Abstract
Fas-mediated T-cell death is known to occur during human immunodeficiency virus (HIV) infection. In this study, we found that HIV type 1 LAI (HIV-1LAI) primes CD8+ T cells from healthy donors for apoptosis, which occurs after Fas ligation. This effect is counteracted by a broad caspase inhibitor (zVAD-fmk). Fas-mediated cell death does not depend on CD8+ T-cell infection, because it occurred in the presence of reverse transcriptase inhibitors. However, purified CD8+ T cells are sensitive to Fas only in the presence of soluble CD4. Finally, we found that interleukin 7 (IL-7) increases Fas-mediated CD4+ and CD8+ T-cell death induced by HIV-1LAI. Since high levels of IL-7 are a marker of poor prognosis during HIV infection, our data suggest that enhancement of Fas-mediated T-cell death by HIV-1LAI and IL-7 is one of the mechanisms involved in progression to AIDS.
During human immunodeficiency virus type 1 (HIV-1) and pathogenic simian immunodeficiency virus infections, T cells exhibit increased spontaneous apoptosis and activation-induced cell death in vitro (2, 3, 10, 16, 26, 37) and in vivo (14, 29, 32). T cells from HIV-1-infected persons show enhanced cell surface expression of Fas and increased sensitivity to Fas-mediated cell death induced either by an agonistic anti-Fas antibody or by a soluble Fas ligand (4, 11, 13, 17, 22, 31). Such cell death has been reported for CD4+ and CD8+ T cells. Whereas CD4+ T-cell loss is a continuous phenomenon during natural HIV infection, CD8+ T cells are progressively depleted during the late stages of the infection, especially when X4 HIV strains are present (39). Since a large number of investigators have suggested that CD8-mediated immune responses play a key role in controlling HIV infections, understanding the mechanisms involved in CD8+ T-cell depletion is of major interest. Several reports have mentioned that HIV-1 may cause CD8+ T-cell death in vitro (9, 18, 46). Vlahakis et al. (46) showed, using gp120, the direct killing of CD8+ T cells that could not be blocked by anti-Fas agonists. Esser et al. (9), using whole inactivated viruses, reported an increase in both Fas expression and cell death after 10 days of culture but did not study Fas sensitivity.
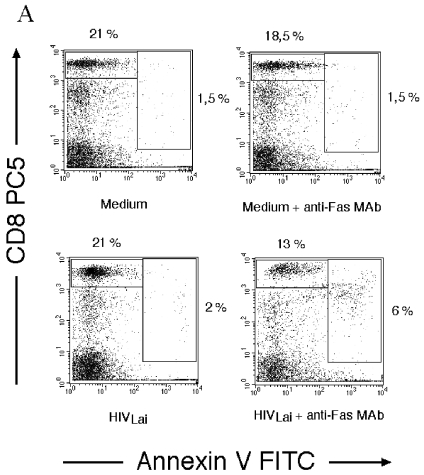
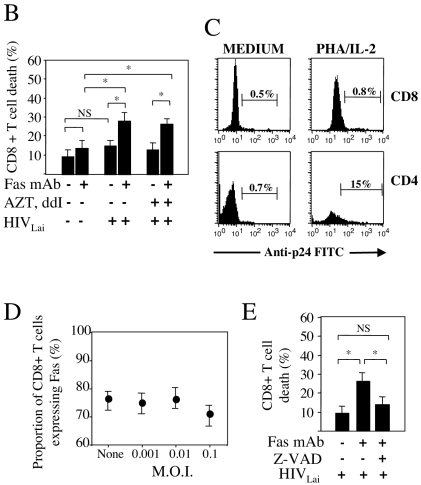
We have previously reported that the incubation of quiescent peripheral blood mononuclear cells (PBMC) from healthy donors in vitro with HIVLAI for 5 days in medium alone, in the absence of any additional T-cell stimuli, leads to the rapid death of CD4+ T cells in response to subsequent antibody-mediated Fas ligation (12, 38). Here we found that HIV-1LAI also primes unstimulated CD8+ T cells from healthy donors to undergo cell death in response to Fas ligation (Fig. 1A and B). A similar percentage of cells died in response to Fas ligation when PBMC were treated with a combination of the antiretroviral drugs (reverse transcriptase inhibitors) dideoxyinosine (ddI) and zidovudine (AZT) before and during incubation with HIV-1LAI. This indicates that the priming of CD8+ T cells for Fas-mediated death does not require productive HIV-1 infection. To confirm that CD8+ T cells were not infected with HIV, we performed three-color flow cytometry, using an anti-p24 monoclonal antibody (MAb) (KC57-fluorescein isothiocyanate [FITC]; Beckman Coulter). Activated CD4+ T cells (Fig. 1C), but not unstimulated or activated CD8+ T cells, were positive for p24. In contrast to what was observed with CD4+ T cells (12), the proportion of CD8+ T cells expressing Fas, as determined by flow cytometry, did not change even when the HIV inoculum was increased (Fig. 1D). This suggests that HIVLAI sensitizes CD8+ T cells to Fas-mediated death independently of its effect on Fas expression. We also found that CD8+ T-cell death in response to Fas ligation was dependent on caspase activation. Indeed, preincubation with the broad caspase inhibitor zVAD-fmk for 1 h prior to Fas antibody treatment significantly reduced, in a dose-dependent manner, Fas-mediated CD8+ T cell-death (Fig. 1E); this effect lasted for at least 36 h.
FIG. 1.
HIV-1LAI mediates priming of quiescent CD8+ T cells for Fas-induced cell death. (A) PBMC were incubated for 5 days in the absence (mock, medium) or presence of HIV-1LAI (MOI of 0.01). On day 5, cells were incubated for a further 18 h in the absence or presence of an agonistic anti-Fas IgM MAb (1 μg/ml). The percentage of dying CD8+ T cells was assessed by using two-color flow cytometry, using PC5-labeled anti-CD8 MAb (Beckman Coulter) and FITC-labeled annexin V (Beckman Coulter) (12). The percentages of living (CD8+ annexin−) and dying (CD8+ annexin+) CD8+ T cells are indicated on the dot plot. (B) PBMC were incubated as in (A) for 5 days in the absence (−) or presence (+) of HIV-1LAI (MOI of 0.01) and in the absence (−) or presence (+) of a combination of the reverse transcriptase inhibitors ddI (0.5 μM) and AZT (1 μM), added at two time points (before addition of HIV-1LAI and 2 days later), and further incubated for 18 h in the absence (−) or presence (+) of an agonistic Fas IgM MAb (1 μg/ml). The percentage of dying CD8+ T cells was calculated as follows: [CD8+ annexin+ cells/(CD8+ annexin− cells + CD8+ annexin+ cells)] × 100. (C) PBMC were incubated with HIV-1LAI (MOI of 0.01) and either stimulated with phytohemagglutinin (10 μg/ml) and IL-2 (10 U/ml) (Chiron Corporation) (PHA/IL-2) or left unstimulated (MEDIUM). Intracellular anti-p24 staining (FITC-labeled KC57 MAb; Beckman Coulter) was gated on CD4+ and CD8+ T cells. Three-color flow cytometry was performed, using PC5-labeled anti-CD8 and APC-labeled anti-CD4. Histograms show the percentage of p24+ cells in each population. (D) PBMC from healthy donors were incubated as in (A) in the absence (None) or presence of HIV-1LAI at different MOIs (0.001, 0.01, or 0.1) for 5 days in medium alone. Fas expression on CD8+ T cells was analyzed by two-color flow cytometry using PC5-labeled anti-CD8 and FITC-labeled anti-Fas MAb (UB2; Beckman Coulter). (E) PBMC incubated with HIVLAI (MOI of 0.01) as in (A) were further incubated in the absence (−) or presence (+) of agonistic anti-Fas MAb and in the absence (−) or presence (+) of the broad caspase inhibitor zVAD-fmk (20 μM). The percentage of dying CD8+ T cells was assessed as in (B). Statistical significance was assessed by the Student t test (*, P < 0.05; NS, no significant difference).
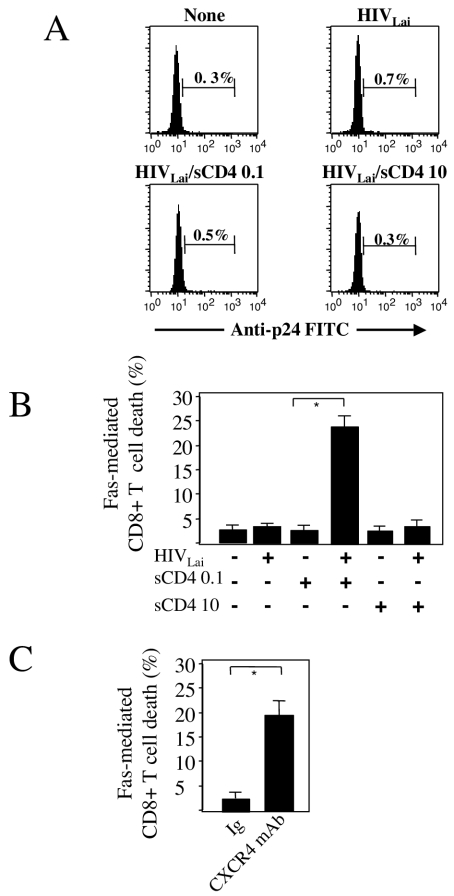
Other cell populations, namely macrophages or CD4+ T cells, seem to be required for HIV-mediated CD8+ T cell death (18, 31, 44). We next explored whether purified CD8+ T cells are sensitive to Fas ligation. Unlike PBMC, purified CD8+ T cells—obtained by negative selection using anti-CD19, -CD56, -CD4, and -CD14 MAbs (Beckman Coulter) and magnetic beads coated with antimouse immunoglobulin G (IgG) (Dynals)—incubated with HIV-1LAI (multiplicity of infection [MOI] of 0.01) were not sensitized to Fas-mediated cell death (Fig. 2B). This suggests that the effect of HIV-1LAI on CD8+ T cells depends on the presence of an additional factor(s). It has been reported that a low dose (0.1 μg/ml) of soluble CD4 alters the conformation of the gp120 envelope glycoprotein, facilitating its interaction with its chemokine receptor and subsequently the entry of virus, even in the absence of cell surface CD4 molecules (43). Thus, we incubated purified CD8+ T cells with different concentrations of human soluble recombinant CD4 (sCD4). Preincubation of HIV-1LAI for 1 h with 0.1 μg of sCD4/ml primed CD8+ T cells for Fas-mediated cell death, whereas 10 μg/ml had no effect (Fig. 2B). sCD4 was initially reported to block viral entry when used at a concentration of 10 μg/ml (8). Fas sensitization mediated by a low dose of soluble CD4 is not associated with viral infection (Fig. 2A). As we observed that sCD4 facilitates Fas-mediated CD8+ T-cell death, we then assessed whether engagement of CXCR4 sensitizes CD8+ T cells to undergo apoptosis following Fas ligation. Using a specific anti-CXCR4 MAb (12G5; R&D System), we observed Fas-mediated CD8+ T-cell death (Fig. 2C). These results indicate that after 5 days in culture, whole particles of HIV-1LAI are able to sensitize not only CD4+ T cells to undergo caspase-dependent death following Fas ligation, as we previously described (12, 38), but also CD8+ T cells after the interaction of CXCR4 with Env.
FIG. 2.
HIV-1LAI requires soluble CD4 for Fas sensitization of quiescent purified CD8+ T cells. (A) CD8+ T cells were purified as previously reported (12) and incubated for 5 days in the absence or presence of HIV-1LAI (MOI of 0.01). HIV-1LAI was also pretreated for 1 h with either 0.1 μg (HIV-1LAI/sCD4 0.1) or 10 μg (HIV-1LAI/sCD4 10) of soluble recombinant CD4/ml. On day 5, flow cytometry was performed with FITC-labeled KC57. Histograms show the percentages of p24+ cells among CD8+ T cells. (B) Purified unstimulated CD8+ T cells were incubated (+) or not (−) with HIV-1LAI (MOI of 0.01) with ddI (0.5 μM) and AZT (1 μM). HIV-1LAI was pretreated (+) or not (−) for 1 h with either 0.1 μg (sCD4 0.1) or 10 μg (sCD4 10) of soluble recombinant CD4/ml. On day 5, an agonistic anti-Fas IgM MAb (1 μg/ml) was added as for Fig. 1A. Dying cells were quantified by flow cytometry using FITC-labeled annexin V. Note the increase in cell death in comparison to results with medium alone. (C) Purified unstimulated CD8+ T cells were incubated either with a plate-immobilized CXCR4-specific MAb (10 μg, 12G5; R&D System) or an isotype control (Ig). On day 5, the cells were incubated with an agonistic anti-Fas IgM MAb (1 μg/ml). Note the increase in cell death in comparison to results with medium alone. All results are means ± standard deviations for three independent experiments, each performed in triplicate. Statistical significance was assessed by the Student t test (*, P < 0.05).
It is generally accepted that the cytokine environment can modulate Fas-mediated cell death (11, 22, 30). In humans, interleukin 7 (IL-7) is involved in normal T lymphopoiesis but also acts as a survival factor for peripheral T lymphocytes (15, 28). IL-7 and IL-2 prevent activated T-cell death in vitro via a signal thought to be mediated by their common gamma chain receptor (45) inducing Bcl-2 expression (1). IL-2 and IL-7 are candidates or are already used for immunotherapy during HIV infection (5, 25, 36). IL-7 is a key cytokine in HIV pathogenesis and is associated with an unfavorable prognosis (27, 33, 34). Serum IL-7 concentrations are inversely correlated with CD4+ and CD8+ T-cell counts during HIV infection (6, 27, 34). Whether increased levels of IL-7 are the cause or the consequence of CD4+ depletion is not clear. In vitro treatment of naive T cells with IL-7 may favor the replication of X4 and R5 HIV strains (7, 41, 42) and the emergence of X4 variants (27, 40). Despite these harmful in vitro effects, exogenous IL-7 has been proposed as a therapeutic adjuvant in HIV infection to stimulate T-cell renewal (23), and IL-7 is considered a good candidate for immunotherapy for AIDS (35).
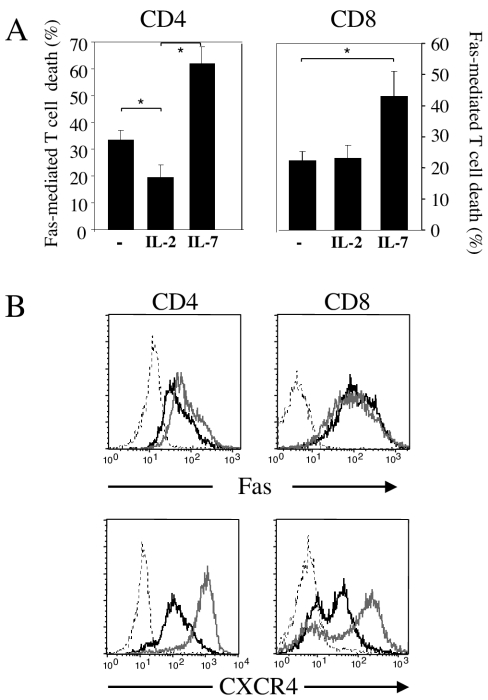
We then compared the effects of these cytokines on Fas priming of CD4+ and CD8+ T cells. IL-2 or IL-7 was added to each medium on day 1, and PBMC were incubated with HIV-1LAI as described above in the presence of antiretroviral drugs to prevent cytokine-mediated viral replication. Our data revealed that IL-7 enhances HIV-1LAI-induced Fas sensitization of both CD4+ and CD8+ T cells (Fig. 3A). In contrast, IL-2 reduced HIV-1LAI-induced Fas sensitization of CD4+ T-cell death, whereas it had no effect on CD8+ T-cell death (Fig. 3A). We assessed the expression of Fas and CXCR4 by flow cytometric analysis of purified CD4+ and CD8+ T cells incubated with either IL-2 or IL-7. Our data revealed that differences in Fas sensitivity in response to IL-2 and IL-7 are related to a difference in CXCR4 but not in Fas expression (Fig. 3B). Previous studies have also shown that IL-7 sensitizes pro-B cells (24) and recent thymic emigrants (21) for Fas-mediated cell death. While IL-7 may inhibit spontaneous apoptosis by increasing the level of the antiapoptotic Bcl-2, Bcl-2 has a poor preventive effect on Fas-mediated T cell death (19, 20). Therefore, the fact that IL-7 is capable of increasing HIVLAI-mediated Fas-induced T-cell death is not a new assertion and is not in contradiction with previous published reports on the antiapoptotic effects of this cytokine.
FIG. 3.
IL-7 but not IL-2 increases HIV-1LAI-mediated priming of quiescent T cells for Fas-induced cell death. (A) PBMC were incubated as for Fig. 1 in the presence of HIV-1LAI (MOI of 0.01) and with ddI (0.5 μM) and AZT (1 μM). IL-2 or IL-7 (20 ng/ml) was added to each medium, and on day 5, PBMC were further incubated with the agonistic anti-Fas IgM MAb (1 μg/ml). The percentages of dying CD4+ and CD8+ T cells were assessed by flow cytometry, using FITC-labeled annexin V and PC5-labeled anti-CD8 or CD4 MAb after 18 h and calculated as for Fig. 1B. All results are means ± standard deviations for three independent experiments, each performed in triplicate. (B) Purified CD4+ and CD8+ T cells were treated with either IL-2 (black lines) or IL-7 (gray lines) (20 ng/ml). The cells were analyzed after 5 days in culture. The expression of Fas and CXCR4 was assessed by flow cytometry, using FITC-labeled Fas MAb (Beckman Coulter) and phycoerythrin-labeled CXCR4 MAb (R&D System). An isotype control is also shown (dotted lines). Histograms are representative of three experiments that gave similar results. Statistical significance was assessed by the Student t test (*, P < 0.05).
In conclusion, our results demonstrate the role of Fas sensitization of uninfected CD8+ T lymphocytes during HIV infection. We also provide new data suggesting that IL-7 has a deleterious effect on HIV pathogenesis and that exogenous IL-7 must be used with caution during HIV infection.
Acknowledgments
J.-D.L. was supported by a fellowship from ANRS (Agence Nationale de Recherche contre le Sida), and F.P. was supported by a fellowship from ECS (Ensemble contre le Sida).
REFERENCES
- 1.Akashi, K., M. Kondo, U. Von Freeden-Jeffry, R. Murray, and I. Weissman. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89:1033-1041. [DOI] [PubMed] [Google Scholar]
- 2.Ameisen, J., and A. Capron. 1991. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol. Today 12:102-105. [DOI] [PubMed] [Google Scholar]
- 3.Arnoult, D., F. Petit, J. Lelievre, D. Lecossier, A. Hance, V. Monceaux, R. Ho Tsong Fang, B. Hurtrel, J. Ameisen, and J. Estaquier. 2003. Caspase-dependent and -independent T-cell death pathways in pathogenic simian immunodeficiency virus infection: relationship to disease progression. Cell Death Differ. 10:1240-1252. [DOI] [PubMed] [Google Scholar]
- 4.Baumler, C. B., T. Bohler, I. Herr, A. Benner, P. H. Krammer, and K. M. Debatin. 1996. Activation of the CD95 (APO-1/Fas) system in T cells from human immunodeficiency virus type-1-infected children. Blood 88:1741-1746. [PubMed] [Google Scholar]
- 5.Chehimi, J., J. D. Marshall, O. Salvucci, I. Frank, S. Chehimi, S. Kawecki, D. Bacheller, S. Rifat, and S. Chouaib. 1997. IL-15 enhances immune functions during HIV infection. J. Immunol. 158:5978-5987. [PubMed] [Google Scholar]
- 6.Chiappini, E., L. Galli, C. Azzari, and M. de Martino. 2003. Interleukin-7 and immunologic failure despite treatment with highly active antiretroviral therapy in children perinatally infected with HIV-1. J. Acquir. Immune Defic. Syndr. 33:601-604. [DOI] [PubMed] [Google Scholar]
- 7.Dardalhon, V., S. Jaleco, S. Kinet, B. Herpers, M. Steinberg, C. Ferrand, D. Froger, C. Leveau, P. Tiberghien, P. Charneau, N. Noraz, and N. Taylor. 2001. IL-7 differentially regulates cell cycle progression and HIV-1-based vector infection in neonatal and adult CD4+ T cells. Proc. Natl. Acad. Sci. USA 98:9277-9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deen, K. C., J. S. McDougal, R. Inacker, G. Folena-Wasserman, J. Arthos, J. Rosenberg, P. J. Maddon, R. Axel, and R. W. Sweet. 1988. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature 331:82-84. [DOI] [PubMed] [Google Scholar]
- 9.Esser, M. T., J. W. Bess, Jr., K. Suryanarayana, E. Chertova, D. Marti, M. Carrington, L. O. Arthur, and J. D. Lifson. 2001. Partial activation and induction of apoptosis in CD4(+) and CD8(+) T lymphocytes by conformationally authentic noninfectious human immunodeficiency virus type 1. J. Virol. 75:1152-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estaquier, J., T. Idziorek, F. de Bels, F. Barre-Sinoussi, B. Hurtrel, A. M. Aubertin, A. Venet, M. Mehtali, E. Muchmore, P. Michel, et al. 1994. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc. Natl. Acad. Sci. USA 91:9431-9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estaquier, J., T. Idziorek, W. Zou, D. Emilie, C. M. Farber, J. M. Bourez, and J. C. Ameisen. 1995. T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J. Exp. Med. 182:1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estaquier, J., J. D. Lelievre, F. Petit, T. Brunner, L. Moutouh-De Parseval, D. D. Richman, J. C. Ameisen, and J. Corbeil. 2002. Effects of antiretroviral drugs on human immunodeficiency virus type 1-induced CD4+ T-cell death. J. Virol. 76:5966-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estaquier, J., M. Tanaka, T. Suda, S. Nagata, P. Golstein, and J. C. Ameisen. 1996. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood 87:4959-4966. [PubMed] [Google Scholar]
- 14.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 15.Fry, T. J., E. Connick, J. Falloon, M. M. Lederman, D. J. Liewehr, J. Spritzler, S. M. Steinberg, L. V. Wood, R. Yarchoan, J. Zuckerman, A. Landay, and C. L. Mackall. 2001. A potential role for interleukin-7 in T-cell homeostasis. Blood 97:2983-2990. [DOI] [PubMed] [Google Scholar]
- 16.Gougeon, M. L., S. Garcia, J. Heeney, R. Tschopp, H. Lecoeur, D. Guetard, V. Rame, C. Dauguet, and L. Montagnier. 1993. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res. Hum. Retrovir. 9:553-563. [DOI] [PubMed] [Google Scholar]
- 17.Gougeon, M. L., H. Lecoeur, A. Dulioust, M. G. Enouf, M. Crouvoiser, C. Goujard, T. Debord, and L. Montagnier. 1996. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J. Immunol. 156:3509-3520. [PubMed] [Google Scholar]
- 18.Herbein, G. M., F. Batliwata, P. Gregersen, T. Pappas, J. Butler, W. A. O'Brien, and E. Verdin. 1998. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV with chemokine receptor CXCR4. Nature 395:189-194. [DOI] [PubMed] [Google Scholar]
- 19.Huang, D. C., M. Hahne, M. Schroeter, K. Frei, A. Fontana, A. Villunger, K. Newton, J. Tschopp, and A. Strasser. 1999. Activation of Fas by FasL induces apoptosis by a mechanism that cannot be blocked by Bcl-2 or Bcl-x(L). Proc. Natl. Acad. Sci. USA 96:14871-14876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, D. C., J. Tschopp, and A. Strasser. 2000. Bcl-2 does not inhibit cell death induced by the physiological Fas ligand: implications for the existence of type I and type II cells. Cell Death Differ. 7:754-755. [DOI] [PubMed] [Google Scholar]
- 21.Jaleco, S., L. Swainson, V. Dardalhon, M. Burjanadze, S. Kinet, and N. Taylor. 2003. Homeostasis of naive and memory CD4+ T cells: IL-2 and IL-7 differentially regulate the balance between proliferation and Fas-mediated apoptosis. J. Immunol. 171:61-68. [DOI] [PubMed] [Google Scholar]
- 22.Katsikis, P. D., E. S. Wunderlich, C. A. Smith, and L. A. Herzenberg. 1995. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J. Exp. Med. 181:2029-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komschlies, K., K. Grzegorzewski, and R. Wiltrout. 1995. Diverse immunological and hematological effects of interleukin 7: implications for clinical application. J. Leukoc. Biol. 58:623-633. [DOI] [PubMed] [Google Scholar]
- 24.Levy, Y., K. Benlagha, A. Buzyn, M. Colombel, J. Brouet, and K. Lassoued. 1997. IL-7 sensitizes human pre-B cells but not pro-B cells to Fas/APO-1 (CD95)-mediated apoptosis. Clin. Exp. Immunol. 110:329-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy, Y., C. Capitant, S. Houhou, I. Carriere, J. P. Viard, C. Goujard, J. A. Gastaut, E. Oksenhendler, L. Boumsell, E. Gomard, C. Rabian, L. Weiss, J. G. Guillet, J. F. Delfraissy, J. P. Aboulker, M. Seligmann, et al. 1999. Comparison of subcutaneous and intravenous interleukin-2 in asymptomatic HIV-1 infection: a randomised controlled trial. Lancet 353:1923-1929. [DOI] [PubMed] [Google Scholar]
- 26.Lewis, D. E., D. S. Tang, A. Adu-Oppong, W. Schober, and J. R. Rodgers. 1994. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J. Immunol. 153:412-420. [PubMed] [Google Scholar]
- 27.Llano, A., J. Barretina, A. Gutierrez, J. Blanco, C. Cabrera, B. Clotet, and J. A. Este. 2001. Interleukin-7 in plasma correlates with CD4 T-cell depletion and may be associated with emergence of syncytium-inducing variants in human immunodeficiency virus type 1-positive individuals. J. Virol. 75:10319-10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackall, C. L., T. J. Fry, C. Bare, P. Morgan, A. Galbraith, and R. E. Gress. 2001. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood 97:1491-1497. [DOI] [PubMed] [Google Scholar]
- 29.Monceaux, V., J. Estaquier, M. Fevrier, M. Cumont, Y. Riviere, A. Aubertin, J. Ameisen, and B. Hurtrel. 2003. Extensive apoptosis in lymphoid organs during primary SIV infection predicts rapid progression towards AIDS. AIDS 17:1585-1596. [DOI] [PubMed] [Google Scholar]
- 30.Mueller, Y., V. Makar, P. Bojczuk, J. Witek, and P. Katsikis. 2003. IL-15 enhances the function and inhibits CD95/Fas-induced apoptosis of human CD4+ and CD8+ effector-memory T cells. Int. Immunol. 15:49-58. [DOI] [PubMed] [Google Scholar]
- 31.Mueller, Y. M., S. C. De Rosa, J. A. Hutton, J. Witek, M. Roederer, J. D. Altman, and P. D. Katsikis. 2001. Increased CD95/Fas-induced apoptosis of HIV-specific CD8(+) T cells. Immunity 15:871-882. [DOI] [PubMed] [Google Scholar]
- 32.Muro-Cacho, C. A., G. Pantaleo, and A. S. Fauci. 1995. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J. Immunol. 154:5555-5566. [PubMed] [Google Scholar]
- 33.Muthukumar, A., A. Wozniakowsli, M. Gauduin, M. Palardini, H. McClure, R. Johnson, G. Silvestri, and D. Sodora. 2004. Elevated interleukin-7 levels not sufficient to maintain T-cell homeostasis during simian immunodeficiency virus-induced disease progression. Blood 103:973-979. [DOI] [PubMed] [Google Scholar]
- 34.Napolitano, L. A., R. M. Grant, S. G. Deeks, D. Schmidt, S. C. De Rosa, L. A. Herzenberg, B. G. Herndier, J. Andersson, and J. M. McCune. 2001. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat. Med. 7:73-79. [DOI] [PubMed] [Google Scholar]
- 35.Nugeyre, M., V. Monceaux, S. Beq, M. Cumont, R. Ho Tsong Fang, L. Chene, M. Morre, F. Barre-Sinoussi, B. Hurtrel, and N. Israel. 2003. IL-7 stimulates T cell renewal without increasing viral replication in simian immunodeficiency virus-infected macaques. J. Immunol. 171:4447-4453. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto, Y., D. Douek, R. McFarland, and R. Koup. 2002. Effects of exogenous interleukin-7 on human thymus function. Blood 99:2851-2858. [DOI] [PubMed] [Google Scholar]
- 37.Oyaizu, N., T. W. McCloskey, M. Coronesi, N. Chirmule, V. S. Kalyanaraman, and S. Pahwa. 1993. Accelerated apoptosis in peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus type-1 infected patients and in CD4 cross-linked PBMCs from normal individuals. Blood 82:3392-3400. [PubMed] [Google Scholar]
- 38.Petit, F., J. Corbeil, J. D. Lelievre, L. Moutouh-de Parseval, G. Pinon, D. R. Green, J. C. Ameisen, and J. Estaquier. 2001. Role of CD95-activated caspase-1 processing of IL-1β in TCR-mediated proliferation of HIV-infected CD4(+) T cells. Eur. J. Immunol. 31:3513-3524. [DOI] [PubMed] [Google Scholar]
- 39.Roederer, M., J. Dubs, M. Anderson, P. Raju, L. Herzenberg, and L. Herzenberg. 1995. CD8 naive T cell counts decrease progressively in HIV-infected adults. J. Clin. Investig. 95:2061-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmitt, N., L. Chene, D. Boutolleau, M. Nugeyre, E. Guillemard, P. Versmisse, C. Jacquemot, F. Barre-Sinoussi, and N. Israel. 2003. Positive regulation of CXCR4 expression and signaling by interleukin-7 in CD4+ mature thymocytes correlates with their capacity to favor human immunodeficiency X4 virus replication. J. Virol. 77:578-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smithgall, M. D., J. G. Wong, K. E. Critchett, and O. K. Haffar. 1996. IL-7 up-regulates HIV-1 replication in naturally infected peripheral blood mononuclear cells. J. Immunol. 156:2324-2330. [PubMed] [Google Scholar]
- 42.Steffens, C., E. Managlia, A. Landay, and L. Al-Harthi. 2002. Interleukin-7-treated naive T cells can be productively infected by T-cell-adapted and primary isolates of human immunodeficiency virus 1. Blood 99:3310-3318. [DOI] [PubMed] [Google Scholar]
- 43.Sullivan, N., Y. Sun, Q. Sattentau, M. Thali, D. Wu, G. Denisova, J. Gershoni, J. Robinson, J. Moore, and J. Sodroski. 1998. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J. Virol. 72:4694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tateyama, M., N. Oyaizu, T. W. McCloskey, S. Than, and S. Pahwa. 2000. CD4 T lymphocytes are primed to express Fas ligand by CD4 cross-linking and to contribute to CD8 T-cell apoptosis via Fas/FasL death signaling pathway. Blood 96:195-202. [PubMed] [Google Scholar]
- 45.Vella, A. T., S. Dow, T. A. Potter, J. Kappler, and P. Marrack. 1998. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc. Natl. Acad. Sci. USA 95:3810-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vlahakis, S., A. Algeciras-Schimnich, G. Bou, C. Heppelmann, A. Villasis-Keever, R. Collman, and C. Paya. 2001. Chemokine-receptor activation by env determines the mechanism of death in HIV-infected and unifected T lymphocytes. J. Clin. Investig. 107:207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]