Abstract
Rationale:
Several studies using diffusion tensor tractography (DTT) have reported on injury in the dentato-rubro-thalamic tract (DRTT) in patients with brain injury. However, there is no study of injury in the DRTT following cerebellar infarct. We report on patients with injury in the DRTT following cerebellar infarct, demonstrated on DTT.
Patient concerns:
Three patients with cerebellar infarct were enrolled in this study. Diffusion tensor imaging data were acquired at 3 weeks (patient 1) and 2 weeks (patients 2 and 3) after onset and the DRTT was reconstructed. The Scale for Assessment and Rating of Ataxiaand the Functional Ambulation Category were used for evaluation of ataxia and gait function.
Diagnoses and Outcomes:
With clinical evaluation, patient 1 scored 18, patient 2 scored 22, and patient 3 scored 28 points on the Scale for Assessment and Rating of Ataxia. On the Functional Ambulation Category patient 1 scored 2, patient 2 scored 2, and patient 3 scored 1 point. DRTT abnormalities were as follows: discontinuation (the upper portion of the left DRTT in the patient 1), narrowing (the lower portion of the left DRTT in patient 2, and the whole right DRTT in the patient 3), and nonreconstruction (the left DRTT in the patient 3).
Lessons:
Using DTT, we demonstrated injury in the DRTT in 3 patients with severe ataxia following cerebellar infarct. We believe that evaluation of the DRTT would be helpful in patients who develop ataxia following cerebellar infarct.
Keywords: ataxia, cerebellar infarct, dentato-rubro-thalamic tract, diffusion tensor imaging
1. Introduction
Cerebellar infarct is uncommon, approximately 2% to 3% in stroke patients. It can cause movement disorders, including tremor, ataxia, and incoordination, because the main role of the cerebellum is movement control by communicating between the cerebrum and cerebellum via the dentato-rubro-thalamic tract (DRTT) and cortico-ponto-cerebellar tract.[1–5] In particular, ataxia is typically defined as a lack of voluntary coordinated movements on hand, leg, and trunk, and it usually occurs after cerebellar injury; however, precise cause of the ataxia is not fully understood. Among causes of the ataxia, injury in the DRTT, a major efferent pathway from the deep cerebellar nuclei to the brainstem and thalamus, is suggested as a major pathogenetic mechanism of ataxia.[6–9] Therefore, in terms of the diagnosis, examination of the DRTT would be important in patients with ataxia following cerebellar infarct. However, research on the DRTT has been limited in the live human brain due to anatomical features of a long, multisynapse, low discrimination with adjacent neural tracts that cross to the opposite side.[7,9]
Recently developed diffusion tensor tractography (DTT), which is derived from diffusion tensor imaging (DTI), has enabled three-dimensional reconstruction and estimation of the microstructural integrity of white matter including the DRTT.[10,11] Several studies using DTI have reported that injury in the DRTT commonly accompanies ataxia in patients with brain injury.[12–15] However, there is limited understanding on study on injury in the DRTT following cerebellar infarct.
In this study, we report 3 patients with injury in the DRTT following cerebellar infarct, demonstrated on DTT.
2. Methods
2.1. Subjects
We recruited 3 patients (male: 1, female: 2, mean age: 64.7 ± 10.7 years, range: 50–75 years) with cerebellar infarct who were admitted to the rehabilitation department of a university hospital. Inclusion criteria for patients were as follows: first ever stroke; an infarct is located in the cerebellum, as confirmed by a neuroradiologist; DTI scanning was performed at an early stage (between 2 and 3 weeks) after the stroke showed ataxia and gait disturbance after the stroke; and no severe apraxia and somatosensory problems (<22 points [full mark: 24] on the subscale for kinesthetic sensation of the Nottingham Sensory Assessment). The patients provided signed, informed consent, and the study protocol was approved by Yeungnam University Hospital institutional review board.
2.2. Clinical evaluation
The Scale for Assessment and Rating of Ataxia (SARA, 0–40 points: a higher score indicates a worse state) and the Functional Ambulation Category (FAC, 0–5 points: a lower score indicates a worse state) were administered to assess ataxia and gait function, respectively.[16,17]
2.3. Diffusion tensor imaging
DTI data were acquired at 3 weeks (patient 1) and 2 weeks (patients 2 and 3) after their strokes using a 6-channel head coil on a 1.5T Philips Gyroscan Intera (Philips, Ltd, Best, the Netherlands) with single-shot echo-planar imaging. For each of the 32 noncollinear diffusion sensitizing gradients, 70 contiguous slices were acquired parallel to the anterior commissure–posterior commissure line. Imaging parameters were as follows: acquisition matrix = 96 × 96; reconstructed to matrix = 192 × 192; field of view = 240 × 240 mm2; repetition time = 10,398 ms; echo time = 72 ms; b = 1000 s/mm2; and a slice thickness of 2.5 mm. Affine multiscale two-dimensional registration at the Oxford Centre for Functional Magnetic Resonance Imaging of Brain (FMRIB) Software Library was used for correction of head motion effect and image distortion.[10,18] Fiber tracking was performed using a probabilistic tractography method based on a multifiber model, and applied in the present study utilizing tractography routines implemented in FMRIB Diffusion (5000 streamline samples, 0.5 mm step lengths, curvature thresholds = 0.2). For the reconstruction of the DRTT, the seed region of interest (ROI) was placed on the dentate nucleus behind the floor of the fourth ventricle on the coronal image.[11] Two target ROIs were given at the junction of the superior cerebellar peduncle between the upper pons and cerebellum on the coronal image and the contralateral red nucleus of the upper midbrain on the axial image.[11] A threshold of 2 streamlines was applied for the results of fiber tracking.
3. Results
The demographic and clinical data for 3 patients are summarized in Table 1. With clinical evaluation, patient 1 scored 18, patient 2 scored 22, and patient 3 scored 28 points on the SARA. On the FAC patient 1 scored 2, patient 2 scored 2, and patient 3 scored 1 point. DRTT abnormalities were discontinuation (the upper portion of the left DRTT in the patient 1), narrowing (the lower portion of the left DRTT in patient 2, and the entire right DRTT in the patient 3), and nonreconstruction (the left DRTT in the patient 3) (see Fig. 1).
Table 1.
Demographic and clinical characteristics.

Figure 1.
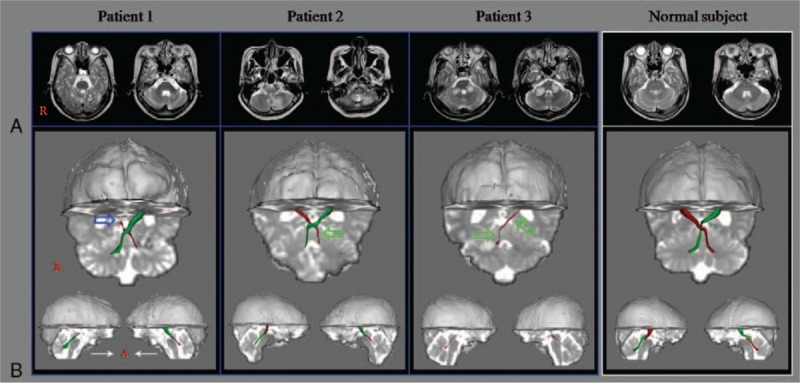
(A) T2-weighted brain MR images at 3 weeks (patient 1) and 2 weeks (patients 2 and 3) after onset show infarct on the left (patients 1 and 2) and both (patient 3) hemispheres cerebellum. (B) Results of DTT. Compared to a normal control, the abnormalities of the DRTT of the patients were discontinuation (the upper portion of the left DRTT in the patient 1 [blue arrow]), narrowing (the lower portion of the left DRTT in patient 2 [green arrow], and the whole right DRTT in the patient 3 [green arrows]), and nonreconstruction (the left DRTT in the patient 3). DRTT = dentato-rubro-thalamic tract, DTT = diffusion tensor tractography, MR = magnetic resonance.
4. Discussion
In this study, we demonstrated injury in the DRTT in 3 patients with severe ataxia following the cerebellar infarct. DRTT injuries were discontinuation (the upper portion of the left DRTT in the patient 1), narrowing (the lower portion of the left DRTT in patient 2, and the whole right DRTT in the patient 3), and nonreconstruction (the left DRTT in the patient 3). Therefore, it appears that ataxia in 3 patients was at least in part attributable to injury in the DRTT. We believe that our results suggest the necessity of evaluation of the DRTT in patients with ataxia after cerebellar infarct.
Several studies using DTI reported on injury in the DRTT in patients following brain injury.[12–15] In 2014, Akhlaghi et al[12] described an injured DRTT by abnormal DTT parameters, including lower fractional anisotropy and higher mean diffusivity in 12 patients with Friedreich ataxia compared with 14 normal controls. During the next year, Marek et al[13] described 6 patients with ataxia and tremor who had injuries of the cerebello-thalamic portion of the DRTT.[13] Jang and Kwon[14] ascribed thinning of the DRTT in the right hemisphere to a patient's ataxia and tremor [SARA: 12 points] following mild traumatic brain injury. In 2015, Schulz et al[15] reported concurrent injuries of the cortico-ponto-cerebellar tract and DRTT related to residual motor function in 26 patients with chronic ischemic stroke.[15] To the best of our knowledge, this is the first study to demonstrate injury in the DRTT in patients with cerebellar infarct and suggest injured DRTT is one of the causes of the ataxia. Therefore, clinicians should consider injury of the DRTT in patients with ataxia following various brain injuries, particularly lesions on the pathways of the DRTT such as thalamus and pontine. However, several limitations should be considered. First, this study is a case report. Second, we could not investigate the degree of ataxia by the state of the DRTT. Third, results of probabilistic DTT might be affected by false positives and negative effects due to the presence of kissing fibers in a voxel or partial volume effect throughout the brain.[19,20] Therefore, we suggest that further studies including large numbers of patients to overcome the limitations should be encouraged.
5. Conclusions
Using DTT, we demonstrated injury in the DRTT in 3 patients with severe ataxia following cerebellar infarct. We believe that evaluation of the DRTT would be helpful in patients with ataxia following cerebellar infarct.
Footnotes
Abbreviations: DRTT = dentato-rubro-thalamic tract, DTI = diffusion tensor imaging, DTT = diffusion tensor tractography, FAC = Functional Ambulation Category, ROI = region of interest, SARA = Scale for Assessment and Rating of Ataxia.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1D1A4A01020385).
The authors have no conflicts of interest to disclose.
References
- [1].Sypert GW, ALvord EC., Jr Cerebellar infarction. A clinicopathological study. Arch Neurol 1975;32:357–63. [DOI] [PubMed] [Google Scholar]
- [2].Macdonell RA, Kalnins RM, Donnan GA. Cerebellar infarction: natural history, prognosis, and pathology. Stroke 1987;18:849–55. [DOI] [PubMed] [Google Scholar]
- [3].Kase CS, Norrving B, Levine SR, et al. Cerebellar infarction. Clinical and anatomic observations in 66 cases. Stroke 1993;24:76–83. [DOI] [PubMed] [Google Scholar]
- [4].Javalkar V, Khan M, Davis DE. Clinical manifestations of cerebellar disease. Neurol Clin 2014;32:871–9. [DOI] [PubMed] [Google Scholar]
- [5].Datar S, Rabinstein AA. Cerebellar infarction. Neurol Clin 2014;32:979–91. [DOI] [PubMed] [Google Scholar]
- [6].Lehericy S, Grand S, Pollak P, et al. Clinical characteristics and topography of lesions in movement disorders due to thalamic lesions. Neurology 2001;57:1055–66. [DOI] [PubMed] [Google Scholar]
- [7].Afifi AK, Bergman RA. Functional Neuroanatomy: Text and Atlas. 2nd ed.New York, NY: Lange Medical Books/McGraw-Hill; 2005. [Google Scholar]
- [8].Marx JJ, Iannetti GD, Thomke F, et al. Topodiagnostic implications of hemiataxia: an MRI-based brainstem mapping analysis. Neuroimage 2008;39:1625–32. [DOI] [PubMed] [Google Scholar]
- [9].Mendoza JE, Foundas AL. Clinical Neuroanatomy: A Neurobehavioral Approach. New York/London: Springer; 2007. [Google Scholar]
- [10].Behrens TE, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 2007;34:144–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kwon HG, Hong JH, Hong CP, et al. Dentatorubrothalamic tract in human brain: diffusion tensor tractography study. Neuroradiology 2011;53:787–91. [DOI] [PubMed] [Google Scholar]
- [12].Akhlaghi H, Yu J, Corben L, et al. Cognitive deficits in Friedreich ataxia correlate with micro-structural changes in dentatorubral tract. Cerebellum 2014;13:187–98. [DOI] [PubMed] [Google Scholar]
- [13].Marek M, Paus S, Allert N, et al. Ataxia and tremor due to lesions involving cerebellar projection pathways: a DTI tractographic study in six patients. J Neurol 2015;262:54–8. [DOI] [PubMed] [Google Scholar]
- [14].Jang SH, Kwon HG. Injury of the dentato-rubro-thalamic tract in a patient with mild traumatic brain injury. Brain Inj 2015;29:1725–8. [DOI] [PubMed] [Google Scholar]
- [15].Schulz R, Frey BM, Koch P, et al. Cortico-cerebellar structural connectivity is related to residual motor output in chronic stroke. Cereb Cortex 2017;27:635–45. [DOI] [PubMed] [Google Scholar]
- [16].Cunha IT, Lim PA, Henson H, et al. Performance-based gait tests for acute stroke patients. Am J Phys Med Rehabil 2002;81:848–56. [DOI] [PubMed] [Google Scholar]
- [17].Weyer A, Abele M, Schmitz-Hubsch T, et al. Reliability and validity of the scale for the assessment and rating of ataxia: a study in 64 ataxia patients. Mov Disord 2007;22:1633–7. [DOI] [PubMed] [Google Scholar]
- [18].Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004;23Suppl. 1:S208–19. [DOI] [PubMed] [Google Scholar]
- [19].Fillard P, Descoteaux M, Goh A, et al. Quantitative evaluation of 10 tractography algorithms on a realistic diffusion MR phantom. Neuroimage 2011;56:220–34. [DOI] [PubMed] [Google Scholar]
- [20].Yamada K, Sakai K, Akazawa K, et al. MR tractography: a review of its clinical applications. Magn Reson Med Sci 2009;8:165–74. [DOI] [PubMed] [Google Scholar]


