Abstract
Endoscopic ultrasound (EUS) and endoscopic resection play an important role in gastric submucosal tumor. However, there were few articles regarding EUS and endoscopic resection of gastric schwannomas. Our aim was to evaluate the role of EUS and endoscopic resection in treating gastric schwannomas.
We retrospectively reviewed 14 patients between March 2012 and April 2016 with gastric schwannomas and who received EUS and endoscopic resection. EUS characteristics, endoscopic resection, tumor features, and follow-up were evaluated in all the patients.
Fourteen patients were enrolled in the present study. The patients’ ages ranged from 25 to 72 years (mean age, 52.6 years). On EUS, all tumors were originating from muscularis propria and hypoechoic. Ten tumors have the extraluminal growth patterns and 4 tumors have the intraluminal growth patterns. Marginal halos were observed in 7 lesions. No cystic change and calcification were found inside the lesions. Complete endoscopic resection was performed in all the patients with no complications occurring in any patients. No recurrence or metastases was found in all patients during the follow-up period.
Gastric schwannoma has some characteristics on EUS, but it is difficult to differentiate gastric schwannoma from gastrointestinal stromal tumor. Endoscopic resection is an effective and safe treatment for gastric schwannoma with an excellent follow-up outcome.
Keywords: endoscopic resection, endoscopic ultrasound, gastric schwannoma, gastric submucosal tumor
1. Introduction
Schwannomas are tumors originating from any nerve with a Schwann cell sheath, which are rarely observed in the gastrointestinal tract. If gastrointestinal tract is involved, the common involved site is the stomach. Most of the gastric schwannomas are asympomatic and are discovered incidentally; sometimes gastric schwannoma may present with epigastric pain and gastrointestinal bleeding. Many schwannomas, however, are misdiagnosed as other mesenchymal tumors, such as gastrointestinal stromal tumors (GISTs). It is therefore of paramount importance to accurately differentiate schwannomas from GISTs. Endoscopic ultrasound (EUS) is a useful imaging technique for diagnosing the gastric submucosal tumors,[1] but only a few reports have been published regarding the EUS characteristics of gastric schwannomas.[2]
At present, the diagnosis of a schwannoma is based on immunohistochemical examination that S-100 protein, a calcium-binding protein found within cell lines of neural crest origin, is positive, whereas GIST is negative for S-100 protein but typically positive for CD-117, DOG-1, and CD34.[3]
All published data to date suggest that gastric schwannomas are benign neoplasms with excellent prognosis after traditional surgery or laparoscopic surgery. With the advancement of endoscopic equipments, some new endoscopic methods, such as endoscopic full-thickness resection (EFTR), have been widely used. However, there were few articles regarding endoscopic resection of gastric schwannomas.[4,5] Thus, our aim is to evaluate the role of EUS and endoscopic resection in treating gastric schwannomas.
2. Materials and methods
2.1. Patients
A database of all patients with gastric schwannomas who was treated by endoscopic resection at shengjing hospital of China Medical University between March 2012 and April 2016 was retrospectively analyzed. The study was approved by the Ethics Committee of China Medical University and all the patients signed written informed consent before the initial endoscopic resection. All patients underwent EUS to evaluate the characteristics of gastric lesions, including the originating layer, the size, echogenicity, and growth pattern and then, endoscopic resection was performed with patient being hospitalized for treatment.
2.2. Endoscopic ultrasound
All EUS procedures were performed with a linear scanning ultrasound endoscope. A single experienced endosonographer reviewed all the EUS images. The following features were recorded: location, the presence of mucosal ulceration, growth pattern, original layer, echogenicity, the presence of marginal halos, cystic change, and calcification.
2.3. Endoscopic resection
2.3.1. Endoscopic submucosal dissection
All endoscopic submucosal dissection (ESD) procedures were performed with propofol sedation and continuous cardiorespiratory monitoring. ESD was performed as follows: APC was used to mark the circumferential of the tumor and then, a mixed solution consisting of 100-mL saline and 1-mL indigo carmine was injected into the submucosa to make a cushion. After the mucosa incision was made, dissection of the tumor was made under direct view to achieve en bloc resection. The hemostasis was performed by electric cautery or APC.
2.3.2. EFTR
The peritumor gastric tissues were incised, and then, the tumor and peritumor gastric tissues were gradually resected in full thickness and electric cautery was used for hemostasis. The tumor was completely removed without injury to the tumor capsule in all cases. Metallic clips or over-the-scope clip was used to close the iatrogenic perforation.
2.3.3. Ligation-assisted endoscopic enucleation
The lesion was first aspirated into the transparent cap attached to the tip of endoscope and then, the elastic band was released around its base. EUS was used to confirm the lesion had been completely ligated. The tumor was exposed by the mucosal and submucosal layers overlying the tumor cut open and gradually dissected. The wound was closed with metallic clips.
2.3.4. Pathological evaluation
The pathological sections were stained by hematoxylin and eosin for regular examination. Immunohistochemical staining, including CD34, CD117, SMA, Dog-1, Ki-67, and S-100 protein, was performed to make differential diagnosis.
2.3.5. Postoperative follow-up
Patients were suggested for the endoscopic follow-up at 1, 3, 6, and 12 months after resection and annually thereafter to observe the wound healing and any residual tumor or recurrence.
3. Results
3.1. Clinical characteristics of patients
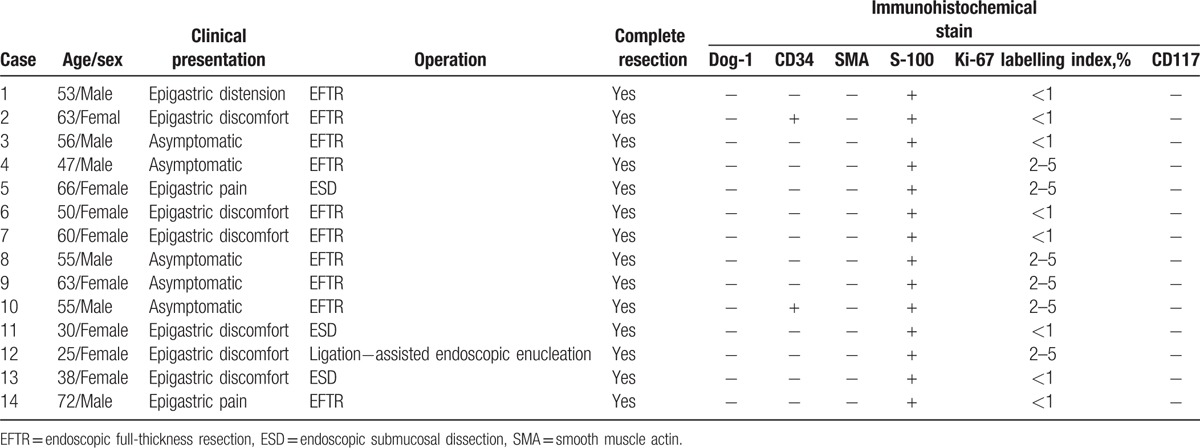
The clinical data for 14 patients (6 male and 8 female) enrolled in the present study were summarized in Table 1. The patients’ ages ranged from 25 to 72 years (mean age, 52.6 years). Nine of 14 patients had epigastric discomfort, pain, or distension; 5 patients were asymptomatic, and their lesions were incidentally found by upper gastroscopy. Ten patients received EFTR; 3 patients were treated by ESD and ligation-assisted endoscopic enucleation was performed for 1 patient. Complete resection was performed in all the patients. No complications, such as delayed perforation and hemorrhage, occurred in any patients.
Table 1.
The patient characteristics, treatment outcomes, and the results of immunohistochemical stain.
3.2. EUS findings
All the patients received EUS. The EUS characteristics of 14 patients with gastric schwannoma were listed in Table 2. Eight lesions were located in antrum; 4 tumors were found in the gastric body, and 2 lesions were in the gastric fundus. No lesions had surface ulceration. The size of tumors ranged from 5 to 25 mm (mean size, 17 mm). All lesions were from muscularis propria and hypoechoic (Fig. 1A). Ten lesions were of the extraluminal growth patterns and 4 tumors were of the intraluminal growth patterns. Marginal halos were observed in 7 lesions (Fig. 1B). No cystic change or calcification was found inside the lesions. No EUS-guided fine needle aspiration was performed.
Table 2.
The endoscopic ultrasound characteristics of 14 patients with gastric schwannoma.
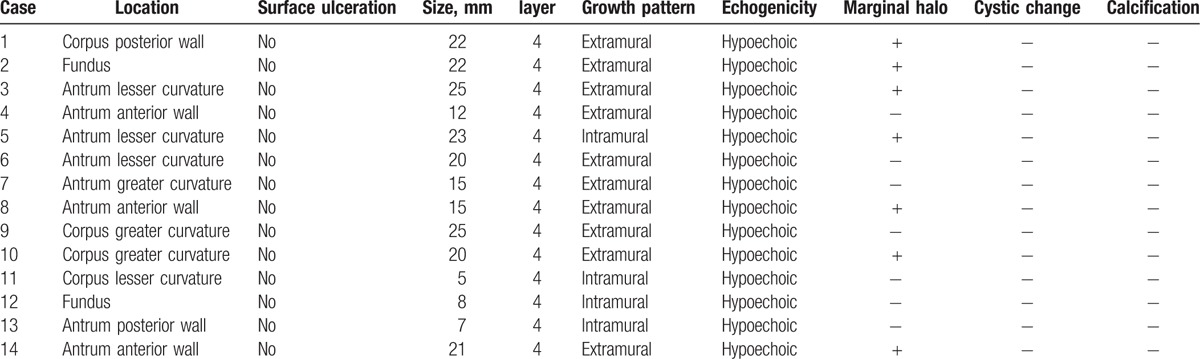
Figure 1.
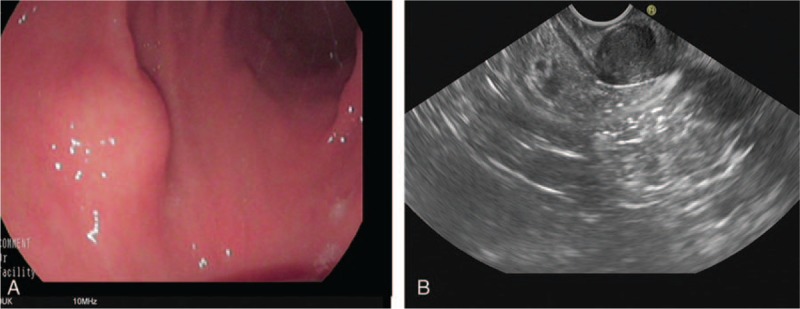
(A) A submucosal elevated lesion was seen in the anterior wall of gastric antrum. (B) Endoscopic ultrasound showed that the hypoechoic lesion was of intraluminal growth pattern and a marginal halo was observed (white arrow).
3.3. Histopathological and immunochemical results
The tumors were composed of arranged spindle cells. All tumors were positive for S-100 protein and negative for SMA, Dog-1, CD34, CD117, and c-kit, except 2 cases were positive for CD34. The Ki-67 labeling index was <5% in all tumors (Table 1).
3.4. Follow-Up
No recurrence or metastases were found in all patients during the follow-up period at a median follow-up time of 28 months (range, 4–53 months).
4. Disscussion
Generally, gastric schwannoma originating from Schwann cells is asymptomatic and usually discovered by routine examinations.[6] However, patients with gastric schwannoma may present with gastrointestinal hemorrhage, epigastric discomfort, and even gastroduodenal intussusceptions.[7,8] Two-thirds of our patients were symptomatic, similar to one previous study.[8] Gastric schwannoma occurs more frequently in patients ranging from 50 to 60 years and female is more likely to happen[9]; in our study, the women:men ratio was 4:3 and the mean age was 52.6 years. Gastric schwannoma mainly occurs in the gastric body, followed by gastric antrum and fundus[3]; 8 (8/14,57.1%) gastric schwannomas were located in the gastric antrum in our study and it might be because our less case number.
However, it is difficult to distinguish between gastric schwannoma and other gastric submucosal tumors, such as GIST. Imaging modalities, such as computed tomography, magnetic resonance imaging, and positron emission tomography, can provide some information for gastric schwannoma, which is, however, not specific and very similar to those of GISTs. With EUS widely used to examine gastric submucosal tumors[10–12], articles have been published regarding the EUS characteristics of gastric schwannoma. Previous EUS studies have reported that gastric schwannoma is a round homogenous mass, usually located in muscularis propria, with marginal halos and without internal echogenic foci[13] and gastric schwannoma exhibited heterogeneously hypoechoic lesions with lower echogenicity compared to the surrounding normal proper muscle layer.[2] In present study, all the gastric schwannomas were from muscularis propria and hypoechoic and did not have cystic change and calcification and half of gastric schwannomas had marginal halos, which can also be frequently seen in GISTs. So it was difficult to diagnose gastric schwannoma by EUS alone, but endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) can be used to help diagnoses.[14–16] The routinely EUS-FNA is not rencommended for primary resectable GIST by National Comprehensive Cancer Network guidelines, owing to the risk of tumor rupture and spread in association with poor prognosis.[17] So we did not perform routinely EUS-FNA for gastric submucosal tumors, which might be GISTs.
The definitive diagnosis of gastric schwannoma is determined by pathologic and immunohistochemical examination of specimens. The tumor consists of spindle cells. Schwannoma shows strong positive staining for S-100 protein and negative staining for CD117, CD34, desmin, and SMA. Occasionally, gastric schwannoma may express CD34.[8] In our cases, we got the similar results that all schwannomas were positive for S-100 protein and 2 cases were positive for CD34.
It is difficult to preoperatively distinguish between gastric schwannoma and other gastric submucosal tumor, such as GIST. Therefore, the presence of such lesion became a psychological burden to patients. For these reasons, the patients preferred to undergo resection. Only a few articles have been reported regarding the endoscopic resection of gastric schwannoma.[4,5] Gastric schwannoma is located in the deep layer and often has the extraluminal growth pattern. In our study, 10 (71.4%) gastric schwannomas had an extraluminal growth pattern. Owing to its characteristics, EFTR (10/14, 71.4%), the same as the number of gastric schwannomas with the extraluminal growth pattern, was often performed to resect the lesions. Three patients were treated by ESD and 1 case was treated by ligation-assisted endoscopic enucleation. Successful endoscopic resection was achieved in all the patients in our study. When performing EFTR, the operator should keep in mind that the tumor should be carefully removed to avoid failing into peritoneal cavity, which may lead to procedure failure. No recurrence or metastasis was found during the follow-up period, as described in the previous studies.[5]
In conclusion, gastric schwannoma is a rare gastric submucosal tumor and mostly originates from muscularis propria on EUS. It has some characteristics on EUS; however, it is not specific, which cannot differentiate it from GIST. Endoscopic resection, including ESD, EFTR, and ligation-assisted endoscopic enucleation, is an effective and safe treatment for gastric schwannoma with an excellent follow-up outcome.
Footnotes
Abbreviations: EFTR = endoscopic full-thickness resection, ESD = endoscopic submucosal dissection, EUS = endoscopic ultrasound, EUS-FNA = endoscopic ultrasound-guided fine-needle aspiration, GIST = gastrointestinal stromal tumor.
The authors report no conflicts of interest.
References
- [1].Altonbary AY, Deiab AG, Negm EH, et al. Endoscopic ultrasound of isolated gastric corrosive stricture mimicking linitis plastica. Endosc Ultrasound 2015;4:66–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yoon JM, Kim GH, Park DY, et al. Endosonographic features of gastric schwannoma: a single center experience. Clin Endosc 2016;49:548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Voltaggio L, Murray R, Lasota J, et al. Gastric schwannoma: a clinicopathologic study of 51 cases and critical review of the literature. Hum Pathol 2012;43:650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li B, Liang T, Wei L, et al. Endoscopic interventional treatment for gastric schwannoma: a single-center experience. Int J Clin Exp Pathol 2014;7:6616–25. [PMC free article] [PubMed] [Google Scholar]
- [5].Cai MY, Xu JX, Zhou PH, et al. Endoscopic resection for gastric schwannoma with long-term outcomes. Surg Endosc 2016;30:3994–4000. [DOI] [PubMed] [Google Scholar]
- [6].Fujiwara S, Nakajima K, Nishida T, et al. Gastric schwannomas revisited: has precise preoperative diagnosis become feasible? Gastric Cancer 2013;16:318–23. [DOI] [PubMed] [Google Scholar]
- [7].Yang JH, Zhang M, Zhao ZH, et al. Gastroduodenal intussusception due to gastric schwannoma treated by Billroth II distal gastrectomy: one case report. World J Gastroenterol 2015;21:2225–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tao K, Chang W, Zhao E, et al. Clinicopathologic Features of Gastric Schwannoma: 8-Year Experience at a Single Institution in China. Medicine 2015;94:e1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zheng L, Wu X, Kreis ME, et al. Clinicopathological and immunohistochemical characterisation of gastric schwannomas in 29 cases. Gastroenterol Res Pract 2014;2014:202960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rana SS, Sharma V, Sharma R, et al. Gastric gastrointestinal stromal tumor mimicking cystic tumor of the pancreas: diagnosed by endoscopic ultrasound-fine-needle aspiration. Endosc Ultrasound 2015;4:351–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Choudhary NS, Puri R, Lipi L, et al. Eosinophilic gastroenteritis mimicking as a malignant gastric ulcer with lymphadenopathy as shown by computed tomography and endoscopic ultrasound. Endosc Ultrasound 2015;4:78–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yegin EG, Duman DG. Small EUS-suspected gastrointestinal stromal tumors of the stomach: an overview for the current state of management. Endosc Ultrasound 2016;5:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jung MK, Jeon SW, Cho CM, et al. Gastric schwannomas: endosonographic characteristics. Abdom Imag 2008;33:388–90. [DOI] [PubMed] [Google Scholar]
- [14].Mohri D, Nakai Y, Isayama H, et al. Malignant peritoneal mesothelioma diagnosed by EUS-guided tissue acquisition. Endosc Ultrasound 2015;4:353–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sharma M, Rafiq A, Kirnake V. Dysphagia due to tubercular mediastinal lymphadenitis diagnosed by endoscopic ultrasound fine-needle aspiration. Endosc Ultrasound 2015;4:348–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hong SW, Cho WY, Kim JO, et al. Gastric schwannoma diagnosed by endoscopic ultrasonography-guided trucut biopsy. Clin Endosc 2013;46:284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Arolfo S, Teggia PM, Nano M. Gastrointestinal stromal tumors: thirty years experience of an institution. World J Gastroenterol 2011;17:1836–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


