Abstract
The aim of this study was to analyze the anatomical characteristics of patients with ruptured abdominal aortic aneurysms (AAAs) using computed tomography (CT) aortography in order to determine the risk factors for rupture.
We retrospectively reviewed the CT aortography findings and medical records of patients with ruptured AAAs who underwent CT aortography between February 2002 and December 2014. Age, sex, blood pressure at the time of rupture, treatment methods used for the ruptured AAAs, and treatment outcomes were analyzed. Statistical analyses were performed to determine the association between the maximum aneurysm diameter, which is considered the standard predictor of aneurysm rupture, and anatomical characteristics such as proximal neck diameter, angle between the suprarenal aorta and the aneurysm neck (α angle), angle between the aneurysm neck and aneurysm sac (β angle), and angles between the abdominal aorta and both iliac arteries.
Data were reviewed for a total of 36 patients. The mean maximum diameter of AAAs was 76.84 ± 21.08 mm. Multivariate analysis adjusted for age and sex indicated statistical correlations between the α and β angles and maximum aneurysm diameter and between the β angle and iliac artery involvement.
Our results suggest that the tortuosity of the aorta tends to be associated with the diameter of AAAs and iliac artery involvement. Investigation of the anatomical characteristics of individual patients using CT aortography is expected to aid in predicting the risk of AAA rupture.
Keywords: 3-dimensional CT angiography, abdominal aortic aneurysm, precipitating factors, ruptured aortic aneurysm
1. Introduction
The prevalence of abdominal aortic aneurysms (AAAs) is increasing with a concomitant increase in the mortality rate, which is approximately 65% to 85% for cases of rupture.[1] Considering this high mortality rate, estimation of the risk factors for AAA rupture is important for preventing aneurysm-related mortality without an unnecessary increase in the rate of intervention.
The maximum diameter of AAA is a well-known standard predictor of rupture. The American Association for Vascular Surgery and the Society for Vascular Surgery recommend intervention if AAA enlarges by 5 mm per year, is >55 mm in diameter, or is symptomatic.[2] However, the risk of rupture of AAAs with a maximum diameter of >55 mm is not equal to or directly proportional to the increment in the diameter. Moreover, the rupture of AAAs with a maximum diameter of <55 mm has been reported, whereas giant AAAs measuring >10 cm have been found to remain intact.[1,2] This indicates that additional parameters should be considered as risk factors for AAA rupture.
From a biomechanical standpoint, rupture is found to occur when the stress exerted on the aneurysm wall exceeds its failure strength.[3] Therefore, to overcome the limitations associated with the use of the maximum AAA diameter as a standard for predicting AAA rupture and to estimate the rupture risk more accurately, studies have examined the effects of mechanical wall stress and revealed features that affect AAA mechanics, such as tortuosity, asymmetry, presence of intraluminal thrombi, and wall thickness.[4–9]
The mechanical wall stress of the abdominal aorta can be used to predict AAA rupture and appears to be more accurate than the maximum AAA diameter.[5] Several studies have assessed the predictive value of biomechanical parameters, with a particular focus on mechanical wall stress.[3,5,10,11] However, the estimation of mechanical wall stress is not feasible or commonly available in clinical practice.
Computed tomography (CT) aortography has become a standard imaging modality for the diagnosis of and treatment planning for AAAs. Therefore, if more detailed and accurate predictors can be individually determined for patients using CT aortography before AAA rupture, more appropriate treatment plans can be charted. According to the computational evaluation of the risk for AAA rupture, the geometric characteristics of aneurysms, such as the AAA neck angle, neck length, diameter, and angle, have been reported as significant predictors of aneurysm rupture.[3,12–14] These anatomical characteristics may be major local hemodynamic factors that affect mechanical wall stress and tend to affect the increase in the AAA diameter. Although several studies have evaluated AAA rupture, few have evaluated the anatomical characteristics of ruptured AAAs.[15] Accordingly, we conducted the present study to determine the relationship between the anatomical characteristics of ruptured AAAs and the maximum AAA diameter, which is the current standard predictor of rupture, using CT aortography.
2. Methods
2.1. Patient selection
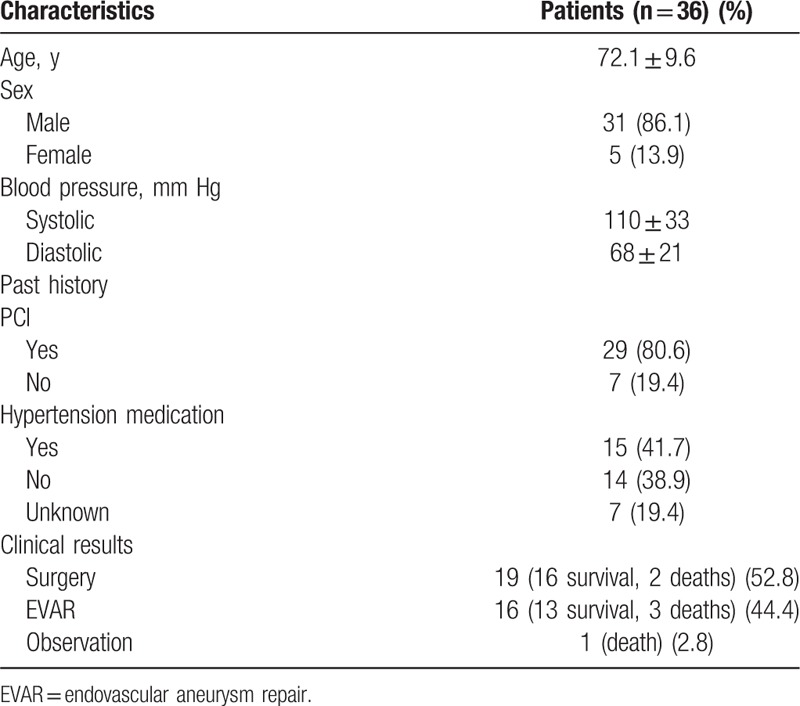
This retrospective study was approved by the institutional review board of our hospital, who waived the need for informed consent because of the retrospective study design (IRB No. 2014-04-001-001). Between February 2002 and December 2014, 39 patients with ruptured AAAs underwent CT aortography. Among these patients, 2 exhibited AAAs involving the suprarenal abdominal aorta and 1 presented with AAA rupture after endovascular repair. The remaining 36 patients exhibited AAAs involving the infrarenal abdominal aorta and reported no history of surgical or endovascular treatment. We included the CT aortography data of these 36 patients in our retrospective analysis. The clinical characteristics of each patient, including age, sex, initial systolic and diastolic blood pressures recorded in the emergency room, treatment methods for the ruptured AAAs, and final treatment outcomes, were documented. Table 1 summarizes the characteristics of the study group. Before analysis, all patient information was anonymized and deidentified.
Table 1.
Clinical characteristics of 36 patients with ruptured abdominal aortic aneurysms.
2.2. Image analysis
All images were evaluated by consensus between 2 radiologists experienced in the diagnosis of aortic aneurysms and interpretation of aortic imaging findings. On the basis of the renal artery ostium, they measured the suprarenal angle (α angle), defined as the angle between the flow axis of the suprarenal aorta and the flow axis of the AAA neck, and the infrarenal angle (β angle), defined as the angle between the flow axis of the aneurysm neck and the flow axis of the AAA sac, on 3-dimensional (3D) volume-rendered images.[11] The angles of the abdominal aorta, right common iliac artery, and left common iliac artery were also measured. RI was recorded as the angle between the abdominal aorta and right common iliac artery, LI as the angle between the abdominal aorta and left common iliac artery, and BI as the angle between both common iliac arteries on the volume-rendered images. The major and minor axes were measured at the point of the maximum AAA diameter on axial CT aortography images, and the cross-sectional diameter difference was calculated from the major and minor axes at the point of the maximum AAA diameter (Fig. 1). The presence of unilateral or bilateral involvement of the common iliac artery was recorded. Sites of contrast extravasation in the ruptured AAAs were assessed on axial images and the quadrant was recorded.
Figure 1.
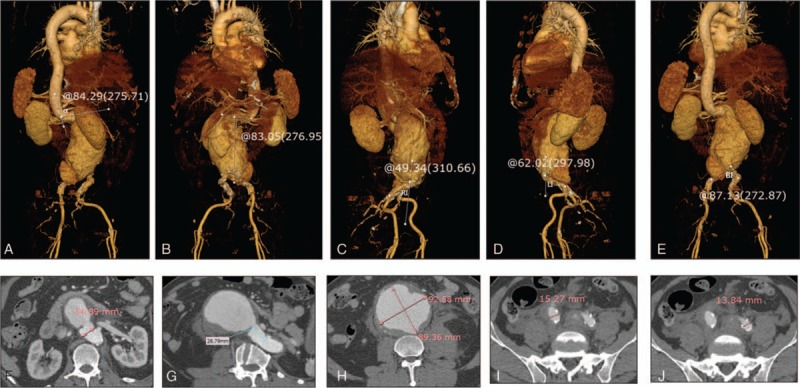
Measurement of anatomical characteristics of ruptured abdominal aortic aneurysms (AAAs) on computed tomography (CT) aortography. Images are reconstructed by Rapidia 2.8 software (Infinite, Seoul, Korea). (A–E) α angle (A), β angle (B), RI∗ (C), LI† (D), and BI‡ (E) are measured on 3-dimensional (3D) volume-rendered images. (F) Proximal neck diameter is measured on an axial image. (G) Proximal neck length is measured on a curved multiplanar reconstruction image. (H) Major and minor axes at the point of the maximum AAA diameter are measured on an axial image. (I, J) Right (I) and left (J) common iliac artery diameters are measured on an axial CT image. ∗Angle between right iliac artery and abdominal aorta, †Angle between left iliac artery and abdominal aorta, ‡Angle between both iliac arteries.
2.3. Statistical analyses
We assessed the relationships between the initial blood pressure values and clinical outcomes using logistic regression analysis. Univariate analyses were performed to determine the correlations between the major axis of AAAs at the point of the maximum diameter and anatomical characteristics, including the α angle, β angle, AAA neck diameter, proximal neck length, RI, LI, and BI. The correlations between the cross-sectional diameter difference and the abovementioned anatomical characteristics were also determined. Then, multivariate linear regression analyses adjusted for age and sex were performed for factors found to be significant in the univariate analyses. The relationship between iliac artery involvement and the aortic axis (α angle, β angle) and the relationship between the anatomical characteristics and the quadrant of the rupture site were statistically assessed using general linear models. We also performed a comparative analysis of CT data obtained before and after AAA rupture and determined the correlations between the major axis of AAA at the point of the maximum diameter and the α and β angles before and after AAA rupture. Kruskal–Wallis tests were used to analyze the correlation between the β angle and iliac artery involvement before and after AAA rupture. Finally, we performed correlation analysis to access the relationship between the maximum AAA diameter and interval change before and after AAA rupture. A P value of <.05 was considered statistically significant. All statistical analyses were performed using SAS software (ver. 9.2; SAS institute Inc., Cary, NC).
3. Results
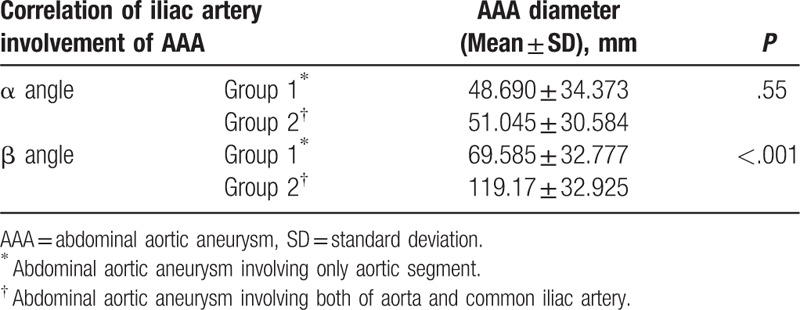
In total, there were 31 men and 5 women with an age range of 49 to 89 (mean, 72.1) years. Thirty of the 36 patients survived after treatment for AAA, and the remaining 6 succumbed to hypovolemic shock or multiorgan failure within 2 weeks after treatment. Three of the 30 survivors died within 3 months of follow-up; 2 died from pneumonia [endovascular aneurysm repair (EVAR) in one and surgery in one] and 1 from metabolic acidosis (surgery). The remaining 27 patients survived for more than 3 months after treatment. Seven of these patients have been alive for more than 30 months after treatment (EVAR in 2 and surgery in 5), while the remaining 20 were lost to follow-up. Among the 36 patients, 30 showed involvement of only a segment of the aorta, 5 showed uni-iliac involvement, and 1 showed bi-iliac involvement. The anatomical characteristics of the 36 patients as observed on CT aortography are summarized in Table 2. Logistic regression analysis showed that neither systolic nor diastolic blood pressure at the initial visit to the emergency room was a significant factor that affected mortality (P = .64 for systolic pressure and P = .86 for diastolic pressure). The findings of statistical analyses of the anatomical characteristics are summarized in Tables 3 and 4. According to linear regression analysis, the major axis of AAA at the point of the maximum diameter was significantly correlated with the α (P < .001) and β (P = .021; Table 3) angles. General linear model analysis revealed a positive correlation between the β angle and iliac artery involvement (P < .001; Table 4). The proximal neck length and neck diameter showed no significant correlation with the major axis of AAA at the point of the maximum diameter, and there was no correlation between the cross-sectional diameter difference and any of the anatomical characteristics. Furthermore, RI, LI, and BI showed no significant correlation with the major axis of AAA at the point of the maximum diameter, and there was no significant correlation between the location of AAA rupture and the α angle, β angle, and major and minor axis differences at the point of the maximum diameter. Only 11 of the 36 patients had available CT data obtained before AAA rupture. Correlation analyses in these 11 patients revealed no significant correlation between the major axis of AAA at the point of the maximum diameter and both α and β angles before and after AAA rupture. The Kruskal–Wallis test showed no correlation between the β angle and iliac artery involvement in these 11 patients. A significant correlation was found between the maximum AAA diameter and interval change (correlation coefficient, 0.9681; P < .001).
Table 2.
Anatomical characteristics of 36 patients with ruptured abdominal aortic aneurysms on CT angiography.
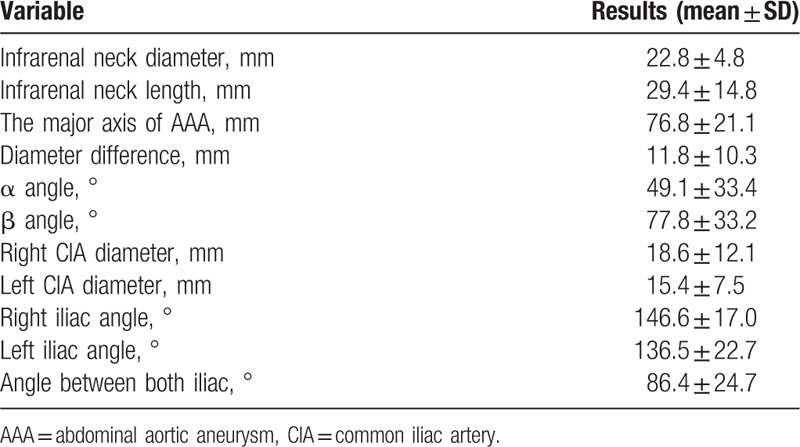
Table 3.
Univariate and multivariate linear regression of correlation of anatomic characteristics on the CT aortography with major axis of the AAAs at the point of maximum AAA diameter.
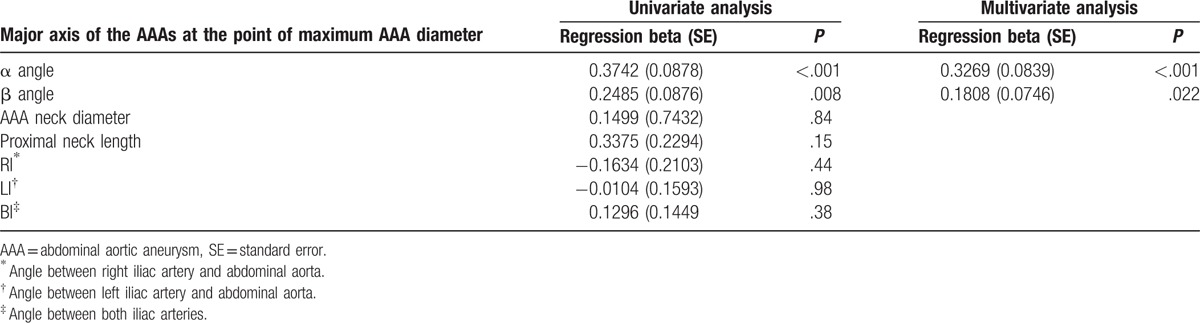
Table 4.
Correlation of iliac artery involvement in AAA with the α and β angles.
4. Discussion
In the present study, we determined the relationship between the anatomical characteristics of ruptured AAAs and the maximum AAA diameter, which is the current standard predictor of rupture, using CT aortography. Although the maximum AAA diameter is considered a standard predictor of AAA rupture, there are some limitations concerning its sole use for the prediction of rupture.[3,5,16,17] Mechanical wall stress is also a well-known predictor of AAA rupture.[3,5,18,19] Several factors affect mechanical wall stress, such as locoregional pressure, aortic wall stiffness, and the anatomical characteristics of the aorta. This mechanical wall stress is possibly a trigger for an increase in the aortic diameter, which results in AAA formation. However, estimation of mechanical wall stress is not feasible or commonly available in clinical practice. Recently, CT aortography has become the standard diagnostic modality for aortic diseases, including AAA. Using this modality, it is easy to acquire images; moreover, the sophisticated anatomical characteristics of the aorta are effectively reflected. Accordingly, we analyzed the anatomical characteristics of the aorta in patients with ruptured AAAs to evaluate any correlation with the maximum AAA diameter on CT aortography, with a focus on the estimation of mechanical wall stress.
We found that both the α and β angles correlated with the maximum AAA diameter, with a positive correlation between the β angle and common iliac artery involvement. These findings suggest that aortic tortuosity is associated with an increase in the AAA diameter and may affect iliac artery involvement. Therefore, aortic tortuosity is a potential risk factor for aneurysm wall stress, and increased tortuosity is expected to be associated with AAA rupture.
Unlike the results of previous studies reporting the computational evaluations of the risk of AAA rupture, our results showed that the AAA neck angle, AAA neck length, and angles between the aorta and both iliac arteries exhibited no significant correlation with the maximum AAA diameter on CT aortography.[6,12] We speculate that the differences in results between the previous studies and our study were caused by the sole use of geometric parameters in the computational evaluations and the omission of detailed anatomical characteristics as observed on CT aortography. In general, AAA geometry has to be relatively uniform, and blood pressure is also uniform in computational evaluations. This results in a gap between computational simulations and actual phenomena. In the present study, we focused on specific anatomical characteristics as observed on CT aortography. First, we excluded evaluations of intraluminal thrombi as an anatomical characteristic. Some studies have considered intraluminal thrombi to prevent AAA rupture, while others have asserted a positive role for intraluminal thrombi in AAA rupture.[20–22] Because of these arguments, we excluded any effects of intraluminal thrombi on AAA rupture in our study. In addition, specific anatomical characteristics of the abdominal aorta, such as vessel diameters and angles, were evaluated. However, patient-specific data regarding the endogenous characteristics of the aortic wall were not considered. Moreover, patient-specific data regarding the distribution of mechanical wall stress in AAA and failure strength or susceptibility to rupture were not considered. Although these patient-specific data could be important factors for AAA rupture, practically, such data could not be acquired in the present study. Another limitation of the study is the small sample size. In general, the vital signs of patients with ruptured AAAs are very unstable because of which all such patients cannot undergo CT aortography. Therefore, the total number of included patients was relatively small in our study. Annual expansion rate of the AAA diameter is a well-known important risk factor of AAA rupture. In this study, our analysis focused on the anatomic characteristic of the present CT aortography. Therefore, we could not perform analysis on the annual expansion rate of maximum AAA diameter as a risk factor caused by small sample size. To overcome this limitation, further study could be needed with a large sample size.
In conclusion, our results suggest that the tortuosity of the aorta tends to be associated with the diameter of AAAs and iliac artery involvement. Although the maximum AAA diameter is a standard predictor of rupture and the annual diameter expansion rate is also an important predictor, we believe that investigation of the anatomical characteristics of individual patients using CT aortography will aid in the more accurate prediction of rupture and overcome the limitations associated with the sole use of the maximum AAA diameter as a predictor of rupture.
Footnotes
Abbreviations: 3D = three-dimensional, AAA = abdominal aortic aneurysm, CIA = common iliac artery, CT = computed tomography.
Funding/support: This study was supported by intramural research promotion grants from Ewha Womans University School of Medicine.
The authors report no conflicts of interest.
References
- [1].Moxon JV, Parr A, Emeto TI, et al. Diagnosis and monitoring of abdominal aortic aneurysm: current status and future prospects. Curr Probl Cardiol 2010;35:512–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brewster DC, Cronenwett JL, Hallett JW, Jr, et al. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg 2003;37:1106–17. [DOI] [PubMed] [Google Scholar]
- [3].Georgakarakos E, Ioannou CV, Papaharilaou Y, et al. Computational evaluation of aortic aneurysm rupture risk: what have we learned so far? J Endovasc Ther 2011;18:214–25. [DOI] [PubMed] [Google Scholar]
- [4].Bonert M, Leask RL, Butany J, et al. The relationship between wall shear stress distributions and intimal thickening in the human abdominal aorta. Biomed Eng Online 2003;2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fillinger MF, Marra SP, Raghavan ML, et al. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg 2003;37:724–32. [DOI] [PubMed] [Google Scholar]
- [6].Kleinstreuer C, Li Z. Analysis and computer program for rupture-risk prediction of abdominal aortic aneurysms. Biomed Eng Online 2006;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Munasinghe A, Brar M, Faiz O. Mortality from ruptured abdominal aortic aneurysms. Lancet 2014;383:2123. [DOI] [PubMed] [Google Scholar]
- [8].Taylor CA, Hughes TJ, Zarins CK. Finite element modeling of three-dimensional pulsatile flow in the abdominal aorta: relevance to atherosclerosis. Ann Biomed Eng 1998;26:975–87. [DOI] [PubMed] [Google Scholar]
- [9].Thubrikar MJ, Robicsek F, Labrosse M, et al. Effect of thrombus on abdominal aortic aneurysm wall dilation and stress. J Cardiovasc Surg (Torino) 2003;44:67–77. [PubMed] [Google Scholar]
- [10].Khosla S, Morris DR, Moxon JV, et al. Meta-analysis of peak wall stress in ruptured, symptomatic and intact abdominal aortic aneurysms. Br J Surg 2014;101:1350–7. [DOI] [PubMed] [Google Scholar]
- [11].Truijers M, Pol JA, Schultzekool LJ, et al. Wall stress analysis in small asymptomatic, symptomatic and ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 2007;33:401–7. [DOI] [PubMed] [Google Scholar]
- [12].Xenos M, Alemu Y, Zamfir D, et al. The effect of angulation in abdominal aortic aneurysms: fluid-structure interaction simulations of idealized geometries. Med Biol Eng Comput 2010;48:1175–90. [DOI] [PubMed] [Google Scholar]
- [13].Li Z, Kleinstreuer C. Effects of blood flow and vessel geometry on wall stress and rupture risk of abdominal aortic aneurysms. J Med Eng Technol 2006;30:283–97. [DOI] [PubMed] [Google Scholar]
- [14].Brady AR, Thompson SG, Fowkes FG, et al. Abdominal aortic aneurysm expansion: risk factors and time intervals for surveillance. Circulation 2004;110:16–21. [DOI] [PubMed] [Google Scholar]
- [15].Fillinger MF, Racusin J, Baker RK, et al. Anatomic characteristics of ruptured abdominal aortic aneurysm on conventional CT scans: implications for rupture risk. J Vasc Surg 2004;39:1243–52. [DOI] [PubMed] [Google Scholar]
- [16].Moll FL, Powell JT, Fraedrich G, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg 2011;41suppl 1:S1–58. [DOI] [PubMed] [Google Scholar]
- [17].Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. The UK Small Aneurysm Trial Participants. Lancet 1998;352:1649–55. [PubMed] [Google Scholar]
- [18].Vorp DA, Raghavan ML, Webster MW. Mechanical wall stress in abdominal aortic aneurysm: influence of diameter and asymmetry. J Vasc Surg 1998;27:632–9. [DOI] [PubMed] [Google Scholar]
- [19].Georgakarakos E, Ioannou CV, Kamarianakis Y, et al. The role of geometric parameters in the prediction of abdominal aortic aneurysm wall stress. Eur J Vasc Endovasc Surg 2010;39:42–8. [DOI] [PubMed] [Google Scholar]
- [20].Speelman L, Schurink GW, Bosboom EM, et al. The mechanical role of thrombus on the growth rate of an abdominal aortic aneurysm. J Vasc Surg 2010;51:19–26. [DOI] [PubMed] [Google Scholar]
- [21].Vorp DA, Mandarino WA, Webster MW, et al. 3rd potential influence of intraluminal thrombus on abdominal aortic aneurysm as assessed by a new non-invasive method. Cardiovasc Surg 1996;4:732–9. [DOI] [PubMed] [Google Scholar]
- [22].Georgakarakos E, Ioannou CV, Volanis S, et al. The influence of intraluminal thrombus on abdominal aortic aneurysm wall stress. Int Angiol 2009;28:325–33. [PubMed] [Google Scholar]


