Supplemental Digital Content is available in the text
Keywords: mainland China, thyroid disease, urinary iodine concentration
Abstract
Background:
Low-iodine intake has historically been an issue in China, causing widespread iodine deficiency diseases (IDD). China started to introduce universal salt iodization in 1995, but reports of increased thyroid disease are a concern and appropriate levels of iodine intake must be considered.
Objective:
To assess the prevalence of thyroid disease with different urinary iodine concentrations (UICs) in the general population of those residing in mainland China. Furthermore, we aimed to analyze the relationship between thyroid disease and UIC, to provide guidance in establishing effective health policies regarding iodine intake.
Methods:
PubMed, Cochrane, Embase, CNKI, Wan fang, and CQVIP databases were searched for random community-based relevant studies with UIC published before January 2016 in mainland China. Two independent reviewers extracted data from eligible citations, and obtained prevalence of thyroid disease for different UICs, as well as the intergroup interaction P values.
Results:
Forty-three articles were included. The prevalence of thyroid nodules was 22.3% (95% confidence interval [CI]: 20.6%–24.1%) for the low-iodine group, 25.4% (95% CI: 20.8%–28.8%) for the medium-iodine group, and 6.8% (95% CI: 2.8%–11.5%) for the high-iodine group. In the high-iodine group, the prevalence of thyroid nodules was lower than the other groups. The prevalence of 8.3% (95% CI: 3.8%–17.3%) for subclinical hypothyroidism in the high-iodine group was significantly higher than the low- and medium-iodine groups (P < .01). The prevalence of hypothyroidism in the medium-iodine group was 0.2% (95% CI: 0.1%–0.4%), and was lower than the prevalence of the other 2 groups (P < .01). There was no difference in prevalence of hyperthyroidism in each group.
Conclusions:
Thyroid nodules are the most easily detectable thyroid disease. These have a lower prevalence in the high-iodine group. The prevalence of most thyroid diseases is lowest for a UIC ranging from 100 to 299 μg/L. This serves as a reference for health policy-making with respect to iodine levels. Further studies on this topic should be carried out according to sufficient thyroid cancer data.
1. Introduction
Iodine is an essential element required for normal thyroid hormone activity and regulation of thyroxine and triiodothyronine. Both insufficient and excessive iodine intake can cause thyroid hormone disorders. Low-iodine intake used to be prevalent in China, resulting in widespread iodine deficiency diseases (IDDs). Salt iodization has been widely adopted around the world since the 1990 s. China started to introduce (universal salt iodization [USI]) in 1995, and salt iodization has been widely adopted since 1996. Since then, increased iodine intake has largely resulted in control of IDDs, but reports of increased thyroid disease are a public health concern and appropriate iodine-intake levels must be carefully considered. For example, what level of iodine intake will not only prevent IDD but also mitigate against thyroid diseases related to iodine excess? Therefore, we reviewed random community-based sample to perform this systematic review and meta-analysis to seek a conclusion about the relationship between iodine intake and thyroid diseases by comparing the prevalence of thyroid diseases with different UIC levels in mainland China during the last 16 years. Our study may help to identify the safe range of UIC and provide guidance on iodine intake, to reduce the prevalence of thyroid diseases.
2. Methods
2.1. Search strategy and selection criteria
The manuscripts in Chinese were collected from CNKI (http://www.cnki.com/), CQVIP (http://www.cqvip.com/), and Wan fang. The English manuscripts were collected from PubMed, Cochrane, and Embase. The search words in English database included “hyperthyroidism” or “hypothyroidism” or “thyroid cancer” or “thyroid tumor” or “thyroid tumour” or “thyriod carcinoma” or “thyroid neoplasms” or “thyroid nodule(s)” and “urine(urinary) iodine(iodides).” In Chinese database, we use Chinese words. Case reports, editorials, letters, management guidelines, studies performed in animals, and ex vivo studies were excluded. We searched the database through January 2016; although we did not limit the time when articles were published, the finally selected reports were from January 2001 to December 2015. The study is a systematic review and meta-analysis, so it does not involve the ethical issues.
Two investigators independently checked all of the retrieved articles according to the following eligibility criteria: participants from a random community-based sample, rather than from volunteers or those receiving routine health examinations; study design were population based instead of hospital-based studies; outcome contained sufficient information (e.g., survey location, survey methodology, diagnostic criteria, sample size, number of participants, urinary iodine concentration); participants must have one of the following thyroid diseases: hyperthyroidism, subclinical hyperthyroidism, hypothyroidism, subclinical hypothyroidism, thyroid cancer, thyroid nodule(s) (TN); TN requiring ultrasound results, thyroid cancer requiring pathology results, and diagnoses of other thyroid diseases had to be based on subject's thyroid function to be included; the survey areas must be in mainland China.
The following studies were excluded: participants suffering from any related diseases, or taking medication known to affect thyroid structure or function; participants were special populations (such as pregnant women, infants, or smokers) or workers in a specific occupation; UIC data taken form historical data instead of through a survey sample; and the number of “No” or “unclear” answers exceeded 5 out of the 14 questions in the QUADAS quality assessment tool.
2.2. Data extraction
Two reviewers (BZ and XZ) independently extracted information relating to the author, year of survey, urine iodine value, study design, patient characteristics, kind of disease, method of diagnosis, sample size, and outcomes. To resolve disagreement between reviewers, a third reviewer assessed all discrepant items, and decision by majority was used for the analysis. In this study, we used the median UIC to classify subjects into 3 subgroups: low-iodine group with median UIC <100 μg/L; medium-iodine group with median UIC in the range of 100 to 299 μg/L; high-iodine group with median UIC >300 μg/L.
2.3. Study design characteristics
We use the QUADAS quality assessment tool to extract relevant study design characteristics from each study. This tool and the definitions of the characteristics are fully described by Penny Whiting.[1] This was the first systematically developed evidence-based quality assessment tool to be used in systematic reviews of diagnostic accuracy studies. Two investigators independently assessed whether each item of QUADAS was fulfilled (yes, no, or unclear).
2.4. Statistical analysis
The Begg rank correlation method was used to assess the potential for publication bias (P < .05 was considered indicative of statistically significant publication bias). The prevalence and 95% confidence intervals (CIs) were used to estimate the prevalence of individual and pooled groups of hypothyroidism, subclinical hyperthyroidism, hyperthyroidism, subclinical hypothyroidism, TN, and thyroid cancer in mainland China. Heterogeneity between studies was calculated with Cochran Q test (reported as χ2 and P values) and the I2 statistic, which describe the percentage of variation between studies. Values of 25%, 50%, and 75% reflected low, moderate, and high degrees of heterogeneity, respectively. For a moderate or high level of heterogeneity, we adopted a random-effects meta-analysis rather than a fixed-effects model. We calculated data using the R software package (version 3.2.2). For analysis of the 3 subgroups, we reported an interaction P value.[2]
3. Results
3.1. Study selection
We identified 1368 potentially relevant publications in the electronic databases. Employing the selection criteria, we obtained quantitative data for our meta-analysis after reading all titles, abstracts, and full texts. A total of 43 studies involving 247 trials were identified for inclusion in the review. Figure 1 portrays our systematic workflow for identifying, screening, and including studies[3–45] in the systematic review.
Figure 1.
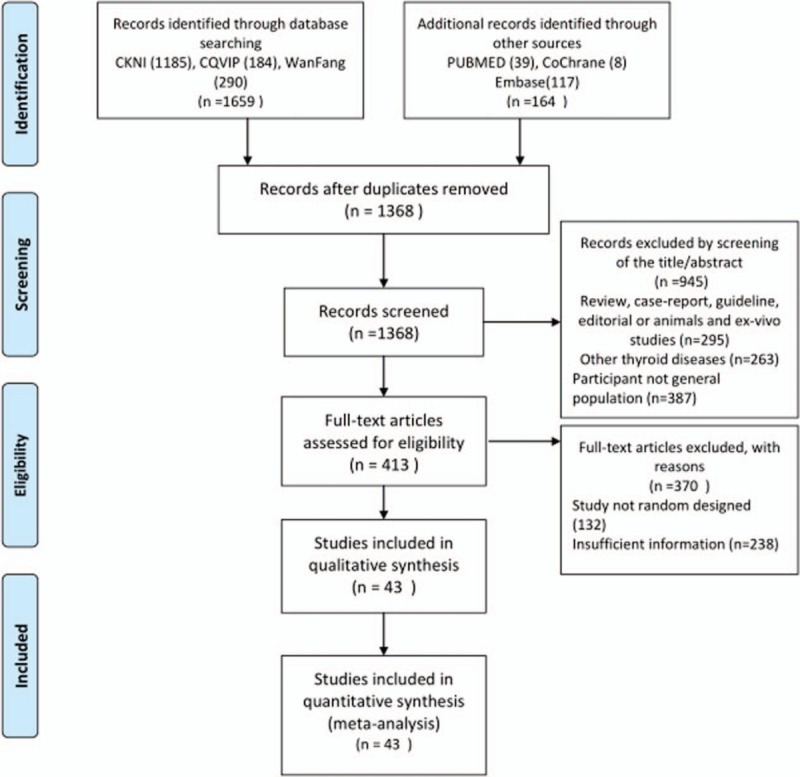
The systematic review workflow.
3.2. Characteristics of papers
The total number of subjects in the selected studies was 178,995, distributed in 14 provinces of mainland China, with ages ranging from 6 to 83. Nineteen studies considered only one type of thyroid disease, and 18 studies considered 4 or more types of thyroid diseases. All studies were based on samples from the general population. The mean QUADAS score, expressed as a percentage of the maximum score, was 85.7% (range, 71.4%–92.9%). Publication bias was observed as assessed by the Begg rank correlation analysis (P = .0016). Table 1 provides a summary of these studies.
Table 1.
Characteristics of studies.
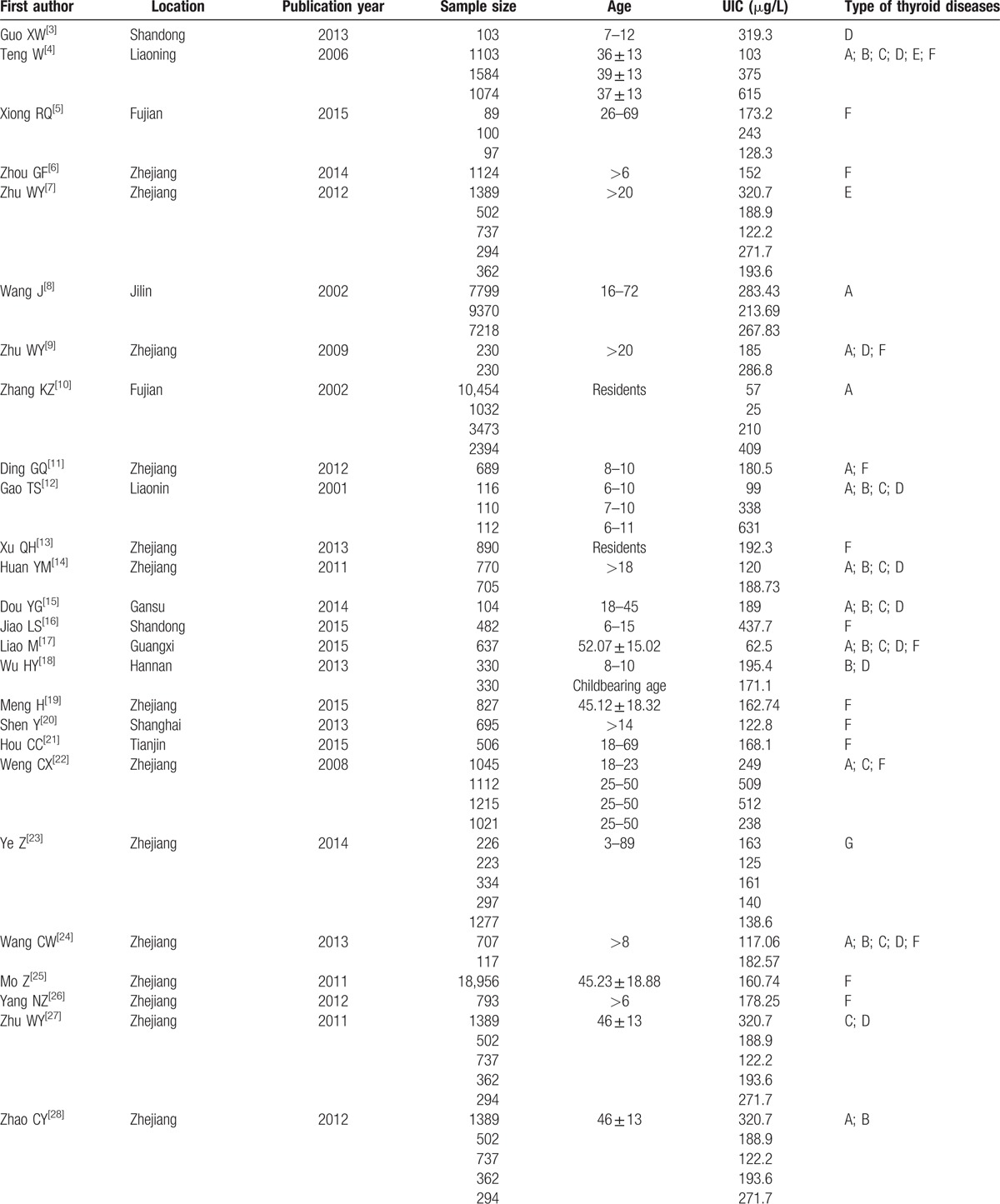
3.3. Pooled prevalence of thyroid diseases
Table 2 and Figures 2 and 3 show the pooled and individual group prevalence of thyroid diseases. Thyroid nodule(s) had the highest pooled prevalence among all thyroid diseases (21.2%, 95% CI: 17%–25.7%) with the second being subclinical hypothyroidism (5%, 95% CI: 3.5%–6.8%) (P < .01). Thyroid cancer had the lowest prevalence (0.1%, 95% CI: 0%–0.3%) for all thyroid diseases.
Table 1 (Continued).
Characteristics of studies.
Figure 2.
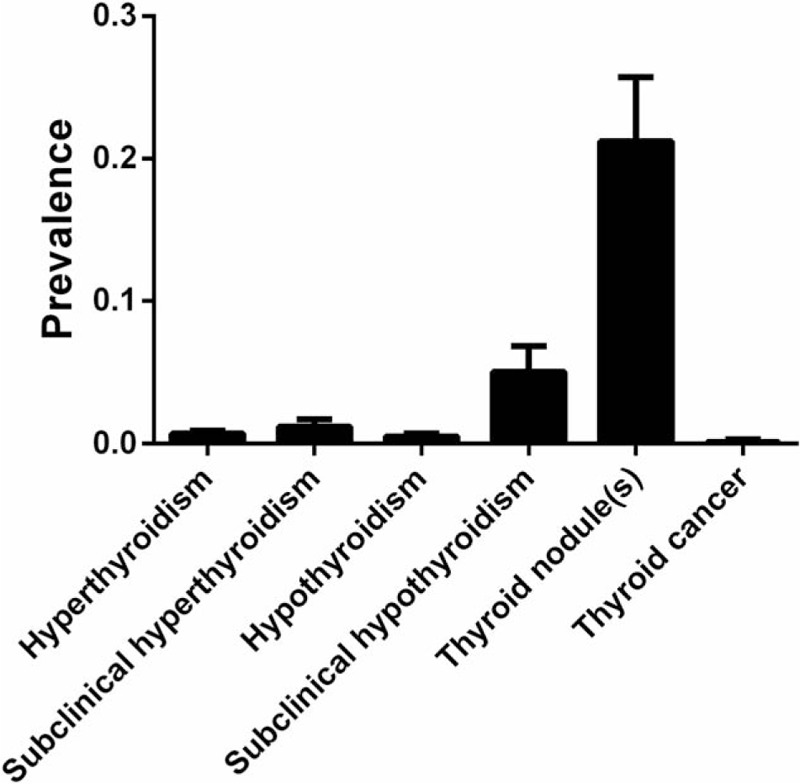
Pooled prevalence of thyroid disease.
Figure 3.
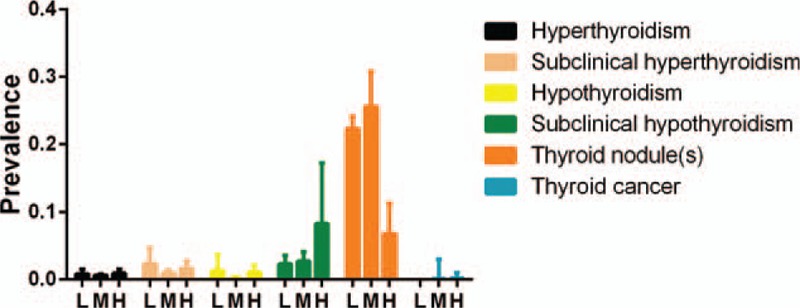
Prevalence of thyroid disease with different urinary iodine concentration.
Table 2.
Prevalence of thyroid disease by different group.
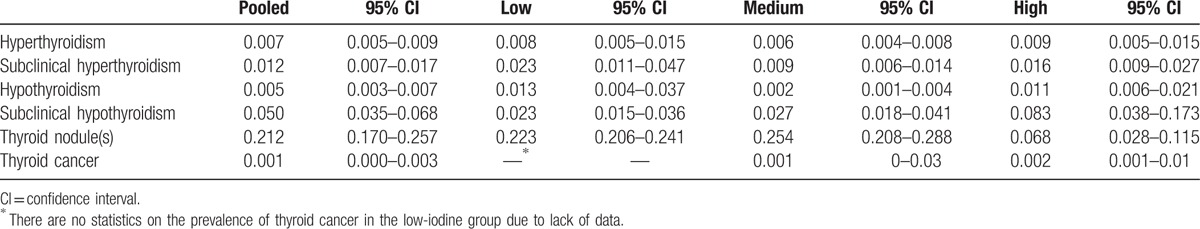
The prevalence of TN in the high-iodine group was 6.8% (95% CI: 2.8%–11.5%) and the prevalence was significantly lower compared with the low- and medium-iodine groups (P < .01). Figures 4 to 6 are the forest plots that show the prevalence of TN with different urinary iodine concentration. The prevalence of subclinical hypothyroidism was 2.7% (95% CI: 1.8%–4.1%) for the medium-iodine group and 8.3% (95% CI: 3.8%–17.3%) for the high-iodine group. The prevalence of the high-iodine group was significantly higher than the low- and medium-iodine groups (P < .01). The prevalence of hypothyroidism in the medium-iodine group was 0.2% (95% CI: 0.1%–0.4%) and it was lower than the prevalence of the other 2 groups (P < .01). The prevalence of hyperthyroidism in each group was not significantly different.
Figure 4.
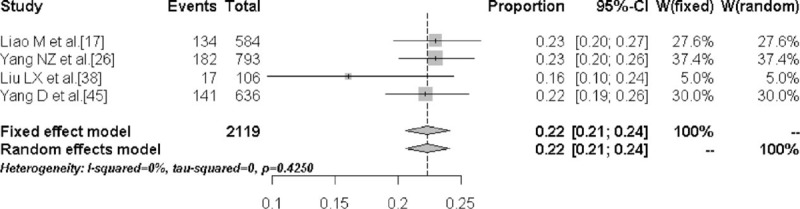
Forest plot displaying the prevalence of thyroid nodules with low urinary iodine concentration.
Figure 6.
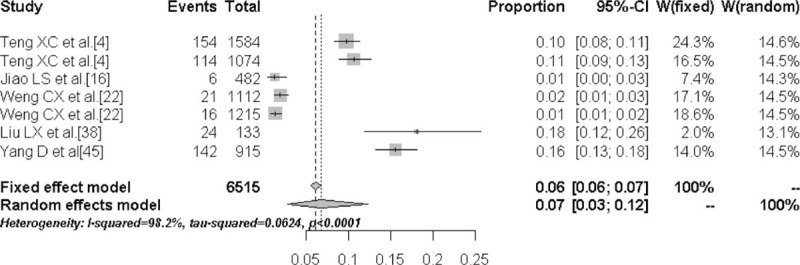
Forest plot displaying the prevalence of thyroid nodules with high urinary iodine concentration.
Figure 5.
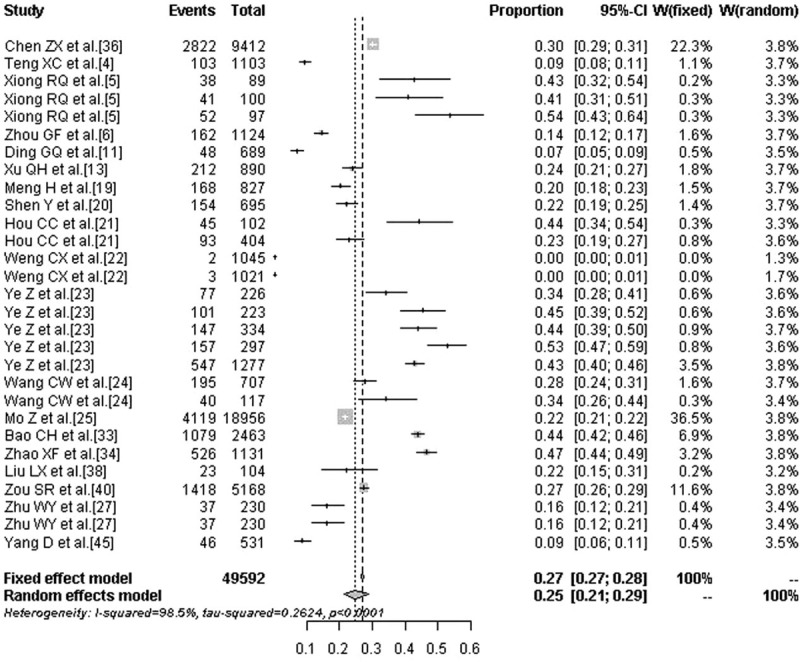
Forest plot displaying the prevalence of thyroid nodules with medium urinary iodine concentration.
4. Discussion
Iodine is mainly derived from the diet and the most absorbed iodine is excreted in the urine. According to the World Health Organization (WHO), the United Nations International Children's Fund (UNICEF), and the International Council for Control of IDD (ICCIDD) recommended standards, a UIC normal range is 100 to 199 μg/L, UIC <100 μg/L determines iodine deficiency, a UIC between 200 and 299 μg/L is exceeding appropriate scope, and a UIC ≥300 μg/L is an excess of iodine nutrition. As USI policy was established, public health authorities have been committed to eliminating IDD and have obtained satisfactory results. For example, China reached its goal of eliminating IDD in 1999. However, the change in iodine intake and its influence on thyroid diseases, especially when UIC ≥300 μg/L, has not been systematically researched.
Our study reveals that TN had the highest prevalence among all thyroid diseases. Other research also shows that TN are one of the commonest types of thyroid disease. The US Marshall Islands has a moderate level of iodine deficiency and has a TN prevalence of 28%.[46] Völzke et al[47] reported an area, which was historically mildly iodine deficient, but now the population consumes more than what is considered normal iodine intake and has a TN prevalence of 20.2%. The prevalence of TN in China is similar to other countries and regions. For example, a recent study in Zhejiang province revealed a TN prevalence of 20.9% for a median UIC of 163 μg/L.[48] These data are consistent with our results.
It is well known that excessive intake of iodine may induce thyroid disease, but we found the prevalence of TN was 6.8% (95% CI: 2.8%–11.5%) for the high-iodine group, which was lower than the other 2 groups. There are few studies that have explored the associations between excessive iodine intake and TN in adult populations. Szabolcs et al[49] reported the prevalence of TN as being 20.2%, 16.2%, and 3.3% for iodine deficiency, iodine prophylaxis, and abundant iodine intake, respectively. One study of company employees found that the prevalence of multiple TN decreased from 25.51% to 12.99% with increasing UIC, with a clear downward trend (P < .01).[50] However, the author of that study concluded that there were no associations between iodine intake and TN based on multivariate logistic regression analysis. In previous studies, the prevalence of TN has depended on sex, age, and head-and-neck radiation exposure history.[51,52] There is no direct evidence to prove that excessive iodine intake can increase the incidence of TN. Whether high-iodine urine levels decrease the risk of TN warrants further research.
In our study, the prevalence of subclinical hypothyroidism was the second most common type of thyroid disease for low-, medium-, and high-iodine groups. The prevalence was higher than in the low- and medium-iodine groups. Szabolcs et al[49] conducted an investigation of 346 senior subjects, and found that the prevalence of subclinical hypothyroidism was 4.2%, 10.4%, and 23.9%, respectively, for regions with UIC of 72, 100, and 513 μg/L, that is, the prevalence increased with increasing iodine intake. The previous conclusion was that subclinical hypothyroidism was related to thyroid antibodies, TPOAb and TgAb. However, a recent study[53] revealed that subjects with subclinical hypothyroidism had only a 20.6% TPOAb seropositivity rate and a 21.2% TgAb seropositivity rate, indicating that autoimmune factors might not be the most important factors in the mechanism of subclinical hypothyrodism. In an animal model,[54] it was shown that prolonged high-iodine intake inhibited pituitary type 2 deiodinase activities and increased the serum TSH level.
The prevalence of hypothyroidism is also related to iodine intake. UIC <100 μg/L indicates iodine deficiency, and iodine-deficiency disorders include hypothyroidism.[55] It is widely reported that high-iodine intake causes an increase in the prevalence of hypothyroidism,[49,53,56] and it is an independent risk factor in precipitating hypothyroidism.[57] This trend is true for children as well as for adults. There is a higher prevalence rate of hypothyroidism among subjects in high-iodine regions compared with other regions. In vitro experiments have proven that excessive iodine intake may cause thyroid follicle apoptosis.[58] Our study reveals that the prevalence of hypothyroidism was 0.2% in the medium-iodine group, 1.3% in the low-iodine group, and 1.1% in the high-iodine group. As hypothyroidism is closely related to hyperlipidemia, heart disease, and neurological diseases, iodine and its correlation with health defects must be taken seriously.
The most common complication of iodine intervention is iodine-induced hyperthyroidism (IIH). When too much iodine is ingested, the thyroid can develop a high tolerance to iodine, with possible regulation mechanisms, including lowering of TSH level; reduction in activity level and amount of sodium–iodine symporter; and the Wolff–Chaikoff effect of short-term blockage of iodine intake.[59] However, individual subjects have very different levels of tolerance to high-iodine intake. After approximately 100 years of iodized salt being ingested around the world, the prevalence of IIH is almost inevitable. There have been multiple reports of IIH in China since the adoption of the policy of iodized salt was introduced in 1995. Since the Netherlands adopted mandatory iodized bread, the prevalence of IIH has increased 20-fold.[60] Delange et al[61] believe that IIH typically occurs after a general increase in increased iodine intake or if medication containing iodine is ingested, and is more common among adults aged >40 with nodular goiter in very low-iodine regions. It is commonly accepted that the increase in IIH is an inevitable consequence of a salt iodization policy, but eventually the prevalence of IIH drops back to levels before salt iodization intervention.[62] Our study indicates that the prevalence of hyperthyroidism was not significantly different among different groups, possibly because the data were gathered 6 to 10 years after USI adoption, hence the prevalence rate of IIH had dropped.
It is commonly accepted that the prevalence of thyroid cancer is related to multiple fractionizing radiation, genetic susceptibility, benign TN, and other determinants. There is no clear correlation between iodine intake and thyroid cancer. A case-control study in Sweden[63] indicated that the prevalence of follicular thyroid carcinoma was closely related to iodine deficiency. An epidemiological study in Greece[64] indicated that papillary carcinoma of the thyroid accounted for 84% of all thyroid diseases among subjects in high-iodine regions, which was much higher than subjects in low-iodine regions. Some other studies have also indicated that the prevalence of thyroid cancer is not very different between subjects in high-iodine regions and low-iodine regions. However, the types of thyroid diseases were different: follicular carcinoma was more common in low-iodine regions, whereas papillary carcinoma was more common in high-iodine regions.[65,66] In this study, there were only 5 papers[4,7,31,35,37] based on random population surveys, and supported by pathology. There were no statistics on the prevalence of thyroid cancer in the low-iodine group due to lack of data, whereas the prevalence of thyroid cancer was 0.1% (95% CI: 0%–3%) in the medium-iodine group and 0.2% (95% CI: 0.1%–1%) in the high-iodine group.
The relationship between the iodine intake level of a population and the occurrence of thyroid diseases is U-shaped with an increase in risk from both low- and high-iodine intake levels.[67] There is a relatively narrow range for optimal intake; disease is more likely to develop in the populations with iodine intake above and below this range. In our study, the prevalence of thyroid diseases was lowest when the UIC was in the range of 100 to 299 μg/L. An individual's iodine intake is determined by multiple factors, including the environmental iodine concentration levels, dietary habits, and absorptive capacity. The environmental iodine concentration level varies widely among different regions in China. A study of IDD in 2005 indicated that the average UIC was 246 μg/L, and >5 provinces had a UIC exceeding 300 μg/L.[68] In our study, different subjects in the same region may have widely varying iodine levels, therefore it may not be sufficient to adopt a unified standard of iodine intake. If possible, UIC measurements and dietary evaluation should be conducted to determine if it is necessary to ingest iodinated salt, and keep a UIC in the recommended range of 100 to 299 μg/L, to prevent thyroid diseases.
There are several limitations to our study. First, the studies were limited to 14 provinces, among a total of 34 administrative regions in China. Second, no definitive conclusions can be drawn on thyroid cancer due to lack of data. Our future work includes collecting more data on thyroid cancer to explore the relationship between thyroid cancer and iodine intake.
5. Conclusions
Thyroid nodules are the most easily detectable thyroid disease. These have a lower prevalence in the high-iodine group. Subclinical hypothyroidism was the second most common type of thyroid disease. The prevalence of most thyroid diseases is lowest for UIC range of 100 to 299 μg/L. This serves as a reference for health policy-making with respect to iodine levels.
Acknowledgments
We acknowledge grants from the National Natural Science Foundation of China (No. 81471704) and Zhejiang Natural Science Foundation (LY16H180002).
Supplementary Material
Footnotes
Abbreviations: CIs = confidence intervals, ICCIDD = International Council for Control of IDD, IDD = iodine deficiency diseases, IIH = iodine-induced hyperthyroidism, TN = thyroid nodule(s), UIC = urinary iodine concentrations, UNICEF = United Nations International Children's Fund, USI = universal salt iodization, WHO = World Health Organization.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Whiting P, Rutjes AW, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Guo XW, Liu Y, Zhai LP, et al. Effects of excessive iodine intake on school-age children's health in high water iodine areas. Chin J Ctrl Endem Dis 2013;28:161–5. [Google Scholar]
- [4].Teng W, Shan Z, Teng X, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med 2006;354:2783–93. [DOI] [PubMed] [Google Scholar]
- [5].Xiong RQ, Dai L, Bao YN, et al. The research of Thyroid nodule with iodine nutritional status and related factors in the current situation. Chin J Clin 2015;9:1746–8. [Google Scholar]
- [6].Zhou GF, Hu SL, Chen JJ, et al. Shangyu city urban and rural population iodine nutritional status investigation and study. Chin J Ctrl Endem Dis 2014;29:50–2. [Google Scholar]
- [7].Zhu WY, Liu XG, Hu XF, et al. Investigation on the iodine nutritional status and the prevalenceof thyroid carcinoma in Zhoushan Archipelago residents. J Hyg Res 2012;41:79–82. [PubMed] [Google Scholar]
- [8].Wang J, Qi Q, Xue XF, et al. Study on epidemiology of hyperthyroidism in different supply time and different concentration of iodized salt. Chin J Ctrl Endem Dis 2002;17:22–6. [Google Scholar]
- [9].Zhu WY, Liu XG, Zhou SQ, et al. Comparative study on iodine nutrition and thyroid health conditions between fishers and buddhists in Zhoushan Archipelago. Chin Prev Med 2009;10:1044–6. [Google Scholar]
- [10].Zhang KZ, Lin YC, Fang ZP, et al. The effect of salt iodization for 10 years on the prevalences of endemic goiter and hyperthyroidism. Chin J Endocrinol Metab 2002;18:342–4. [Google Scholar]
- [11].Ding GQ, Mo Z, Lou XM. Iodine nutritional status and the thyroid nodules prevalence of school-age children. Chin J Sch Health 2012;33:111341–3. [Google Scholar]
- [12].Gao TS, Teng WP, Shan ZY, et al. Effect of iodine intake on thyroid diseases and intelligence among schoolchildren in rural areas. Natl Med J China 2001;81:453–6. [PubMed] [Google Scholar]
- [13].Xu QH. Analysis of iodine nutrition status survey results in rural populations of Changshan county. Chin J PHM 2013;29:99–101. [Google Scholar]
- [14].Huang YM, Xu WM, Deng J, et al. Research on status of iodine nutrition and prevalence of thyroid diseases in the population of rural and urban areas of Hangzhou city. Zhejiang Prev Med 2011;23:13–5. [Google Scholar]
- [15].Dou YG, Wang YL, Wang TC, et al. Analysis of iodine nutrition and thyroid function of adults in urban areas of Wuwei city Gansu province. J Hyg Res 2014;43:58–62. [PubMed] [Google Scholar]
- [16].Jiao LS, Chen DQ, Zhao RC, et al. The investigation and analysis of thyroid nodules in children of high iodine areas. Chin J Ctrl Endem Dis 2015;30:89–91. [Google Scholar]
- [17].Liao M, Liu J, Ning RJ, et al. Investigation of iodine nutrition and thyroid function in people of the coastal salt-producing area in Guangxi. Chin J Dis Control Prev 2015;19:536–8. [Google Scholar]
- [18].Wu Hy, Wang HM, Su YD, et al. Survey of iodine nutrition and thyroid function of childbearing age women and 8-10 year-old children in Hainan Province. China Trop Med 2013;13:1349–51. [Google Scholar]
- [19].Meng H, Ceng CY. The investigation of Liandu area resident iodine nutrition and thyroid nodules disease situation. Zhejiang Prev Med 2015;27:591–3. [Google Scholar]
- [20].Shen Y, Zheng YY, Yuan H, et al. Survey of iodine nutritional status of the residents in Jiading District of Shanghai. China Trop Med 2013;13:307–9. [Google Scholar]
- [21].Hou CC, Liu ZH, Wang Y, et al. Surveys on iodine nutrional status and thyroid related diseases among adults in areas with different water ioding levels in Tianjin. Chin Prev Med 2015;16:839–42. [Google Scholar]
- [22].Weng CX, Shi MS, Wang GM, et al. The survey of Xiangshan peninsula residents thyroid disease prevalence. Mod Pract Med 2008;20:346. [Google Scholar]
- [23].Ye Z, Chen L, Pei GJ, et al. The analysis of the residents iodine nutrition and thyroid disease situation in Xiangshan peninsula after salt iodization 18 years. Chin J PHM 2014;30:232–4. [Google Scholar]
- [24].Wang CW, Han SZ, Zhang B. Survey of iodine level and prevalence of thyroid diseases in residents in areas using iodized salt or non-iodized saltin Daishan. Zhejiang Dis Surveill 2013;28:71–5. [Google Scholar]
- [25].Mo Z. Effect Factors of the Prevalence of Thyroid Nodules in Zhejiang Province. Ningbo: School of Medicine, Ningbo University; 2011. [Google Scholar]
- [26].Yang NZ, Chen Y, Yang WY, et al. A survey on iodine nutrition condition of residents in Jiaojiang District, Taizhou City, Zhejiang Province. J Environ Occup Med 2012;29:148–53. [Google Scholar]
- [27].Zhu WY, Liu XG, Zhou SQ, et al. The investigation of the prevalence of hypothyroidism in Zhoushan archipelago. Chin J Health Lab Technol 2011;21:1252–4. [Google Scholar]
- [28].Zhao CY, Zhu WY, Liu XG, et al. Investigation of the prevalence of hyperthyroidism in Zhoushan Archipelago. Wei Sheng Yan Jiu 2012;4:37–9. [PubMed] [Google Scholar]
- [29].Yan SL, Wang YG, Wang F, et al. Relationgship between iodine in urine and Graves disease along coastal district in Shandong. Chin J Endemiol 2004;23:245–7. [Google Scholar]
- [30].Zhang GQ, Sang ZN, Wei W, et al. Effects of excessive iodine on thyroid disease. In: Hunan: The 14th annual symposium of Danone institute China; 2011:206–210. [Google Scholar]
- [31].Miao Huihui. A Cross-Section Study on Iodine Nutrition Status and Thyroid Diseases in Fujian Province. Fu zhou: Fujian Medical University; 2015. [Google Scholar]
- [32].Li HQ, Sang ZN, Tan L, et al. Thyroid function and serum lipids of adults living in areas of excessive iodine in water in Hebei Province. Wei Sheng Yan Jiu 2012;4:536–9. [PubMed] [Google Scholar]
- [33].Bao CH. The investigation and analysis of Xiangshan peninsula resident iodine nutrition and thyroid nodules disease situation. Mod Pract Med 2014;26:748–50. [Google Scholar]
- [34].Zhao XF, Dong HJ, Zhang J, et al. An analysis on the prevalence of thyroid nodules and risk factors among residents from coastal area. Zhejiang Prev Med 2015;27:120–3. [Google Scholar]
- [35].Zhou YL. Effects of Iodine Excess on Spectrum of Thyroid Diseases. Nanjing: Nanjing Medical University; 2008. [Google Scholar]
- [36].Chen ZX. A Cross-Sectional Study on the Associations Between Iodized Salt, Thyroid Function and Thyroid Nodule. Zhejiang: Zhejiang University; 2013. [Google Scholar]
- [37].Liu L. A Study of Correlation Between High Iodine Intake and Thyroid Neoplasm Based on Epidemiological Investigation. Tianjin: Tianjin Medical University; 2011. [Google Scholar]
- [38].Liu L, Wang D, Liu P, et al. The relationship between iodine nutrition and thyroid disease in lactating women with different iodine intakes. Br J Nutr 2015;114:1487–95. [DOI] [PubMed] [Google Scholar]
- [39].Peng NC, Shi LX, Zhang Q, et al. An epidemiological survey of the prevalence of thyroid diseases in mild iodine deficiency city after salt iodization. Chin J Intern Med 2013;52:16–20. [PubMed] [Google Scholar]
- [40].Zou S, Wu F, Guo C, et al. Iodine nutrition and the prevalence of thyroid disease after salt iodization: a cross-sectional survey in Shanghai, a coastal area in China. PLoS One 2012;7:e40718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chen W, Sang ZN, Liu H, et al. Investigation of thyroid function abnormalities in children in high water iodine areas of Hebei province. Chin J Prev Med 2012;46:148–51. [PubMed] [Google Scholar]
- [42].Teng XC, Shan Z, Chen Y, et al. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: a cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol 2011;164:943–50. [DOI] [PubMed] [Google Scholar]
- [43].Teng XC, Shi X, Shan Z, et al. Safe range of iodine intake levels: a comparative study of thyroid diseases in three women population cohorts with slightly different iodine intake levels. Biol Trace Elem Res 2008;121:23–30. [DOI] [PubMed] [Google Scholar]
- [44].Gu XL, Zhang DL, Gao ZN, et al. The epidemiological studies of thyroid dysfunction in Dalian zhangzi island. Chin J Mod Drug Appl 2013;7:99–100. [Google Scholar]
- [45].Du Y, Gao Y, Meng F, et al. Iodine deficiency and excess coexist in china and induce thyroid dysfunction and disease: a cross-sectional study. PLoS One 2014;9:e111937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Takahashi T, Fujimori K, Simon SL, et al. Thyroid nodules, thyroid function and dietary iodine in the Marshall islands. Int J Epidemiol 1999;28:742–9. [DOI] [PubMed] [Google Scholar]
- [47].Völzke H, Lüdemann J, Robinson DM, et al. The prevalence of undiagnosed thyroid disorders in a previously iodine-deficient area. Thyroid 2003;13:803–10. [DOI] [PubMed] [Google Scholar]
- [48].Gu F, Ding G, Lou X, et al. Incidence of thyroid diseases in Zhejiang Province, China, after 15 years of salt iodization. J Trace Elem Med Biol 2016;36:57–64. [DOI] [PubMed] [Google Scholar]
- [49].Szabolcs I, Podoba J, Feldkamp J, et al. Comparative screening for thyroid disorders in old age in areas of iodine deficiency, long-term iodine prophylaxis and abundant iodine intake. Clin Endocrinol (Oxf) 1997;47:87–92. [DOI] [PubMed] [Google Scholar]
- [50].Zhu HF, Yang Y, Li JY, et al. Prevalence of thyroid nodules and influencing factors among employees of a company in Qingda. Chin J Prev Med 2012;46:228–32. [PubMed] [Google Scholar]
- [51].Rojeski MT, Gharib H. Nodular thyroid disease: evaluation and management. N Engl J Med 1985;313:428–36. [DOI] [PubMed] [Google Scholar]
- [52].Gharib H. Changing concepts in the diagnosis and management of thyroid nodules. Endocrinol Metab Clin North Am 1997;26:77–80. [DOI] [PubMed] [Google Scholar]
- [53].Shan Z, Chen L, Lian X, et al. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in China: a cross-sectional study in 10 cities. Thyroid 2016;26:1125–30. [DOI] [PubMed] [Google Scholar]
- [54].Li N, Jiang Y, Shan Z, et al. Prolonged high iodine intake is associated with inhibition of type 2 deiodinase activity in pituitary and elevation of serum thyrotropin levels. Br J Nutr 2012;107:674–82. [DOI] [PubMed] [Google Scholar]
- [55].Dunn JT. What's happening to our iodine? J Clin Endocrinol Metab 1998;83:3398–400. [DOI] [PubMed] [Google Scholar]
- [56].Laurberg P, Pedersen KM, Hreidarsson A, et al. Iodine intake and the pattern of thyroid disorders: a comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland. Denmark J Clin Endocrinol Metab 1998;83:765–9. [DOI] [PubMed] [Google Scholar]
- [57].Chong W, Shi XG, Teng WP, et al. Multifactor analysis of relationship between the biological exposure to iodine and hypothyroidism. Natl Med J China 2004;84:1171–4. [PubMed] [Google Scholar]
- [58].Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489–99. [DOI] [PubMed] [Google Scholar]
- [59].Eng PH, Cardona GR, Previti MC. Regulation of the thyroid iodide symporter by iodide in FRTL-5 cells. Eur J Endocrinol 2001;144:139–44. [DOI] [PubMed] [Google Scholar]
- [60].Delange F, de Benoist B, Alnwick D. Risks of iodine induced hyperthyroidism after correction of ioding deficiency by iodized salt. Thyroid 1999;9:545–56. [DOI] [PubMed] [Google Scholar]
- [61].Foutoulakis S, Philippou G, Tsatsoulis A. The role of iodine in the evolution of thyroid disease in Greece: from endemic goitertothy-roidauto immunity. Hormones (Athens) 2007;6:25–35. [PubMed] [Google Scholar]
- [62].Stanbury JB, Ermans AE, Bourdoux P, et al. Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid 1998;8:83–100. [DOI] [PubMed] [Google Scholar]
- [63].Lv YY, Zhu BZ. The relationship between Iodine intake and incidence of thyroid cancer. Foreign Med 2008;19:33–4. [Google Scholar]
- [64].Ilias I, Alevizaki M, Lakka PE. Differentiated thyroid cancer in Greece: 1963–2000. Relation to demographic and environmental factors. Hormones (Athens) 2002;1:174–8. [DOI] [PubMed] [Google Scholar]
- [65].Mack WJ, Preston-Martin S, Bernstein L, et al. Reproductive and hormonal risk factors for thyroid cancer in Los Angeles County females. Cancer Epidemiol Biomarkers Prev 1999;8:991–7. [PubMed] [Google Scholar]
- [66].Knobel M, Medeiros-Neto G. Relevance of iodine intake as a reputed predisposing factor for thyroid cancer. Arq Bras Endocrinol Metabol 2007;51:701–12. [DOI] [PubMed] [Google Scholar]
- [67].Laurberg P, Bülow Pedersen I, Knudsen N, et al. Environmental iodine intake affects the type of nonmalignant thyroid disease. Thyroid 2001;11:457–69. [DOI] [PubMed] [Google Scholar]
- [68].Fu ZY, Zhang JY. Iodized salt and current situation of iodized-induced diseases. Med Recapitulate 2009;15:1520–3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


