the plasma membrane of microplasmodia of Physarum polycephalum. J Membr Biol. 1982;68(1):67–77. doi: 10.1007/BF01872255. [DOI] [PubMed] [Google Scholar]
- Gradmann D., Hansen U. P., Long W. S., Slayman C. L., Warncke J. Current-voltage relationships for the plasma membrane and its principal electrogenic pump in Neurospora crassa: I. Steady-state conditions. J Membr Biol. 1978 Mar 20;39(4):333–367. doi: 10.1007/BF01869898. [DOI] [PubMed] [Google Scholar]
- Groves P. M., Gamow R. I. Intracellular recordings from phycomyces. Plant Physiol. 1975 May;55(5):946–947. doi: 10.1104/pp.55.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halachmi D., Eilam Y. Cytosolic and vacuolar Ca2+ concentrations in yeast cells measured with the Ca2+-sensitive fluorescence dye indo-1. FEBS Lett. 1989 Oct 9;256(1-2):55–61. doi: 10.1016/0014-5793(89)81717-x. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Indge K. J. Polyphosphates of the yeast cell vacuole. J Gen Microbiol. 1968 May;51(3):447–455. doi: 10.1099/00221287-51-3-447. [DOI] [PubMed] [Google Scholar]
- Johnson L. M., Bankaitis V. A., Emr S. D. Distinct sequence determinants direct intracellular sorting and modification of a yeast vacuolar protease. Cell. 1987 Mar 13;48(5):875–885. doi: 10.1016/0092-8674(87)90084-5. [DOI] [PubMed] [Google Scholar]
- Kitamoto K., Yoshizawa K., Ohsumi Y., Anraku Y. Dynamic aspects of vacuolar and cytosolic amino acid pools of Saccharomyces cerevisiae. J Bacteriol. 1988 Jun;170(6):2683–2686. doi: 10.1128/jb.170.6.2683-2686.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn S. J., Horn R. Influence of sodium-calcium exchange on calcium current rundown and the duration of calcium-dependent chloride currents in pituitary cells, studied with whole cell and perforated patch recording. J Gen Physiol. 1989 Nov;94(5):789–812. doi: 10.1085/jgp.94.5.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. D., Schlenk F. Examination of isolated yeast cell vacuoles for active transport. J Bacteriol. 1974 Apr;118(1):314–316. doi: 10.1128/jb.118.1.314-316.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi S., Koyama Y., Sakamoto T., Soda M., Kairiyama C. B. Expression of rat alpha-fetoprotein cDNA in Escherichia coli and in yeast. J Biochem. 1988 Dec;104(6):968–972. doi: 10.1093/oxfordjournals.jbchem.a122592. [DOI] [PubMed] [Google Scholar]
- Ohsumi Y., Anraku Y. Active transport of basic amino acids driven by a proton motive force in vacuolar membrane vesicles of Saccharomyces cerevisiae. J Biol Chem. 1981 Mar 10;256(5):2079–2082. [PubMed] [Google Scholar]
- Ohsumi Y., Kitamoto K., Anraku Y. Changes induced in the permeability barrier of the yeast plasma membrane by cupric ion. J Bacteriol. 1988 Jun;170(6):2676–2682. doi: 10.1128/jb.170.6.2676-2682.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohya Y., Anraku Y. Functional expression of chicken calmodulin in yeast. Biochem Biophys Res Commun. 1989 Jan 31;158(2):541–547. doi: 10.1016/s0006-291x(89)80083-x. [DOI] [PubMed] [Google Scholar]
- Preston R. A., Murphy R. F., Jones E. W. Assay of vacuolar pH in yeast and identification of acidification-defective mutants. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7027–7031. doi: 10.1073/pnas.86.18.7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J. A., Vacata V., McCusker J. H., Haber J. E., Mortimer R. K., Owen W. G., Lecar H. ATP-sensitive K+ channels in a plasma membrane H+-ATPase mutant of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7866–7870. doi: 10.1073/pnas.86.20.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saimi Y., Martinac B., Gustin M. C., Culbertson M. R., Adler J., Kung C. Ion channels in Paramecium, yeast and Escherichia coli. Trends Biochem Sci. 1988 Aug;13(8):304–309. doi: 10.1016/0968-0004(88)90125-9. [DOI] [PubMed] [Google Scholar]
- Sato T., Ohsumi Y., Anraku Y. Substrate specificities of active transport systems for amino acids in vacuolar-membrane vesicles of Saccharomyces cerevisiae. Evidence of seven independent proton/amino acid antiport systems. J Biol Chem. 1984 Sep 25;259(18):11505–11508. [PubMed] [Google Scholar]
- Schroeder J. I., Hedrich R. Involvement of ion channels and active transport in osmoregulation and signaling of higher plant cells. Trends Biochem Sci. 1989 May;14(5):187–192. doi: 10.1016/0968-0004(89)90272-7. [DOI] [PubMed] [Google Scholar]
- Schroeder J. I., Raschke K., Neher E. Voltage dependence of K channels in guard-cell protoplasts. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Burstein Y., Patchornik A. Selective oxidation of methionine residues in proteins. Biochemistry. 1975 Oct 7;14(20):4497–4503. doi: 10.1021/bi00691a025. [DOI] [PubMed] [Google Scholar]
- Slayman C. L., Slayman C. W. Whole cells for the study of transport linked to membrane potential: Neurospora crassa. Methods Enzymol. 1979;55:656–666. doi: 10.1016/0076-6879(79)55073-3. [DOI] [PubMed] [Google Scholar]
- Sorgato M. C., Keller B. U., Stühmer W. Patch-clamping of the inner mitochondrial membrane reveals a voltage-dependent ion channel. Nature. 1987 Dec 3;330(6147):498–500. doi: 10.1038/330498a0. [DOI] [PubMed] [Google Scholar]
- Tanifuji M., Sato M., Wada Y., Anraku Y., Kasai M. Gating behaviors of a voltage-dependent and Ca2+-activated cation channel of yeast vacuolar membrane incorporated into planar lipid bilayer. J Membr Biol. 1988 Nov;106(1):47–55. doi: 10.1007/BF01871766. [DOI] [PubMed] [Google Scholar]
- Tedeschi H., Mannella C. A., Bowman C. L. Patch clamping the outer mitochondrial membrane. J Membr Biol. 1987;97(1):21–29. doi: 10.1007/BF01869611. [DOI] [PubMed] [Google Scholar]
- Trout G. E. The estimation of microgram amounts of methionine by reaction with chloramine-T. Anal Biochem. 1979 Mar;93(2):419–422. doi: 10.1016/s0003-2697(79)80173-6. [DOI] [PubMed] [Google Scholar]
- Valls L. A., Hunter C. P., Rothman J. H., Stevens T. H. Protein sorting in yeast: the localization determinant of yeast vacuolar carboxypeptidase Y resides in the propeptide. Cell. 1987 Mar 13;48(5):887–897. doi: 10.1016/0092-8674(87)90085-7. [DOI] [PubMed] [Google Scholar]
- Wada Y., Ohsumi Y., Tanifuji M., Kasai M., Anraku Y. Vacuolar ion channel of the yeast, Saccharomyces cerevisiae. J Biol Chem. 1987 Dec 25;262(36):17260–17263. [PubMed] [Google Scholar]
- Weik R., Neumcke B. ATP-sensitive potassium channels in adult mouse skeletal muscle: characterization of the ATP-binding site. J Membr Biol. 1989 Sep;110(3):217–226. doi: 10.1007/BF01869152. [DOI] [PubMed] [Google Scholar]
- Wiemken A., Dürr M. Characterization of amino acid pools in the vacuolar compartment of Saccharomyces cerevisiae. Arch Microbiol. 1974;101(1):45–57. doi: 10.1007/BF00455924. [DOI] [PubMed] [Google Scholar]