Abstract
Benzodiazepines are used as first-line treatments for status epilepticus. Fosphenytoin (FPHT) is recommended for second-line therapy; however, intravenous injection of levetiracetam (LEV) may also be effective against status epilepticus. Herein, we compared the efficacy and safety of LEV as a second-line treatment for status epilepticus with FPHT in Japanese patients.
Patients with status epilepticus were selected from the database of the Emergency and Critical Care Center of Hitachi General Hospital. The subjects were patients whose status epilepticus was successfully stopped by diazepam, and in whom FPHT or LEV was administered after diazepam. As LEV injections recently became clinically available in Japan, the choice of drug was determined by the treatment period. Thus, 21 patients who were intravenously injected with LEV as a second-line therapy and 42 matched patients (historical controls) who were treated with FPHT (1:2) were selected.
The subjects had a mean age of 64.0 ± 2.2 years, and included 48 males and 15 females. The status epilepticus control rates of the FPHT and LEV groups did not differ significantly (81.0% [34/42] vs 85.1% [18/21], respectively; P = .69). As for serious adverse events, a reduction in blood pressure was observed in the FPHT group, but not in the LEV group. The oral anticonvulsant switching rates of the 2 groups were similar, but the same-drug switching rates of the FPHT and LEV groups were 8.1% and 77.8%, respectively.
The efficacy of intravenous LEV injections after status epilepticus was equivalent to that of FPHT, and the incidence of adverse events was lower in the LEV group. LEV is effective and safe at preventing recurrent seizures after status epilepticus following benzodiazepine treatment.
Keywords: epilepsy, fosphenytoin, levetiracetam, status epilepticus
1. Introduction
Status epilepticus refers to a state in which convulsions persist and recovery is not achieved. It is life-threatening and can cause irreversible cerebral damage; therefore, it is necessary to promptly stop such convulsions and prevent their recurrence.[1] As an initial treatment for status epilepticus, potent gamma aminobutyric acid agonists, such as benzodiazepines and barbiturates, must be administered quickly to stop the patient's convulsions.[2] Lorazepam and diazepam are recommended as first-line drugs, based on their efficacy in clinical studies, and hence, are commonly used.[3]
Lorazepam and diazepam are short-acting drugs that can produce immediate effects. However, treatment with another long-acting anticonvulsant drug is necessary to prevent recurrent convulsions. For this purpose, phenytoin (PHT) has previously been used to treat patients with status epilepticus. Since fosphenytoin (FPHT) was developed, it has been associated with a lower incidence rate of adverse reactions than PHT and has been recommended as a second-line therapy for use after benzodiazepine treatment.[4] However, both PHT and FPHT can induce adverse reactions such as a reduction in blood pressure, arrhythmia, and allergic symptoms. Although FPHT exhibits a lower incidence of adverse reactions, it can still cause blood pressure reduction and arrhythmia, which is an issue.[5,6]
On the other hand, levetiracetam (LEV), which primarily binds to the synaptic vesicle protein 2A SV2A and regulates the release of neurotransmitters, is effective against convulsions.[7] It has also been demonstrated to be effective against status epilepticus, and such treatment is associated with a low incidence of adverse reactions. Thus, both LEV and FPHT have been recommended as second-line therapies for status epilepticus in some international guidelines.[8–10] However, to the best of our knowledge, no clinical studies have directly compared LEV with FPHT; therefore, it remains unclear which drug is more effective. In addition, no study has examined the efficacy of LEV injections in status epilepticus in Japan. LEV injections became clinically available in Japan in December 2015 and are covered by the Japanese health insurance system. Since then, LEV has often been used as a second-line therapy for status epilepticus in Japan.
Herein, we analyzed Japanese patients with status epilepticus who received FPHT or LEV as a second-line therapy following benzodiazepine treatment at the Emergency and Critical Care Center of our hospital, and used the frequency of recurrent convulsions and other outcomes to evaluate the efficacy of LEV after status epilepticus.
2. Materials and methods
Using the database of the Emergency and Critical Care Center of Hitachi General Hospital, we selected patients with status epilepticus who were admitted to the intensive care unit (ICU) or emergency admission unit and treated between April 2013 and May 2016. Patients whose convulsions stopped after the administration of diazepam to treat acute-phase status epilepticus and who were subsequently administered FPHT or LEV were included. In Japan, diazepam is first administered for status epilepticus before second-line therapy. Therefore, diazepam was administered immediately after the patients arrived at our hospital. Five or 10 mg of diazepam was administered to stop the patients’ convulsions, and only patients whose convulsions were stopped by the infusion of diazepam were included. LEV or FPHT was administered in 100 mL of normal saline over approximately 30 minutes.
As LEV injections became clinically available in Japan in December 2015, only FPHT was used until November 2015, and LEV was mainly used from December 2015; therefore, the choice of drug was determined by the treatment period, that is, historical controls were used in this study.
We excluded patients aged <15 years, those who had drug poisoning, and those who experienced alcohol withdrawal. Only patients who were diagnosed with status epilepticus by neurosurgeons or neurologists were included. Thus, 21 patients who were intravenously injected with LEV as a second-line therapy to prevent recurrent convulsions and 42 sex-/age-/previous oral anticonvulsant drug-/convulsion type-matched patients who were treated with FPHT (1:2) (historical controls) were selected.
Epileptic seizures meeting the following criteria were regarded as status epilepticus: convulsions persisting for ≥5 minutes and different convulsions that persisted for ≥2 minutes without consciousness recovery between the 2 seizures.[11] The duration of status epilepticus was estimated based on inquiry records from witnesses or rescue workers.
As a primary outcome, we analyzed the presence or absence of recurrent convulsions after the administration of FPHT or LEV and regarded the patients who did not exhibit recurrent convulsions as having achieved epilepsy control. Convulsion recurrence was assessed based solely on the presence/absence of apparent convulsions. As secondary outcomes, we analyzed the presence or absence of serious adverse events and switching from intravenous injections to oral administration. As adverse events, only serious events that occurred on the first day (changes in circulatory kinetics, arrhythmia, consciousness disorders, and respiratory suppression) were analyzed from the patients’ course tables and medical records.
This study was approved by the ethics review board of our hospital.
3. Statistical analysis
Differences were assessed using Student's t-test and one-way analysis of variance. All statistical analyses were performed using statistical software (JMP 10; SAS Institute Inc.). Results are expressed as mean ± standard deviation values. P-values of <.05 were considered to be significant.
4. Results
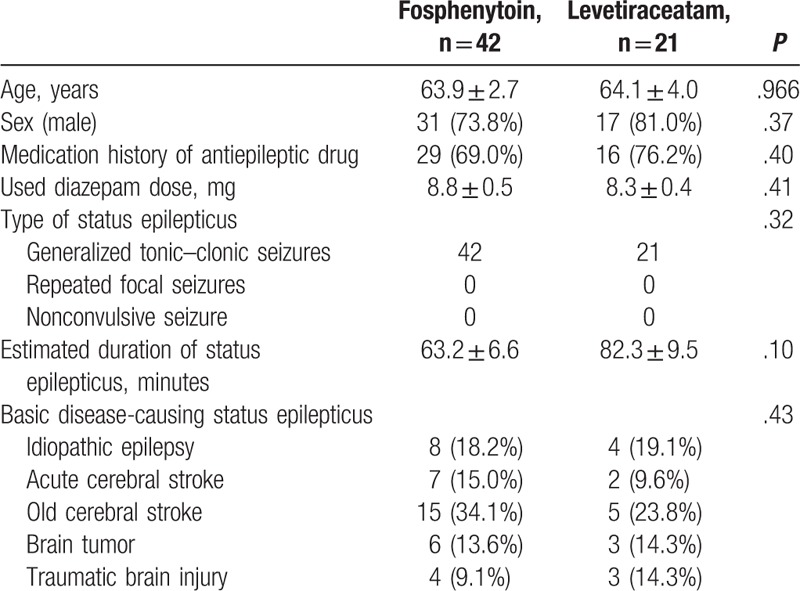
The subjects consisted of 42 FPHT-treated and 21 LEV-treated patients. Their mean age was 64.0 ± 2.2 years, and they included 48 males and 15 females. The subjects’ basic characteristics are shown in Table 1. There were no significant differences in age, sex, the frequency of the previous oral administration of anticonvulsant drugs, the administered diazepam dose, or the underlying diseases that caused the patients’ status epilepticus between the groups. The type of status epilepticus was evaluated as generalized tonic–clonic seizures in all subjects. The estimated duration of status epilepticus in the FPHT and LEV groups was 63.2 ± 6.6 and 82.3 ± 9.5 minutes, respectively (P = .10), and it was ≥30 minutes in all subjects.
Table 1.
Basic characteristics.
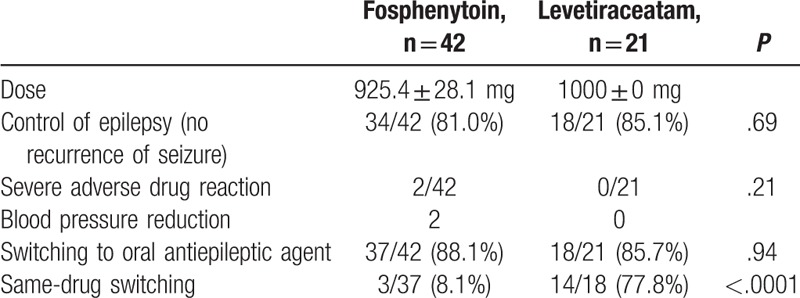
The study outcomes are shown in Table 2. FPHT (dose: 22.5 mg/kg) was administered over 30 minutes. The dose was adjusted in accordance with the patient's age and liver/kidney function levels. The mean dose was 925.4 ± 28.1 mg. On the other hand, LEV was administered at a dose of 1000 mg (dissolved in 100 mL of normal saline) to all subjects in the LEV group. As a primary outcome, epilepsy control that is, the absence of recurrent seizures, was achieved in 34 (81.0%) of the 42 patients in the FPHT group and 18 (85.1%) of the 21 patients in the LEV group (P = .69). As for the patients (in both groups) in whom epilepsy control was not initially achieved, some patients’ seizures were brought under control using combination therapy involving other antiepileptic drugs, whereas other patient's seizures could not be controlled (refractory epilepsy). As for the secondary outcomes, severe adverse reactions (reduction in blood pressure) were observed in 2 patients in the FPHT group, but there were no adverse events in the LEV group. Switching to oral antiepileptic drugs was conducted in <90% of the patients in both groups, and the frequency of such switching did not differ significantly between the 2 groups. However, same-drug switching, such as switching from FPHT to an oral PHT preparation, was performed in 3 (8.1%) of the 37 patients in the FPHT group and in 14 (77.8%) of the 18 patients in the LEV group; the percentage was markedly higher in the latter group (P <.0001).
Table 2.
Outcome.
5. Discussion
In this study, we compared the efficacy of LEV and FPHT against the recurrence of seizure after status epilepticus. To the best of our knowledge, this is the first report to compare LEV with FPHT as 2nd-line therapies for use after benzodiazepine treatment in patients with status epilepticus. In patients with status epilepticus, the preventive effects of LEV against recurrent convulsions were equivalent to those of FPHT. In addition, no marked adverse reactions occurred in the LEV group. As for switching to oral anticonvulsant drugs, the proportion of patients in whom same-drug switching was conducted was markedly higher in the LEV group than in the FPHT group, suggesting that LEV is more useful than FPHT as a second-line therapy for status epilepticus.
LEV exhibits several anticonvulsive actions. Although its mechanism of action remains to be clarified, it is considered to involve its ability to inhibit presynaptic neurotransmitter release by binding to SV2A.[7,12,13] LEV is reportedly useful for preventing epileptic seizures, and the incidence of serious adverse reactions after LEV treatment is low, suggesting that it is safe.[14,15]
In regard to the treatment of status epilepticus, PHT or FPHT has been used as a second-line therapy after benzodiazepine treatment for many years. Some studies have compared the efficacy of LEV against status epilepticus with that of PHT[16–18] and reported that LEV is similarly effective and safer than PHT. However, to the best of our knowledge no clinical studies have compared FPHT with LEV, and this is the first study to compare their efficacy. FPHT is a water-soluble prodrug of PHT. It was developed as a drug that would carry a lower risk of adverse cardiovascular reactions, such as changes in blood pressure and the induction of arrhythmia or phlebitis, than PHT, but some clinical studies have indicated that PHT and FPHT display similar adverse event rates.[5,6] The present study found that LEV was safer than FPHT and did not cause any serious adverse events.
In addition, the intravenous injection of PHT or FPHT has some limitations with regard to switching to an oral PHT preparation, for example, such preparations can cause allergic reactions, such as hypersensitivity syndrome and Stevens–Johnson syndrome, and systemic lupus erythematosus-like symptoms.[19] In this study, FPHT was switched to an oral PHT preparation in an extremely small number of patients. On the other hand, LEV, which may not induce fatal adverse reactions, was efficiently switched to an oral LEV preparation. Same-drug switching may facilitate the continuous, stable control of epileptic seizures. Regarding the treatment provided before switching to oral drugs, the intravenous injection of LEV may be more effective than PHT or FPHT.
Indeed, some international studies have recommended LEV, as an equivalent substitute for PHT or FPHT, as a 2nd-line therapy for status epilepticus.[9] In the Guidelines for the Management of Status Epilepticus published by the American Epilepsy Society in 2016, LEV and FPHT are also recommended equivalently.[10] According to expert opinion regarding the use of anticonvulsant drugs, especially in the Emergency Department, LEV injections produced the most favorable results.[8] In Japan, the Japanese Society of Neurology published guidelines regarding status epilepticus, but only FPHT is recommended as a second-line therapy. In this study, we investigated the efficacy of LEV and FPHT for the recurrence of seizure after status epilepticus involving Japanese patients for the first time and demonstrated the safety of LEV. In the future, LEV should be considered as a treatment option for status epilepticus in Japan.
In this study, the response rate (the primary outcome) was higher than previously reported in clinical studies of status epilepticus. This can be explained as follows: reasons: LEV and FPHT were used as second-line therapies after benzodiazepine treatment, and the subjects were patients in whom benzodiazepine treatments led to the cessation of their convulsions. This study was conducted at an Emergency and Critical Care Center (not at a neurological center in which a large number of patients with refractory epilepsy are treated). Nonresponders to FPHT and LEV with recurrent convulsions are regarded as having refractory epilepsy, and their convulsions are also resistant to other drugs. The rate at which such patients visit an institution may markedly influence the response rate. Many of the patients’ convulsions had been caused by an acute or old stroke in this study because Japanese society is aging and strokes are more frequent.
This study had several limitations. First, it was a retrospective study involving a single institution and a relatively small sample size; thus, there may have been a bias in the subject inclusion process or the convulsion control analysis. This study included a relatively high proportion of elderly people whose status epilepticus had been caused by an acute or old stroke. In the future, a prospective, randomized, controlled trial should be conducted to compare the efficacy of LEV with that of FPHT. Second, this study analyzed the efficacy of LEV or FPHT as a second-line therapy following benzodiazepine treatment. The efficacy of these drugs as first-line therapies for status epilepticus should also be clarified. Third, as we do not perform continuous electroencephalogram monitoring in our ICU, the convulsion recurrence was determined based solely on the presence/absence of apparent convulsions. Therefore, nonconvulsive seizures could not be detected in this study, and the epilepsy control rate may actually have been lower in both groups. Fourth, the dose of FPHT given in this study was relatively lower than the traditional 20 mg/kg dose. PHT or FPHT is often administered in lower doses to status epilepticus patients in Japan because the patients include a lot of elderly patients and their bodies are often small. Fifth, the dose of LEV was also restricted that is, 1000 mg LEV was administered in this study, but internationally LEV is administered at higher doses to patients with status epilepticus. In the guidelines established by the American Epilepsy Society, 60 mg/kg (maximum: 4500 mg) LEV is recommended,[10] which markedly exceeds the dose covered by the Japanese national health insurance system. Although the safety of LEV was considered, its optimal dose for status epilepticus must be reviewed in the future.
6. Conclusions
LEV and FPHT exhibited similar efficacy at preventing recurrent seizures after the termination of status epilepticus by benzodiazepines, but the incidence rate of adverse events was lower after LEV treatment, which facilitated switching to oral drugs. A large double-blind controlled study comparing LEV with FPHT should be performed to confirm the efficacy of LEV against status epilepticus.
Footnotes
Abbreviations: FPHT = fosphenytoin, GABA = gamma aminobutyric acid, ICU = intensive care unit, LEV = levetiracetam, PHT = phenytoin, SV2A = synaptic vesicle 2A.
The authors have no conflicts of interest to disclose.
References
- [1].Chapman MG, Smith M, Hirsch NP. Status epilepticus. Anaesthesia 2001;56:648–59. [DOI] [PubMed] [Google Scholar]
- [2].Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci 1997;17:7532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med 1998;339:792–8. [DOI] [PubMed] [Google Scholar]
- [4].Chang CW, Bleck TP. Status epilepticus. Neurol Clin 1995;13:529–48. [PubMed] [Google Scholar]
- [5].Coplin WM, Rhoney DH, Rebuck JA, et al. Randomized evaluation of adverse events and length-of-stay with routine emergency department use of phenytoin or fosphenytoin. Neurol Res 2002;24:842–8. [DOI] [PubMed] [Google Scholar]
- [6].Swadron SP, Rudis MI, Azimian K, et al. A comparison of phenytoin-loading techniques in the emergency department. Acad Emerg Med 2004;11:244–52. [DOI] [PubMed] [Google Scholar]
- [7].Meehan AL, Yang X, McAdams BD, et al. A new mechanism for antiepileptic drug action: vesicular entry may mediate the effects of levetiracetam. J Neurophysiol 2011;106:1227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Karceski S, Morrell MJ, Carpenter D. Treatment of epilepsy in adults: expert opinion, 2005. Epilepsy Behav 2005;7suppl 1:S1–64. quiz S65–67. [DOI] [PubMed] [Google Scholar]
- [9].Rossetti AO, Lowenstein DH. Management of refractory status epilepticus in adults: still more questions than answers. Lancet Neurol 2011;10:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American Epilepsy Society. Epilepsy Curr 2016;16:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- [12].Rogawski MA. Diverse mechanisms of antiepileptic drugs in the development pipeline. Epilepsy Res 2006;69:273–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Meehan AL, Yang X, Yuan LL, et al. Levetiracetam has an activity-dependent effect on inhibitory transmission. Epilepsia 2012;53:469–76. [DOI] [PubMed] [Google Scholar]
- [14].Kwan P, Lim SH, Chinvarun Y, et al. Efficacy and safety of levetiracetam as adjunctive therapy in adult patients with uncontrolled partial epilepsy: the Asia SKATE II Study. Epilepsy Behav 2010;18:100–5. [DOI] [PubMed] [Google Scholar]
- [15].Trinka E, Dobesberger J. New treatment options in status epilepticus: a critical review on intravenous levetiracetam. Ther Adv Neurol Disord 2009;2:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chakravarthi S, Goyal MK, Modi M, et al. Levetiracetam versus phenytoin in management of status epilepticus. J Clin Neurosci 2015;22:959–63. [DOI] [PubMed] [Google Scholar]
- [17].Cock HR, Group E. Established status epilepticus treatment trial (ESETT). Epilepsia 2011;52suppl 8:50–2. [DOI] [PubMed] [Google Scholar]
- [18].Yasiry Z, Shorvon SD. The relative effectiveness of five antiepileptic drugs in treatment of benzodiazepine-resistant convulsive status epilepticus: a meta-analysis of published studies. Seizure 2014;23:167–74. [DOI] [PubMed] [Google Scholar]
- [19].Ye YM, Thong BY, Park HS. Hypersensitivity to antiepileptic drugs. Immunol Allergy Clin North Am 2014;34:633–43. ix. [DOI] [PubMed] [Google Scholar]


