Abstract
The difference of PD-L1 expression between only HPV-positive patients and premalignant cervical lesion patients did not be reported in present studies. And to test the PD-L1 expression in some cervical cell lesion studies using cervical exfoliated cells sample also was ignored. Meanwhile, the PD-L1 expression as a predictive biomarker still existed controversy. So in the study, first to compare the expression of PD-L1 between only HPV-positive patients and premalignant cervical lesion patients, then to research the association between PD-L1 and HPV status, lastly to explore the possible prognostic value for HPV treatment in premalignant cervical lesion patients.
Cervical exfoliated cells samples of 54 premalignant cervical lesion patients with HPV16 infection were collected; meanwhile the cervical exfoliated cells samples from 20 healthy women without HPV infection and 20 patients with only HPV16 infection but cervical cytology normal were collected as 2 control groups. Flow-through hybridization and gene chip (FHGC) was used to detect the HPV type, the PD-L1 expression was tested by Flow cytometry analysis, the methylation-sensitive high-resolution melting (MS-HRM) was used to test the HPV16 L1 gene methylation. The 54 premalignant cervical lesion patients were followed up in 18 months to assess the prognostic value of PD-L1 for HPV treatment.
The PD-L1 positive cell rate and mean fluorescence intensity of PD-L1 positive cell in premalignant cervical lesion patients with HPV16 infection were higher than 2 control groups. Mean fluorescence intensity of PD-L1 positive cell were increased in 54 cases when existing multiple HPV status and high HPV16-L1 gene methylation (L1 gene methylation more than 50%). High PD-L1 expression (PD-L1 positive cell rate more than 10%), high HPV16-L1 gene methylation, and multiple HPV infection status could prolong the time to clean HPV infection by Kaplan–Meier analysis. Multivariate Cox proportional hazards analysis also showed that all of high PD-L1 expression, high HPV-L1 methylation, and multiple HPV infection status should increase the risk of HPV unclearance in premalignant cervical lesion patients; the hazard ratio (HR) was 2.043 (CI: 1.050–3.973), 2.797 (CI: 1.277–6.122), and 3.050 (CI: 1.406–6.615).
PD-L1 expression only was correction with HPV infection when the infection induced the cervical cells to create the lesion. PD-L1 was the risk factor of HPV unclearance in premalignant cervical lesion patients, so anti-PD-L1 therapy could be a potential effectiveness way of HPV infection in premalignant cervical lesion patients.
Keywords: cervical lesions, human papillomavirus, prognostic, programmed cell death 1 ligand 1
1. Introduction
Programmed cell death 1 ligand 1 (PD-L1) was a kind of protein on the cell surface, also called B7-H1 protein, which was edited by the CD274 gene. PD-L1 could combine with Programmed cell death protein 1 (PD-1) on the cell surface of effector T cells, then conduct immunosuppressive signals in order to inhibit the activity of immune effector T cells. PD-L1 expressed on the surface of many types cells, including placental cells, vascular endothelial cells, liver cells, epithelial cells, mesenchymal stem cells, B cells, T cells, dendritic cells, macrophages, and tumor cells. PD-L1 expression on the surface of tumor cells became the driving factor, which induced tumor cells to escape the attack of immune cells. The present study showed that there were 4 ways to upregulate the tumor cell PD-L1 expression, including the activation of EGFR, MAPK or PI3K-Akt pathway, high STAT3 and HIF-1proteins expression, the amplification of PD-L1 code gene (9p24.1, CD274 gene), the induce of EB virus (EB virus positive gastric cancer and nasopharyngeal cancer, existing the PD-L1 high expression without CD274 gene amplification), epigenetic regulation mechanisms such as DNA methylation.[1] Recent studies found that HPV as a kind of common virus was closely related to some tumors, its infection status was closely related to PD-L1 expression in some tumor cells such as tonsillar cancer,[2] but some researches also reported that there were no correlation between HPV infection and PD-L1 expression in some tumors.[3–4] Cervical cancer as a tumor had the most closely relation to HPV infection, the previous studies revealed that PD-L1 was a solid biomarker of productive HPV infection in the cervix,[5] and the overexpress of HPV16 E7 oncoprotein in the cervical epithelial carcinoma increased the expression level of the PD-L1 protein.[6] But all the reports first ignored the difference of PD-L1 between only HPV infection but cervical normal patients and HPV infection with cervical lesion patients; meanwhile, the samples of these studies were tissue specimens, neglecting the important of cervical exfoliated cells sample in cervical cell lesion study. At last, the previous studies did not include the relation between the specific HPV infection status and PD-L1 expression.
HPV infection was divided into the single infection and multiple infection, transient infection and persistent infection. Different HPV infection induced different changes of cervical cells. The L1 protein as the main capsid protein of HPV, played an important role to recognize the host cell and keep persistent infection, which was a good index to evaluate the infection state in host cells.[7] Previous studies showed that the quantity of L1 protein was declining with cervical cell lesion's severity. The L1 gene was the code gene of L1 protein; its methylation was the major reason of L1 protein low-expression, which showed positive correlation to the degree of cervical lesions.[8–9] L1 gene methylation deeply involved in the progression and heterogeneity of cervical lesions, which also was the good index to evaluate the HPV persistent infection state.
In the study, we completed the study using cervical exfoliated cells sample; we first compared the PD-L1 expression between HPV infection but cervical cytology normal patients and cervical lesion patients with HPV infection, and then explored the relation between the specific HPV infection status and PD-L1 expression. Finally, we studied the prognostic value for HPV infection, in order to clarify the value of PD-L1 test for cervical lesion patients with HPV infection.
2. Materials and methods
2.1. Sample source
The cervical exfoliated cells samples sourced from the women, who initially visited the gynecology department of the Tumor Hospital Affiliated to Xinjiang Medical University from January 2015 to June 2015. The chosen women must not accept any HPV related treatment and HPV vaccine. The samples with inadequate cervical exfoliated cells or a lot of blood contamination must be ruled out. At the same time to collect the results of HPV types and Thin Prep Cytologic Test (TCT)detection. First, 54 patients were collected, who were first detected premalignant cervical lesion with HPV16 infection (including 29 cases Atypical Squamous Cells of Undetermined Significance, 16 cases Lower-grade Squamous Intraepithelial Lesions, 9 cases Higher-grade Squamous Intraepithelial Lesions), their median age was 49 year-old, and all patients were followed up in next 18 months and re-registered after 3 months in every 2 follow-up time. Then, selected 20 healthy women without HPV infection and 20 cases with HPV16 infection without cell lesion as control, and their median age was 43 year-old. The ethics committee of the tumor hospital affiliated to Xinjiang Medical University approved the study procedure.
2.2. Reagents and instruments
The HPV genetype test used the 21 HPV GenoArray Diagnostic Kit from ChaoZhou Hybribio Biological Chemical Co. Ltd., People's Republic of China. The method of HPV genetype test was flow-through hybridization and gene chip (FHGC), the equipments for the test such as Thermal Cycler and HybriMax devices (Flow-through Hybridization HybriMax).
The L1 gene methylation levels were tested by the methylation-sensitive high resolution melting (MS-HRM). The major instrument was Roche LightCycler type 480 sensitivity analyzer. The completely methylated and unmethylated HPV-16 L1 gene standards of MS-HRM were synthesized (Genscript, Nanjing,China); the specific primers also were synthesized (Genscript, Nanjing,China). The EpiTect Bisulfite Kit and EpiTect HRM PCR Kit were bought from Germany QIAGEN company.
The PD-L1 flow cytometry analysis kit (BB515 Mouse Anti-Human CD274, Lot: 564554) and Isotype control reagent kit (BB515 Mouse BALB/c IgG1-κ, Lot: 564416) were from the BD BIOSCIENCES PHARMINGEN (USA); the flow cytometer was Beckman CytomicsTM FC500 (USA).
2.3. Experimental procedure
2.3.1. FHGC for the HPV genetype test
The HPV genetype test was carried out by the steps of HPV GenoArray Diagnostic Kit, which could detect 21 HPV genotypes, including 6, 11, 16, 18, 31, 33, 35, 39, 42, 43,44, 45, 51, 52, 53, 56, 58, 59, 66, and 68 types, and CP8304 types.
-
1.
Extracted the HPV viral DNA by the DNA extraction kit.
-
2.
Took 1 μL of extracted DNA solution and then did PCR amplification according to the instructions in the reaction system by PCR amplification.
-
3.
Made diversion hybridization amplification for amplified DNA samples.
-
4.
Made hybridization results analysis of hybrid membrane after coloration; corresponding color parts’ classification is the result.[10]
2.3.2. MS-HRM analysis of the HPV-16 L1 gene
The sequence HPV-16 L1 localized from nucleotide (nt) 5576 to (nt) 5636 (NCBI accession no. NC_001526.2), which contains 4 CpG sites (nt 5602, nt 5608, nt5611, nt 5617), was tested.
-
1.
Mixed the completely methylated and unmethylated HPV-16 L1 gene standards in 0%, 10%, 25%, 50%, 75%, and 100% methylated to unmethylated template ratios, which served as the methylation standards for MS-HRM.
-
2.
Extracted the HPV viral DNA by DNA extraction kit.
-
3.
The methylation standards and all extracted HPV viral DNA were bisulfite modified; the detailed steps referred to the instruction book of EpiTect Bisulfite Kit.
-
4.
The specific PCR primers used were that, Forward primer:5′ GCGCATTATTGTTGATGTAGGTGATTTTTATTTATATTTTAG3′, reverse prime: 5′: GCCGCACTAAACAACCAAAAAAACATCTAAAAAAAAATA 3′. The detailed steps of MS-HRM PCR referred to the handbook of EpiTect HRM PCR.
-
5.
The HRM data were analyzed using the Genescanning Software (Roche).[11]
2.3.3. Flow cytometry PD-L1 analysis
-
1.
Collected the exfoliative cytology specimens, which were in the cell preservation solution. Then, through the 300 mesh nylon mesh filter, 1500 r/s centrifugal for 10 minutes, discarded the liquid supernatant, and then repeated this process by adding PBS fluid to the sediment; finally, suspended the exfoliated cells with the 1 mL phosphate buffered saline (PBS) solution. Then, regulated cell concentration to 2–4 × 104/mL using the PBS solution.
-
2.
Added 5 μL BB515 Mouse Anti-Human CD274 reagent into the test tube; meanwhile, added 5 μL BB515 Mouse BALB/c IgG1-κinto the Isotype control tube. Then, respectively added 1 ml cell suspension solution into the test and control tubes, mixed the tube by mechanical oscillator. Incubated for 20 minutes in dark place. Lastly, tested the specimen by the FC500 flow cytometer. The example of PD-L1 test was in Figure 1.
Figure 1.
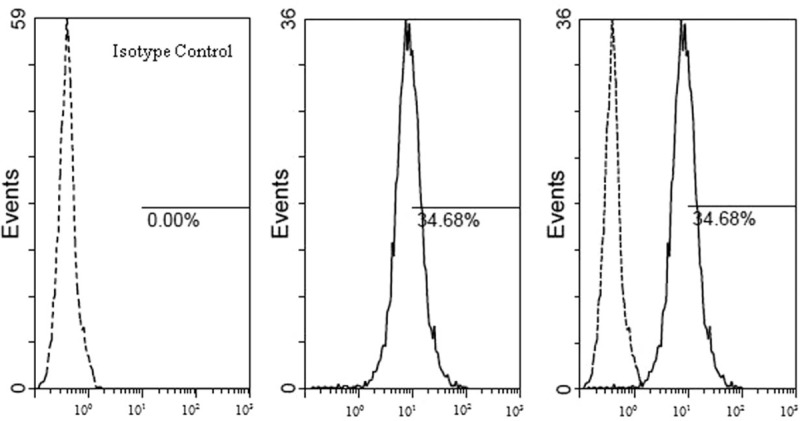
The detection results of PD-L1 (BB515 fluorescence label), in the example, the PD-L1 positive cell rate was 34.68%, mean fluorescence intensity of PD-L1 positive cell was 27.9. HPV = human papillomavirus, PD-L1 = programmed cell death 1 ligand 1.
2.4. Statistical analysis
Data were analyzed by SPSS software (version 18.0; SPSS, Inc., Chicago, IL). Data were expressed as the mean ± standard deviation. Differences between 2 groups were analyzed with Student's t test. Log-rank was used to compared the treatment time for HPV in different PD-L1, HPV L1 methylation, and HPV infection status subgroups. Multivariate Cox proportional hazards analysis explored the prognostic value of PD-L1, HPV16-L1 methylation, and HPV infection status. α=0.05 was the inspection level, and P<.05 was considered to indicate statistically significant differences.
3. Results
3.1. PD-L1 expression in the different cervical cell
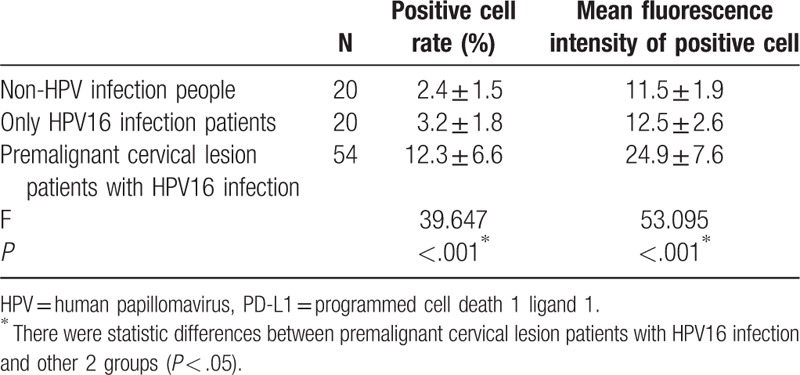
Table 1 showed the PD-L1 expression in 3 groups population, not only the PD-L1 positive cell rate but also mean fluorescence intensity of positive cell were compared in different population; the 2 indicators were highest in the group of premalignant cervical lesion patients with HPV16 infection.
Table 1.
Comparison of PD-L1 expression in 3 different populations.
3.2. The distribution of PD-L1 in different HPV16-L1 methylation subgroups and HPV16 infection status subgroups in 54 cases
The results of HPV16-L1 gene methylation were divided into 2 subgroups, including low methylation group (L1 gene methylation less than 50%) and high methylation group (L1 gene methylation more than 50%). The HPV infection included single HPV16 infection subgroup (only HPV16 infection) and multiple HPV16 infection subgroup (existing HPV16 infection and other HPV genotype infection at the same time). Compared to the PD-L1 positive cell rate and mean fluorescence intensity of PD-L1 positive cells between different subgroups, the result was shown in Figure 2; it showed that mean fluorescence intensity of PD-L1 positive cells was higher in high HPV16-L1 gene methylation or multiple HPV16 infection subgroups.
Figure 2.

Compared to the PD-L1-positive cell rate and mean fluorescence intensity of PD-L1-positive cells in different HPV16-L1 methylation subgroups and in different HPV16 infection subgroups. HPV = human papillomavirus, PD-L1 = programmed cell death 1 ligand 1.
3.3. Association of PD-L1 expression, HPV16-L1 gene methylation, HPV infection status, and the prognosis of HPV treatment in 54 cases premalignant cervical lesion patients
The high PD-L1 protein expression was determined the PD-L1 positive cell rate more than 10%. The low PD-L1 expression was defined as the PD-L1 positive cell rate less than 10%. In followed-up 18 months, 10 cases were censored. The Kaplan–Meier analysis compared to the HPV clean time in different subgroups. The result was shown in Figure 3. High PD-L1 expression, high HPV16-L1 gene methylation, and multiple HPV infection status could prolong the time to clean HPV infection.
Figure 3.
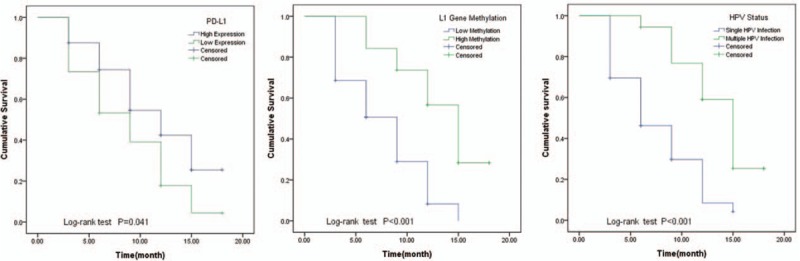
Compared to the HPV clean time in different subgroups by Log-rank analysis, the HPV clean time had statistical differences in different PD-L1 expressions, HPV16-L1 gene methylation, and HPV infection status subgroups (P < .05). HPV = human papillomavirus, PD-L1 = programmed cell death 1 ligand 1.
3.4. Multivariate Cox proportional hazards analysis explored the hazard ratio (HR) of PD-L1 expression, HPV16-L1 gene methylation, and HPV infection status to clean HPV infection in premalignant cervical lesion patients with HPV infection
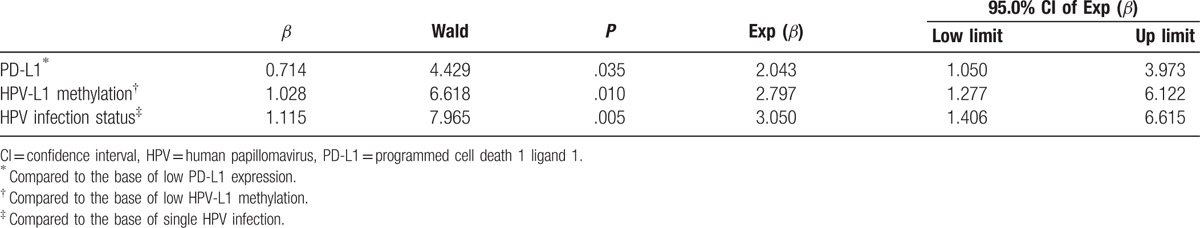
From Table 2, it was 2.043 time that the hazard of HPV infection untreatment in PD-L1 high expression than low expression in premalignant cervical lesion patients, also it was 2.797 time that the hazard of HPV infection untreatment in HPV-L1 high methylation than low methylation, 3.050 time that the hazard of HPV infection untreatment in multiple HPV infection than single infection.
Table 2.
Multivariate Cox proportional hazards analysis of HPV clearance.
4. Discussion
We conducted this study to examine the relationship between HPV infection and the PD-L1 expression of cervical exfoliated cell. We first showed that only HPV infection without lesion in the cervical cell did not create high PD-L1 expression, but the HPV infection with cervical lesion could increase the high-level expression of PD-L1. We also showed that the association of increased PD-L1 expression with high HPV-L1 gene methylation and multiple HPV infection status. At last, we showed that high PD-L1 expression, high HPV-L1 gene methylation, and multiple HPV infection status strengthened the risk of HPV infection uncleaned in premalignant cervical lesion patients with HPV infection.
The detection of PD-L1 was based the PD-L1 protein level on the cell membrane surface; therefore, the priority detection method was immunohistochemical (IHC) in clinical trials, the result of IHC overdependented on the subjective judgment of pathologistes.[12–13] The flow cytometric method (FCM) not only detected the percentage of PD-L1 positive cell, but also paid attention to the PD-L1 content of PD-L1 positive cell by the mean fluorescence intensity of PD-L1 positive cells. In tale 1, we could find that the positive cell rate and mean fluorescence intensity of PD-L1 positive cells were no differences between only HPV infection group and non-HPV infection group, but the 2 indicators were higher in premalignant cervical lesion with HPV infection group than only HPV infection group. So, we speculated that only HPV infection was not the key factor of PD-L1 expression; only when the HPV infection leads to the lesion of cervical cell, the PD-L1 expression became high. Kataoka et al[14] reported that HPV genome integrated into or around the PD-L1 gene locus, which could induce the high expression of PD-L1 through 3′-UTR disruption of PD-L1 gene. The mechanism should be a major way to explain the high PD-L1 expression in premalignant cervical lesion patients with HPV infection.
PD-L1 was higher expression in premalignant cervical lesion patients with HPV infection, so it was meaningful to explore the relation between PD-L1 expression and HPV infection in the population. HPV L1 gene methylation and HPV infection status were the major factors that HPV infection led to the lesion of cervical cell,[10,15–16] so in the study, we researched the relationship of PD-L1 expression with L1 gene methylation and HPV infection status. In Figure 2, it showed that PD-L1-positive cell rate did not increase along with the deepen of L1 gene methylation or HPV infection status, but mean fluorescence intensity of PD-L1 positive cells was increased along with the deepen of L1 gene methylation and HPV infection status. It proved that PD-L1 expression intensity was connection with HPV infection in PD-L1 positive cervical cells, so serious HPV infection could inflected the PD-L1 expression.
Mezache et al[5] speculated that the strong correlation of PD-L1 and CIN lesions raised the possibility that PD-L1 may have a role in the spontaneous clearance of HPV infections. In the study, we followed up the clearance time of HPV infection in premalignant cervical lesion patients with HPV infection. We found that high PD-L1 expression, high HPV L1 gene methylation, and multiple HPV infection status could increase the clearance time of HPV infection. High HPV L1 gene methylation means HPV persistent infection in cervical cell, multiple HPV infection status also created HPV persistent infection, all these prolonged the clearance time. PD-L1 was the ligand of PD-1, which was a primary immunosuppressive driver, PD-L1 overexpression may be an important facilitator for virus infection and tumor progress.[17–22] High PD-L1 expression could create an immunosuppressive condition in cervix uterus, which led the long time of HPV clearance.
Though PD-L1 expression as a predictive biomarker still existed controversy,[23–24] but in the study, multivariate Cox proportional hazards analysis showed that all high PD-L1 expression, high HPV-L1 methylation, and multiple HPV infection status were the risk factors of HPV clearance in premalignant cervical lesion patients; the hazard ratio (HR) was 2.043 (CI: 1.050–3.973), 2.797 (CI: 1.277–6.122), and 3.050 (CI: 1.406–6.615). Most of cervical lesion was derived from the HPV infection; multivariate analysis could take an adequate consideration between the virus infection and host cell immune state, so the result of Cox analysis could better evaluate the prognosis of HPV infection in premalignant cervical lesion patients.
In conclusion, we demonstrated that PD-L1 expression only was connection with HPV infection when inducing cervical cell lesion. We also explored that PD-L1 was the risk factor of HPV clearance in premalignant cervical lesion patients, so anti-PD-L1 therapy could be a potential effectiveness of HPV infection in premalignant cervical lesion patients.
Acknowledgments
The authors thank Professor Fang Xin-zhi and his colleagues for their assistance in providing the cytology specimens.
Footnotes
Abbreviations: 3′-UTR = the three prime untranslated region, CIN = cervical intraepithelial neoplasia, FCM = flow cytometry, FHGC = flow-through hybridization and gene chip, HPV = human papillomavirus, HR = hazard ratio, IHC = immunohistochemical, MS-HRM = methylation-sensitive high resolution melting, PD-1 = programmed cell death 1, PD-L1 = programmed cell death 1 ligand 1, TCT = thin prep cytologic test.
Funding Support: This research was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous.
Region, People's Republic of China, (No. 2015211C133).
The authors have no conflicts of interest to disclose.
References
- [1].Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med 2016;375:1767–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hong AM, Vilain RE, Romanes S, et al. PD-L1 expression in tonsillar cancer is associated with human papillomavirus positivity and improved survival: implications for anti-PD1 clinical trials. Oncotarget 2016;7:77010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim HS, Lee JY, Lim SH, et al. Association between PD-L1 and HPV status and the prognostic value of PD-L1 in oropharyngeal squamous cell carcinoma. Cancer Res Treat 2016;48:527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ottenhof SR, Djajadiningrat RS, de Jong J, et al. Expression of programmed death ligand 1 in penile cancer is of prognostic value and associated with HPV status. J Urol 2017;197:690–7. [DOI] [PubMed] [Google Scholar]
- [5].Mezache L, Paniccia B, Nyinawabera A, et al. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol 2015;28:1594–602. [DOI] [PubMed] [Google Scholar]
- [6].Liu C, Lu J, Tian H, et al. Increased expression of PD-L1 by the human papillomavirus 16 E7 oncoprotein inhibits anticancer immunity. Mol Med Rep 2016;15:1063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oka N, Kajita M, Nishimura R, et al. L1 gene methylation in high-risk human papillomaviruses for the prognosis of cervical intraepithelial neoplasia. Int J Gynecol Cancer 2013;23:235–43. [DOI] [PubMed] [Google Scholar]
- [8].Wang J, Tian Q, Zhang S, et al. Clinical significance of HPV L1 capsid protein detection in cervical exfoliated cells in high-risk HPV positive women. Zhonghua Fu Chan Ke Za Zhi 2015;50:253–7. [PubMed] [Google Scholar]
- [9].Xu X, Yang J, Lin N, et al. Semi-quantitative detection of HPV L1 capsid protein in exfoliative cytological examination facilitates the differential diagnosis of cervical lesions. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2014;30:1194–7. [PubMed] [Google Scholar]
- [10].Feng YC, Yang J, Liu CM, et al. DNA ploidy of cervical epithelial cells should be a cure criterion of high-risk HPV infection in Xinjiang Uygur women. Onco Targets Ther 2015;8:827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Feng YC, Zhang Y, Liu CM, et al. Increased HPV L1 gene methylation and multiple infection status lead to the difference of cervical epithelial cell lesion in different ethnic women of Xinjiang, China. Medicine 2017;96:e6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hutarew G. PD-L1 testing, fit for routine evaluation? From a pathologist's point of view. Memo 2016;9:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gaule P, Smithy JW, Toki M, et al. A quantitative comparison of antibodies to programmed cell death 1 ligand 1. JAMA Oncol 2017;3:256–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kataoka K, Shiraishi Y, Takeda Y, et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature 2016;534:402–6. [DOI] [PubMed] [Google Scholar]
- [15].Liu P, Iden M, Fye S, et al. Targeted, deep sequencing reveals full methylation profiles of multiple HPV types and potential biomarkers for cervical cancer progression. Cancer Epidemiol Biomarkers Prev 2017;26:642–50. [DOI] [PubMed] [Google Scholar]
- [16].Niyazi M, Sui S, Zhu K, et al. Correlation between methylation of human papillomavirus-16 L1 gene and cervical carcinoma in Uyghur women. Gynecol Obstet Invest 2017;82:22–9. [DOI] [PubMed] [Google Scholar]
- [17].Kim WY, Jung HY, Nam SJ, et al. Expression of programmed cell death ligand 1 (PD-L1) in advanced stage EBV-associated extranodal NK/T cell lymphoma is associated with better prognosis. Virchows Arch 2016;469:581–90. [DOI] [PubMed] [Google Scholar]
- [18].Ma C, Patel K, Singhi AD, et al. Programmed death-ligand 1 expression is common in gastric cancer associated with Epstein–Barr virus or microsatellite instability. Am J Surg Pathol 2016;40:1496–506. [DOI] [PubMed] [Google Scholar]
- [19].Zhou ZQ, Tong DN. Guan J, et al. Follicular helper T cell exhaustion induced by PD-L1 expression in hepatocellular carcinoma results in impaired cytokine expression and B cell help, and is associated with advanced tumor stages. Am J Transl Res 2016;8:2926–36. [PMC free article] [PubMed] [Google Scholar]
- [20].Howitt BE, Sun HH, Roemer MG, et al. Genetic basis for PD-L1 expression in squamous cell carcinomas of the cervix and vulva. JAMA Oncol 2016;2:518–22. [DOI] [PubMed] [Google Scholar]
- [21].Chen Z, Pang N, Du R, et al. Elevated expression of programmed death-1 and programmed death ligand-1 negatively regulates immune response against cervical cancer cells. Mediators Inflamm 2016;2016:6891482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bhaijee F, Anders RA. PD-L1 expression as a predictive biomarker: is absence of proof the same as proof of absence? JAMA Oncol 2016;2:54–5. [DOI] [PubMed] [Google Scholar]
- [24].Oguejiofor K, Galletta-Williams H, Dovedi SJ, et al. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV- oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget 2017;8:14416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


