Abstract
Background:
The study aims to explore the correlations of the single nucleotide polymorphisms (SNPs) of CRYAA and CRYAB with the risk and clinicopathological features of children with congenital cataract.
Methods:
The study enrolled 168 children diagnosed as congenital cataract (case group) and 172 normal children (control group) from May 2015 to May 2016. Genomic DNA extraction was performed using a QIAamp DNA blood mini kit. Polymerase chain reaction (PCR) products were genotyped using an ABI direct sequencer. Haplotype, allele, and genotype frequencies of CRYAA and CRYAB gene polymorphisms analyses were carried out using the SHEsis software. Logistic regression analysis was performed in order to analyze the risk factors for children suffering from congenital cataract.
Results:
Presence of significant differences between the case and control groups’ genotype and allele frequencies of CRYAA rs7278468 and CRYAB rs370803064/rs387907338. TA of CRYAB gene might increase congenital cataract risk in children, while GCG of CRYAA gene and GC of CRYAB gene might decrease congenital cataract risk in children. CRYAA rs7278468, CRYAB rs370803064/rs387907338 polymorphisms were significantly correlated to uncorrected visual acuity, best-corrected visual acuity, nystagmus, visual axis opacification, microcornea, lens opacity, posterior capsular thickening, and degrees of posterior capsule opacification after operation in children with congenital cataract. Logistic regression analysis revealed that the T allele of CRYAA rs7278468, A allele of CRYAB rs370803064, T allele of CRYAB rs387907338, family history, and TA haplotype of CRYAB gene were risk factors for children with congenital cataract.
Conclusion:
Our findings demonstrated that CRYAA rs7278468 and CRYAB rs370803064/rs387907338 are correlated with the risk and clinicopathological features of children suffering from congenital cataract.
Keywords: clinicopathological features, congenital cataract, CRYAA, CRYAB, risk, single nucleotide polymorphism
1. Introduction
Congenital cataract is the most common treatable cause of pediatric visual disability, due to the metabolic disorders of embryonic lens transparency during early fetal period.[1] It is characterized by ocular lens opacification, which usually results from an injury to the lens micro-architecture leading to light scatter, or the formation of protein augment causing loss of transparency.[2] Congenital cataract prevalence is estimated to range from 0.6 to 6 per 10,000 live births, with an incidence rate of about 2.2 to 2.49 in every 10,000 live births, and an approximate 40% cases with congenital cataract are reported to be inherited in isolation or due to ocular syndrome or abnormalities.[3] Nearly a-third of congenital cataract cases are familial with an autosomal dominant or recessive inheritance.[4] Despite the remarkable improvements in the clinical cataract management and updated information of lens structure and function, the correlations among cataract morphology, etiology, and mechanisms remain unclear. Accumulating reports demonstrate that genetic functions are greatly involved in the whole process.[5,6] At present, most advances have been made to identify the role of genes in causing autosomal congenital cataract.[7,8]
Various gene mutations have been linked to congenital cataract, including structural protein genes, transcription factors, transport molecules, and cell adhesion molecules.[9] Families with heritable cataract have indicated gene mutations associations with lens crystallins, among which α-crystallins are key water soluble proteins which are expressed in the lens to contribute to lens clarity maintenance.[10] α-Crystallins are mainly comprised of 2 proteins, namely, αA- and αB-crystallins, at a molar ratio of 3:1,[11] which are encoded by individual genes localized on disparate chromosomes, crystallin αA (CRYAA), and crystallin αB (CRYAB) in the small heat-shock protein (sHSP) family.[12] They can form hetero-oligomers that bind and isolate injured proteins, inhibiting the formation of particulates that are able to scatter light.[13] The CRYAA gene is expressed densely in the lens, while CRYAB is ubiquitously expressed in a broad variety of tissues and is correlated with neurologic, cardiac, and muscular dysfunctions.[14] The CRYAA gene is mapped to chromosome 21q22.3, which consists of 3 exons.[15] Located on chromosome 11q23, CRYAB encodes for a member of the sHSP family composing of 175 amino acid protein,[4] and functions as a molecular chaperone, restraining the accumulation of denatured proteins after exposure to stresses, including radiation, heat shock, and oxidative stress.[13] Currently, over 40 loci have been mapped in congenital cataract development.[3] Our study targets to elucidate the effects of mutations on loci rs7278468, rs3761382, and rs13053109 of CRYAA and rs370803064 and rs387907338 of CRYAB on risks of pediatric congenital cataract to provide more genetic information on the cause of congenital cataract.
2. Materials and methods
2.1. Study subjects
The study case group included a total of 168 children (107 male and 61 female) with a calculated mean age of 5.2 ± 1.2 years, who were diagnosed with congenital cataract in Linyi People's Hospital from May 2015 to May 2016. The inclusion criteria were as follows: a diagnosis of monocular or binocular congenital cataract[16]; an excess of 3 mm-limit in opacification of crystallin lens, nucleus of the lens or posterior pole; patients capable of performing the Snellen visual acuity test; no complications post successful surgery and patients present with complete post-operative review; and no ocular complications like congenital glaucoma, persistent hyperplastic primary vitreous (PHPV), or retinopathy of prematurity (ROP). The exclusion criteria were as follows: patients with a history of ocular injury; congenital ocular anomaly; mentally disturbed patients; indications for surgery; and other ocular or systemic diseases. An additional 172 normal children (92 male and 80 female) with a calculated mean age of 5.3 ± 1.3 years, who underwent physical examinations, were recruited as the control group during the same period. It was ensured that the case group had no blood relations with the control group. The study was approved by the Ethics Committee of Linyi People's Hospital, and signed informed consents were obtained from all study subjects.
2.2. Blood sampling and DNA extraction
Fasting peripheral venous blood samples (5 mL) were collected from the case group patients within 24 hours of admission. Two percent ethylenediamine tetraacetic acid (EDTA) was added to the samples followed by preservation at −80 °C. DNA was extracted from the entire blood sample (200 μL) using a QIAamp DNA blood mini kit (Qiagen, Hilden, Germany). Purity of the extracted DNA was determined by an ultraviolet spectrophotometer with A260/A280 ratio in the range of 1.8 to 2.0. After agarose gel electrophoresis for DNA verification, the required DNA template concentration for polymerase chain reaction (PCR) was then calculated. Extracted genomic DNA was stored in the TE buffer at −80 °C prior to analyses.
2.3. Detection of single nucleotide polymorphisms (SNPs) in CRYAA and CRYAB genes
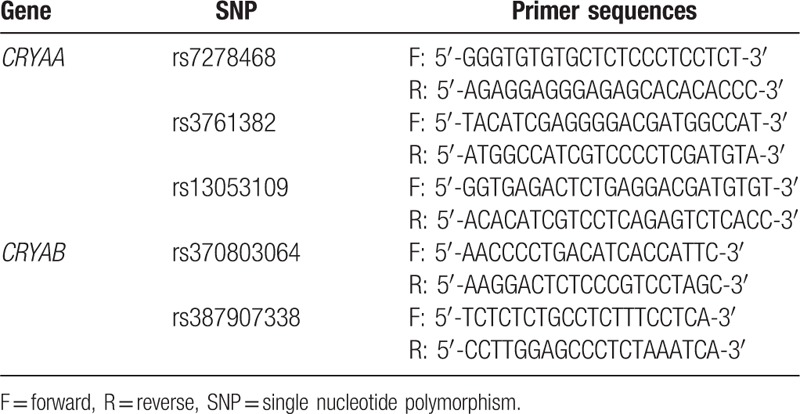
Genotypes of CRYAA and CRYAB genes were examined using an ABI3100-Avant sequencer. CRYAA and CRYAB gene sequences were obtained from the GenBank. PCR primers for rs7278468, rs3761382, and rs13053109 sequences in the CRYAA gene and rs370803064 and rs387907338 sequences in the CRYAB gene were designed using the Primer Premier 5.0 software (Table 1).
Table 1.
Primer sequences for CRYAA SNPs (rs7278468, rs3761382, and rs13053109) and CRYAB SNPs (rs370803064 and rs387907338).
The total reaction volume was 50 μL, which included 5 μL of 10 × buffer solution, 1 μL of DNA template (5 ng/pL), 0.75 μL of upstream primer and downstream primers each, 5 μL of dNTPs, 0.5 μL (2.5 U) of Prime STAR DNA polymerase, and 32 μL of ddH2O. A gradient PCR instrument was used to conduct the qRT-PCR, and amplification conditions were as follows: 3 minutes of predenaturation at 94 °C, 35 cycles of 30 seconds at 94 °C, 1 minute at 55 °C and 65 second at 72°C, and a final extension for 10 minutes at 72 °C. Two percent agarose gel electrophoresis was employed in order to purify the obtained PCR products after detection. Denaturation was performed and the sequences and genotypes were detected by an ABI3100-Avant sequencer (Applied Biosystems, Inc., CA) (Fig. 1).
Figure 1.
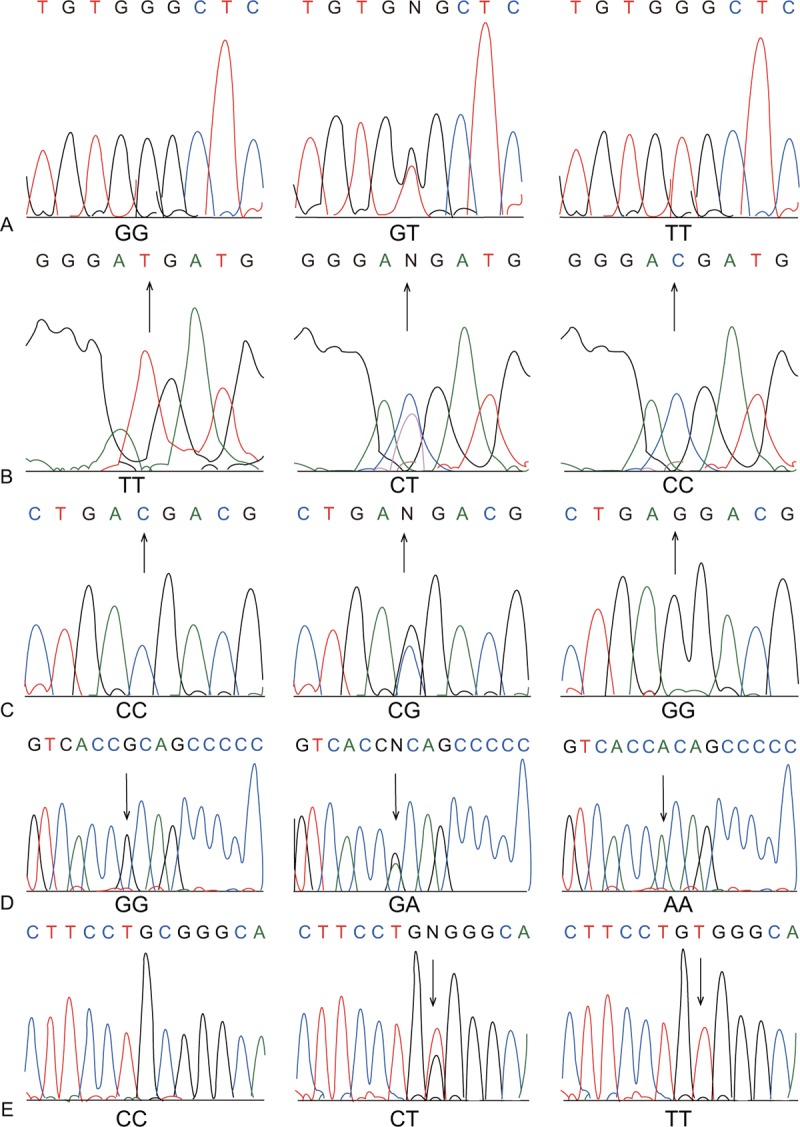
Sequencing results of CRYAA SNPs (rs7278468, rs3761382, and rs13053109) and CRYAB SNPs (rs370803064 and rs387907338). A, CRYAA rs7278468; B, CRYAA rs3761382; C, CRYAA rs13053109; D, CRYAB rs370803064; and E, CRYAB rs387907338. SNP = single nucleotide polymorphism.
2.4. Operative procedures and postoperative observation
Anesthesia evaluations were conducted before operations, and patients were operated on only when under the influence of general anesthesia. The same doctor conducted all procedures (sclera tunnel incision, lens extraction, posterior capsulotomy, anterior vitrectomy, and posterior chamber lens implantation) on all patients. After successful operations, patients received various routine eye examinations for uncorrected visual acuity, corrected visual acuity in mydriatic optometry, visual field, intraocular pressure, fundus, external eyes, anterior junction under slit lamp, etc. and follow-up visits. Best-corrected visual acuity was recorded based on the Snellen chart 1 month after the operation.[17]
Efficacy evaluations of the opacification of posterior pole in patients were made postoperation.[18] The classification and evaluation criteria were as follows: 0, no opacification; 1, appearance of microfold or lens epithelial cells in posterior capsule; 2, appearance of honeycomb opacification and lens epithelial cells or fiber membrane in posterior capsule; 3, appearance of Elschnig pearl or thick fiber membrane; 4, appearance of fundus-blocking Elschnig pearl.
2.5. Statistical methods
Data analyses were performed using the SPSS 20.0 integrated software. Measurement data were presented as mean ± standard deviation. Comparisons between 2 groups, and comparisons among 3 groups were performed using the t test and one-way analysis of variance, respectively. Categorical data were shown as percentage or rate, which was further examined by a chi-square test. Odds ratio (OR) with 95% confidence interval (95% CI) was used to estimate the correlations of SNPs with children with congenital cataract. Examinations of representativeness of the population were made using the Hardy–Weinberg equilibrium. Haplotype analyses were performed by the SHEsis software, and a chi-square test was used for frequency comparison between groups. Logistic regression analysis was used to analyze the risk factors for children with congenital cataract. All tests were two-sided, with P < .05 was considered as statistically significant.
3. Results
3.1. Baseline characteristics of the subjects in the case and control groups
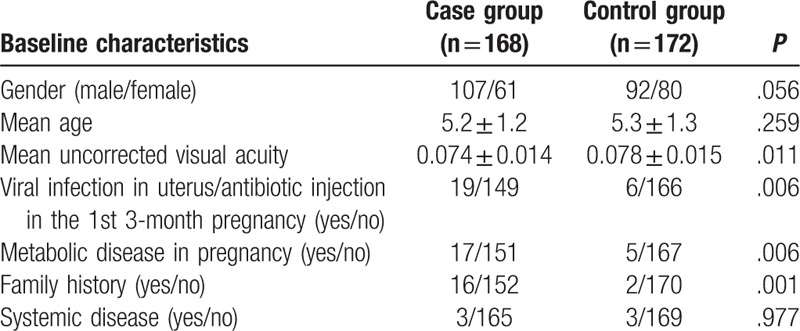
As shown in Table 2, the case and control groups revealed no remarkable differences in terms of age, gender, and state of systemic disease (all P > .05). The case group showed significant differences in terms of mean visual acuity, family history, and viral infection in uterus or antibiotic injection in the 1st 3-month pregnancy, metabolic disease in pregnancy in comparison with the control group (all P > .05).
Table 2.
Comparisons of baseline characteristics between the case and control groups.
3.2. Distributions of genotype and allele frequencies of SNPs in CRYAA and CRYAB genes
The Hardy–Weinberg equilibrium demonstrated that rs7278468, rs3761382, and rs13053109 distribution in the CRYAA gene and rs370803064 and rs387907338 distribution in the CRYAB gene were consistent with the Hardy–Weinberg equilibrium (P > .05), further indicating that the selected groups were representative.
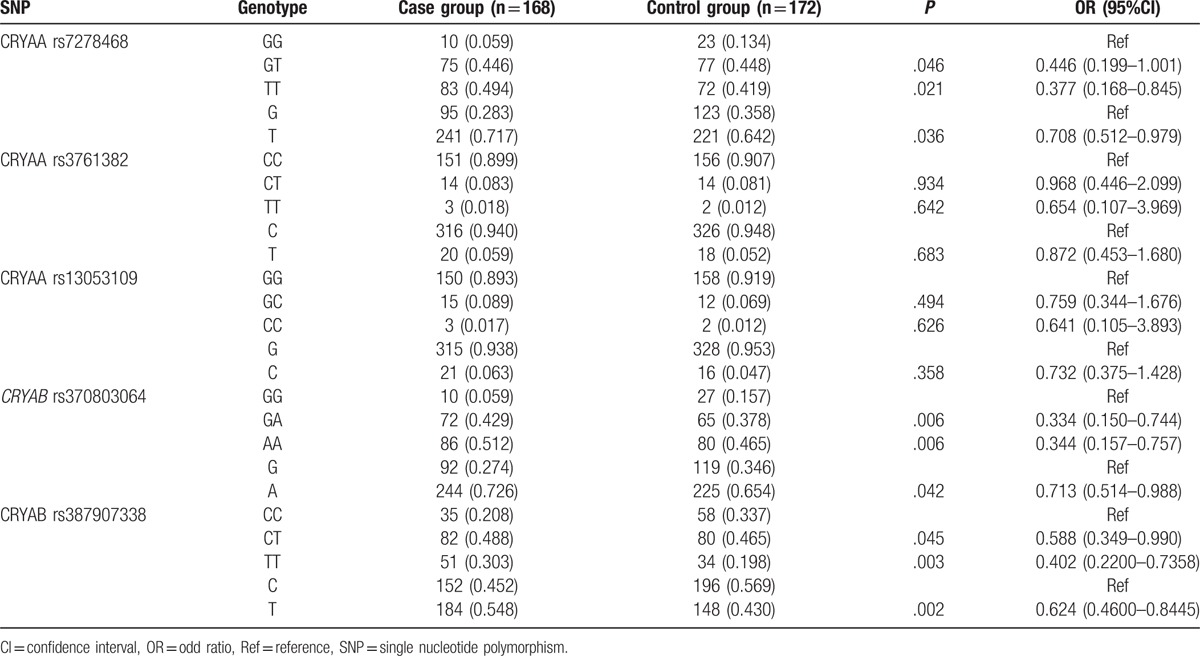
As shown in Table 3, the genotype and allele frequencies of rs7278468 in the CRYAA gene and rs370803064 and rs387907338 in the CRYAB gene showed evident differences between the case and control groups (all P < .05). The case group showed a higher frequency of T allele of rs7278468 in the CRYAA gene (OR = 0.7151, 95%CI = 0.514–0.993, P < .05), A allele of rs370803064 in the CRYAB gene (OR = 0.7129, 95%CI = 0.514–0.988, P < .05), and T allele of rs387907338 in the CRYAB gene (OR = 0.6238, 95%CI = 0.46–0.8445, P < .05) in comparison with the control group. However, no evident difference was identified in the frequencies of rs3761382 and rs13053109 in the CRYAA gene (both P > .05).
Table 3.
Distribution of genotypes and allele frequencies of 3 SNPs (rs7278468, rs3761382, and rs13053109) in CRYAA and 2 SNPs (rs370803064 and rs387907338) in CRYAB.
3.3. Haplotype analyses for SNPs in CRYAA and CRYAB genes
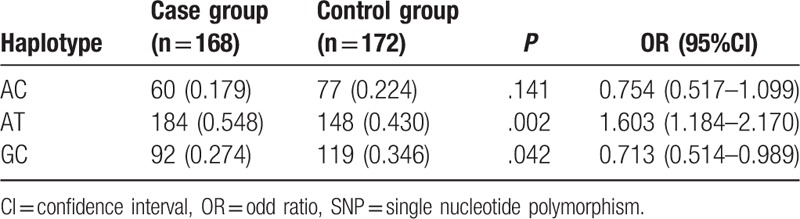
Haplotype analyses for rs7278468, rs3761382, and rs13053109 sequences in the CRYAA gene and rs370803064 and rs387907338 sequences in the CRYAB gene were feasible owing to the presence of a strong linkage disequilibrium among these 5 SNPs (r2 > 0.8) and those with minor allele frequency (MAF) more than 0.1 were further analyzed. The results indicated that haplotype GCG of the CRYAA gene might decrease congenital cataract risk for in newborn infants (OR = 0.705, 95%CI = 0.51–0.975, P < .05). TA of the CRYAB gene might increase congenital cataract risk, on the other hand GC might decrease congenital cataract risk in newborn infants (TA: OR = 1.603, 95%CI = 1.184–2.17, P < .05; GC: OR = 0.713, 95%CI = 0.514–0.989, P < .05) (Tables 4 and 5).
Table 4.
Haplotype analyses for 3 SNPs (rs7278468, rs3761382, and rs13053109) in CRYAA.
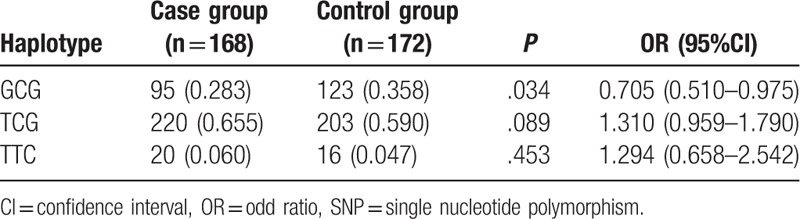
Table 5.
Haplotype analyses of 2 SNPs (rs370803064 and rs387907338) in CRYAB.
3.4. Relation between the clinicopathological features of children with congenital cataract and SNPs in CRYAA and CRYAB genes
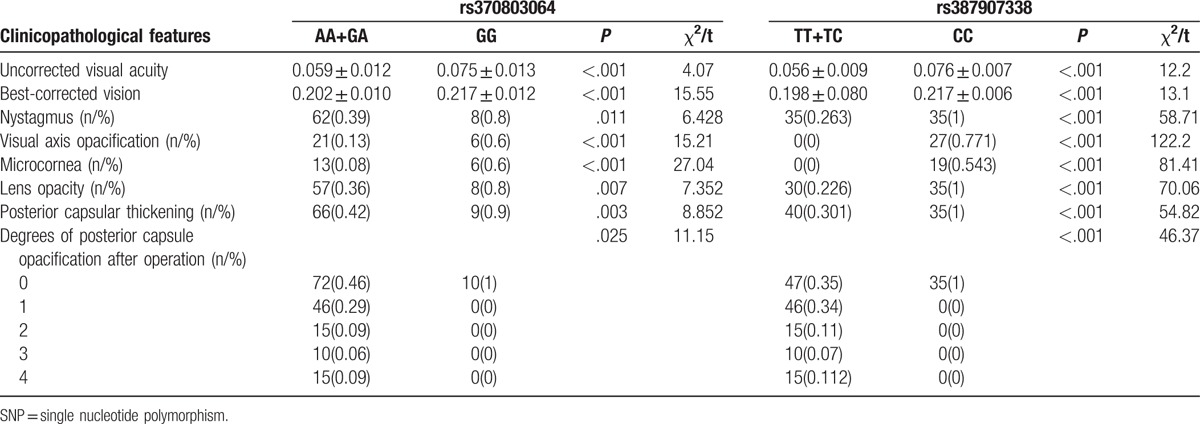
As shown in Tables 6 and 7, patients presenting with a T allele in CRYAA rs7278468 (GT + TT) and CRYAB rs387907338 (TT + TC), and an A allele in CRYAB rs370803064 (AA + GA), respectively, showed a weaker uncorrected visual acuity and the best-corrected visual acuity and severer nystagmus, visual axis opacification, microcornea deformity, lens opacity, posterior capsular thickening, and degrees of posterior capsule opacification after the operation than the patients presenting with corresponding homozygote GG, CC, and GG (all P < .05).
Table 6.
Relation between the clinicopathological features of children with congenital cataract and three SNPs (rs7278468, rs3761382 and rs13053109) in CRYAA.
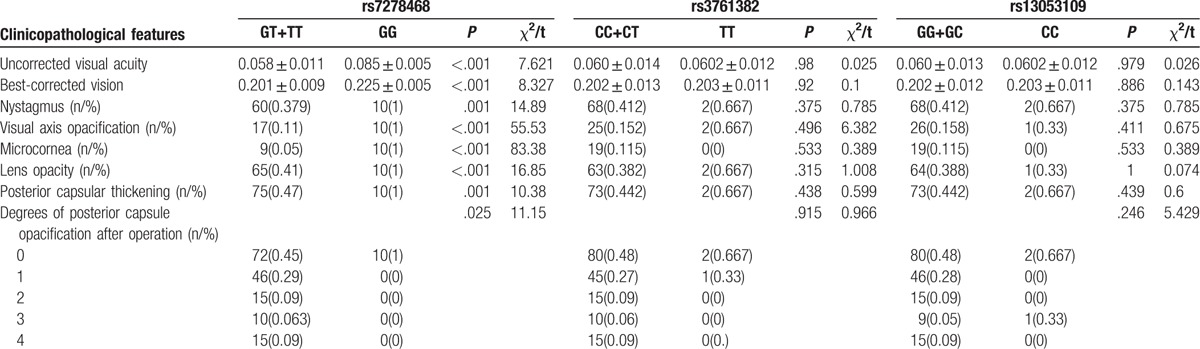
Table 7.
Relation between the clinicopathological features of congenital cataract and two SNPs (rs370803064 and rs387907338) in CRYAB.
3.5. Logistic regression analysis of the risk factors for children with congenital cataract
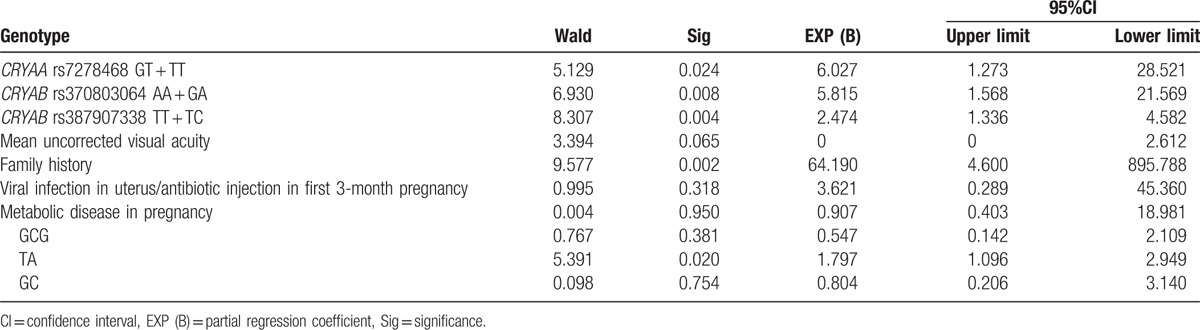
Logistic regression analyses were performed taking congenital cataract development as the dependent variable and furthermore, mean visual acuity, family history, viral infection in the uterus or antibiotic injection in the 1st 3-month pregnancy, metabolic disease in pregnancy, CRYAA rs7278468, CRYAB rs370803064 rs387907338, GCG, TA, and GC were chosen as the independent variables. The results revealed that the presence of a T allele in CRYAA rs7278468, A allele in CRYAB rs370803064, T allele in CRYAB rs387907338, family history, and TA are the risk factors for children suffering from congenital cataract (all P < .05) (Table 8).
Table 8.
Logistic regression analysis of the risk factors for children with congenital cataract.
4. Discussion
CRYAA and CRYAB mutations have been reported to play a critical role in congenital cataract.[13,19] Currently, over 40 loci have been mapped in congenital cataract development.[3] However, the mechanism of CRYAA and CRYAB mutations leading to congenital cataract remains unclear. The present study tried to investigate the effects of mutations on loci rs7278468, rs3761382, and rs13053109 on the CRYAA gene and rs370803064 and rs387907338 on the CRYAB gene on risks of pediatric congenital cataract. Consequently, the study indicated that CRYAA rs7278468, CRYAB rs370803064, and CRYAB rs387907338 were associated with the risk and clinicopathological features of children with congenital cataract.
Initially, the research findings revealed a significant difference between the case and control groups’ genotype and allele frequencies of CRYAA rs7278468, CRYAB rs370803064, and CRYAB rs387907338. Being members of the sHSP family, CRYAA and CRYAB genes can function as molecular chaperone-like agents and accumulate related proteins in large soluble gatherings of about 30 to 40 subunits.[1] The CRYAA gene is expressed at a higher concentration in the lens and critically participates in lens clarity maintenance, thus preventing opacification.[11]CRYAA gene is not highly expressed in the lens epithelium, whereas it shows an elevated expression in the elongation zone and plays a significant role in the differentiation from epithelial cells to fiber cells in the lens.[20]CRYAB gene mutations can result in diverse clinical phenotypes, such as isolated cataract, myopathy, myofibrillar, cardiomyopathy, as well as a multisystemic disorder with a combination of these features.[13] To date, a number of mutations in CRYAA and CRYAB genes have been identified in patients or families with a congenital cataract background.[13,20–22] For example, c.246_248delCGC (p.117delR), a novel mutation of the CRYAA gene, has been detected in a Chinese family with perinuclear congenital cataracts of autosomal type.[15] Su et al[23] also identified a disease-causing mutation in the CRYAA gene, c.161G > C (p.R54P), with autosomal dominant Y-suture cataracts. Jiaox et al[4] have reported 2 novel missense mutations, namely p.R11C and p. R12C of the CRYAB gene, show relations with autosomal recessive congenital nuclear cataracts. It is also identified that c.59C > G (P20R) in the CRYAB gene was a mutant in a 5-generation family with a hereditary posterior polar cataract background.[13] Moreover, evidence identified a novel missense R11H mutation of the CRYAB gene to be correlated with congenital nuclear cataract in a 4-generation family.[11] Ma et al[24] confirmed that the T allele on rs7278468 is capable of contributing to the increased frequency in patients with age related cataract (ARC), which is consistent with our results. We further indicated that CRYAA rs7278468, CRYAB rs370803064, and CRYAB rs387907338 show correlations with uncorrected visual acuity, best-corrected visual acuity, nystagmus, visual axis opacification, microcornea, lens opacity, posterior capsular thickening, and degrees of posterior capsule opacification after operation.
Furthermore, our study data indicated that TA of the CRYAB gene might increase congenital cataract risk in children, while GCG of the CRYAA gene and GC of the CRYAB gene might decrease congenital cataract risk in children. Ma et al[24] found that the C-G-T haplotype could function as a risk factor for ARC, yet the T-C-G haplotype appeared to be an ARC protective factor, and the rs7278468 T allele on the CRYAA gene was responsible for a decrease in the transcriptional activity imparted by the original risk haplotype, and the T allele can increase its binding affinity in KLF10's binding motif, reducing CRYAA transcription and αA-crystallin protein levels. Logistic regression analysis also confirmed that T allele of CRYAA rs7278468, A allele of CRYAB rs370803064, T allele of CRYAB rs387907338, family history, and TA haplotype of CRYAB were risk factors for children suffering from congenital cataract.
In summary, the present study provided evidence of risk factors on the congenital cataract genetic background. CRYAA rs7278468, CRYAB rs370803064, and CRYAB rs387907338 were associated with the risks and clinicopathological features of children with congenital cataract. However, the etiology and pathogenesis of hereditary congenital cataract is complicated and affected by multiple factors. More researches are required to further investigate the relationship between congenital cataract and CRYAA and CRYAB genes.
Acknowledgments
The authors thank all participants enrolled in the present study.
Footnotes
Abbreviations: CRYAA = crystallin αA, PCR = polymerase chain reaction, sHSP = small heat-shock protein, SNP = single nucleotide polymorphism.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Yi J, Yun J, Li ZK, et al. Epidemiology and molecular genetics of congenital cataracts. Int J Ophthalmol 2011;4:422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hejtmancik JF. Congenital cataracts and their molecular genetics. Semin Cell Dev Biol 2008;19:134–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gonzalez-Huerta LM, Messina-Baas O, Urueta H, et al. A CRYGC gene mutation associated with autosomal dominant pulverulent cataract. Gene 2013;529:181–5. [DOI] [PubMed] [Google Scholar]
- [4].Jiaox X, Khan SY, Irum B, et al. Missense mutations in CRYAB are liable for recessive congenital cataracts. PLoS One 2015;10:e0137973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pichi F, Lembo A, Serafino M, et al. Genetics of congenital cataract. Dev Ophthalmol 2016;57:1–4. [DOI] [PubMed] [Google Scholar]
- [6].Dave A, Martin S, Kumar R, et al. Epha2 mutations contribute to congenital cataract through diverse mechanisms. Mol Vis 2016;22:18–30. [PMC free article] [PubMed] [Google Scholar]
- [7].Wang L, Chen Y, Chen X, et al. Further evidence for P59L mutation in GJA3 associated with autosomal dominant congenital cataract. Indian J Ophthalmol 2016;64:508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Irum B, Khan SY, Ali M, et al. Mutation in LIM2 is responsible for autosomal recessive congenital cataracts. PLoS One 2016;11:e0162620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Laurie KJ, Dave A, Straga T, et al. Identification of a novel oligomerization disrupting mutation in CRYAlphaA associated with congenital cataract in a South Australian family. Hum Mutat 2013;34:435–8. [DOI] [PubMed] [Google Scholar]
- [10].VanderVeen DK, Andrews C, Nihalani BR, et al. Crystalline cataract caused by a heterozygous missense mutation in gammaD-crystallin (CRYGD). Mol Vis 2011;17:3333–8. [PMC free article] [PubMed] [Google Scholar]
- [11].Chen Q, Ma J, Yan M, et al. A novel mutation in CRYAB associated with autosomal dominant congenital nuclear cataract in a Chinese family. Mol Vis 2009;15:1359–65. [PMC free article] [PubMed] [Google Scholar]
- [12].Graw J. Genetics of crystallins: cataract and beyond. Exp Eye Res 2009;88:173–89. [DOI] [PubMed] [Google Scholar]
- [13].Xia XY, Wu QY, An LM, et al. A novel P20R mutation in the alpha-B crystallin gene causes autosomal dominant congenital posterior polar cataracts in a Chinese family. BMC Ophthalmol 2014;14:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lu H, Peng L, Yuan X, et al. Concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a treatment paradigm also applicable to patients in Southeast Asia. Cancer Treat Rev 2009;35:345–53. [DOI] [PubMed] [Google Scholar]
- [15].Kong XD, Liu N, Shi HR, et al. A novel 3-base pair deletion of the CRYAA gene identified in a large Chinese pedigree featuring autosomal dominant congenital perinuclear cataract. Genet Mol Res 2015;14:426–32. [DOI] [PubMed] [Google Scholar]
- [16].Uemura Y. Diagnosis of congenital cataract. Ganka 1971;13:613–20. [PubMed] [Google Scholar]
- [17].Devi RR, Yao W, Vijayalakshmi P, et al. Crystallin gene mutations in Indian families with inherited pediatric cataract. Mol Vis 2008;14:1157–70. [PMC free article] [PubMed] [Google Scholar]
- [18].Findl O, Buehl W, Menapace R, et al. Comparison of 4 methods for quantifying posterior capsule opacification. J Cataract Refract Surg 2003;29:106–11. [DOI] [PubMed] [Google Scholar]
- [19].Khoshaman K, Yousefi R, Tamaddon AM, et al. The impact of hydrogen peroxide on structure, stability and functional properties of human R12C mutant alphaA-crystallin: the imperative insights into pathomechanism of the associated congenital cataract incidence. Free Radic Biol Med 2015;89:819–30. [DOI] [PubMed] [Google Scholar]
- [20].Javadiyan S, Craig JE, Souzeau E, et al. Recurrent mutation in the crystallin alpha A gene associated with inherited paediatric cataract. BMC Res Notes 2016;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang Z, Su D, Li Q, et al. A R54L mutation of CRYAA associated with autosomal dominant nuclear cataracts in a Chinese family. Curr Eye Res 2013;38:1221–8. [DOI] [PubMed] [Google Scholar]
- [22].van der Smagt JJ, Vink A, Kirkels JH, et al. Congenital posterior pole cataract and adult onset dilating cardiomyopathy: expanding the phenotype of alphaB-crystallinopathies. Clin Genet 2014;85:381–5. [DOI] [PubMed] [Google Scholar]
- [23].Su D, Guo Y, Li Q, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol 2012;13:163–71. [DOI] [PubMed] [Google Scholar]
- [24].Ma X, Jiao X, Ma Z, et al. Polymorphism rs7278468 is associated with age-related cataract through decreasing transcriptional activity of the CRYAA promoter. Sci Rep 2016;6:23206. [DOI] [PMC free article] [PubMed] [Google Scholar]


