Abstract
Murine gammaherpesvirus 68 (MHV-68), Kaposi's sarcoma-associated herpesvirus (HHV-8), and Epstein-Barr virus (EBV) are all members of the gammaherpesvirus family, characterized by their ability to establish latency in lymphocytes. The RTA protein, conserved in all gammaherpesviruses, is known to play a critical role in reactivation from latency. Here we report that HHV-8 RTA, not EBV RTA, was able to induce MHV-68 lytic viral proteins and DNA replication and processing and produce viable MHV-68 virions from latently infected cells at levels similar to those for MHV-68 RTA. HHV-8 RTA was also able to activate two MHV-68 lytic promoters, whereas EBV RTA was not. In order to define the domains of RTA responsible for their functional differences in viral promoter activation and initiation of the MHV-68 lytic cycle, chimeric RTA proteins were constructed by exchanging the N-terminal and C-terminal domains of the RTA proteins. Our data suggest that the species specificity of MHV-68 RTA resides in the N-terminal DNA binding domain.
Gammaherpesviruses are distinguished by their ability to establish latency in lymphocytes and are associated with malignancies, such as various B-cell lymphomas (1-3, 31, 33) and Kaposi's sarcoma (5, 20, 22, 24). Murine gammaherpesvirus 68 (MHV-68) is classified as a type 2 gammaherpesvirus and is currently used as a mouse model for human gammaherpesviruses Kaposi's sarcoma-associated herpesvirus/human herpesvirus-8 (HHV-8) and Epstein-Barr virus (EBV) (11, 21, 27, 29). The knowledge gained from the use of MHV-68 is instrumental in understanding human gammaherpesvirus pathogenesis.
RTA is an immediate-early viral transactivator protein conserved among gammaherpesviruses (14, 18, 30, 37). The RTA protein of HHV-8 has been shown to be necessary and sufficient for reactivation of HHV-8 in latently infected cells (18, 30). Ectopic expression of MHV-68 RTA is sufficient and necessary for reactivation of MHV-68 from latency and for activation of the lytic cycle of MHV-68 during de novo infection (36, 37). This is in contrast to EBV, a type 1 gammaherpesvirus, which generally requires the cooperativity of two viral proteins, Zebra and RTA, for lytic replication and reactivation (6-8, 12, 38). To define the functional similarity or difference among these RTA proteins, we tested whether the RTA protein of HHV-8 or EBV could activate MHV-68 lytic promoters and reactivate MHV-68 from latency. The comparison of these three RTAs has allowed us to determine which domains of RTA are necessary for reactivation of MHV-68 and transactivation of viral lytic promoters.
Activation of heterologous promoters by the RTA proteins.
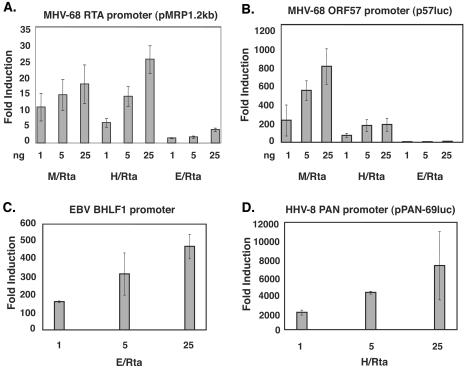
All three RTAs are known to activate their respective autologous RTA promoters in addition to the promoters of downstream lytic viral genes (9, 10, 13, 15-17, 25, 28). In order to determine if the RTA proteins also have the ability to transactivate promoters from heterologous viruses, we tested the ability of HHV-8 RTA and EBV RTA to transactivate two MHV-68 lytic promoters in a reporter assay. A 1.2-kb fragment of the MHV-68 RTA promoter was cloned into pGL3 Basic (pMRP1.2kb) and tested alongside the ORF57/Mta promoter construct in pGL2 Basic (p57Luc) (16). 293T cells (106) and 5 × 104 BHK-21 cells were transfected with 50 ng of the pMRP1.2kb or p57luc construct and increasing amounts of pFLAG RTA expression vectors MHV-68 RTA (M/RTA), HHV-8 RTA (H/RTA), EBV RTA (E/RTA), or pFLAG-CMV alone. Luciferase results were assayed 24 h posttransfection by using a dual luciferase reporter system (Promega, Madison, Wis.). Figure 1A shows that H/RTA activates pMRP1.2kb to the same degree as M/RTA. E/RTA, however, activates the promoter to a much lower level. In Fig. 1B, H/RTA was also able to activate the MHV-68 ORF57 promoter, although to a lower level than did MHV-68 RTA. E/RTA did not activate the promoter to a significant level. Thus, H/RTA had the ability to activate two MHV-68 lytic promoters, whereas E/RTA did not.
FIG. 1.
Activation of MHV-68, EBV, and HHV-8 promoters by homologous RTA proteins. A reporter construct consisting of either the MHV-68 ORF50 promoter (pMRP1.2kb) (A), the MHV-68 ORF57/Mta promoter (p57luc) (B), the EBV BHLF1 promoter (C), or the HHV-8 PAN promoter (pPAN-69luc) (D) was cotransfected with each construct or pFLAG-CMV in the amounts indicated. All transfections were done in BHK-21 or 293T cells. Cell lysates were harvested at 36 h posttransfection and assayed for luciferase activity.
In order to confirm that the H/RTA and E/RTA constructs are transcriptionally active, they were tested on autologous HHV-8 or EBV lytic promoters in reporter assays. The abilities of the pFLAG constructs to express HHV-8 RTA or EBV RTA were confirmed by Western blotting (see Fig. 3B). In Fig. 1C and D, various amounts of pFLAG-H/RTA or pFLAG-E/RTA were transfected into 293T cells along with 50 ng of the indicated promoter construct. The H/RTA construct was transfected with pPAN-69luc, a promoter construct consisting of 69 bp of the PAN promoter cloned into pGL3 Basic (28), and the E/RTA construct was transfected with an EBV BHLF1 promoter cloned into pGL3 basic (19). The results show that both H/RTA and E/RTA are transcriptionally active on their autologous promoters. The inability of E/RTA to activate the MHV-68 RTA and ORF57 promoters is therefore not due to the production of inactive protein.
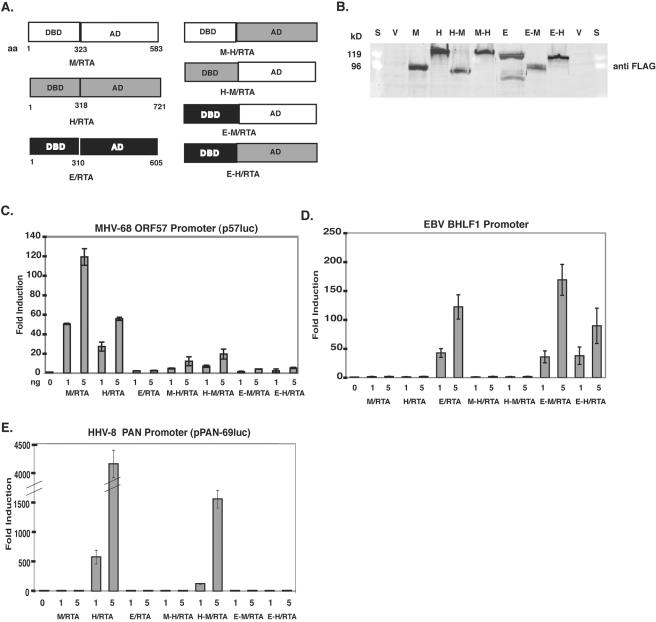
FIG. 3.
Construction of chimeric RTA proteins and their activation of gammaherpesvirus promoters. (A) The DBD and activation domain (AD) are indicated for the three RTA proteins. The activation domains of the proteins were swapped as indicated in the diagram, and the amino acid sequences that were used are labeled. (B) 293T cells were transfected with pFLAG (V), pFLAG-H/RTA (H), pFLAG-M/RTA (M), pFLAG-E/RTA (E), pFLAG-M-H/RTA (M-H), pFLAG-H-M/RTA (H-M), pFLAG-E-M/RTA (E-M), or pFLAG-E-H/RTA (E-H). Cell lysates were harvested 24 h posttransfection, and a Western blot was performed, using a monoclonal antibody against FLAG (Sigma). The lane marked S is the protein marker standard (Bio-Rad). A reporter construct consisting of either the HHV-8 Pan promoter (E), the EBV BHLF1 promoter (D), or the MHV-68 ORF57 promoter (C was cotransfected with each construct or pFLAG-CMV in the amounts indicated into 293T cells. Cell lysates were harvested at 36 h posttransfection and assayed for luciferase activity.
Reactivation of MHV-68 from latency.
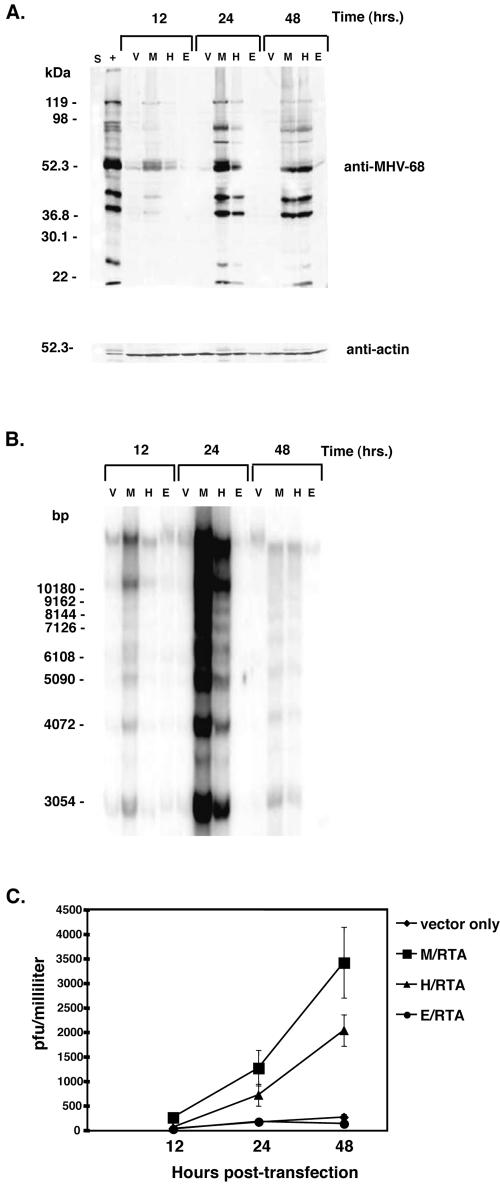
Based on the reporter assay data, we determined whether H/RTA could reactivate MHV-68 from latency. S11-E cells (107) (an MHV-68-latently infected B-cell lymphoma cell line [34]) were electroporated with 10 μg of the M/RTA, H/RTA, or E/RTA construct or pFLAG-CMV alone. By use of Western blotting, we determined that all of the pFLAG-CMV RTA constructs were expressed to similar levels in S11-E cells (data not shown). Cells were harvested at 12, 24, and 48 h posttransfection, and whole-cell extracts were analyzed for viral protein expression by Western blotting, using a rabbit hyperimmune serum against MHV-68-infected rabbit cells (32) (Fig. 2A). Extract from 105 cells was compared, along with extract from 104 MHV-68-infected BHK-21 cells as a positive control (+). A murine monoclonal antibody against actin was used as a control for protein loading. As expected, M/RTA transfection resulted in lytic protein expression with the peak of production at 24 h posttransfection. H/RTA transfection also resulted in MHV-68 protein production, although the number and intensity of expression were less than those for M/RTA. This indicates that M/RTA reactivates MHV-68 more efficiently than H/RTA. No detectable MHV-68 lytic protein expression was seen after transfection of E/RTA, suggesting that E/RTA is incapable of inducing MHV-68 reactivation. There was also no evidence of MHV-68 lytic proteins in the transfection of pFLAG-CMV.
FIG. 2.
Transfection of homologous RTA proteins into MHV-68-latently infected cells. (A) MHV-68-latently infected S11-E cells were electroporated with pFLAG-CMV (V), pFLAG-M/RTA (M), pFLAG-H/RTA (H), or pFLAG-E/RTA (E). Protein from 104 MHV-68-infected BHK-21 cells (+) was included as a positive control, and S is the protein marker standard (Bio-Rad). Western analysis was performed with rabbit hyperimmune serum against MHV-68-infected rabbit cell lysates and a murine monoclonal actin antibody as a control for protein loading. (B) MHV-68-latently infected S11-E cells were elec-troporated with of pFLAG (V), pFLAG-M/RTA (M), pFLAG-H/RTA (H), or pFLAG-E/RTA (E). Total DNA from transfected cells was harvested at the indicated time points posttransfection. The DNA was digested with HindIII and subjected to Southern analysis with a probe derived from a 0.8-kb region adjacent to the terminal repeat region of MHV-68. (C) Supernatants from the transfected S11E cells were collected at the time points indicated, and the viral titer was measured by plaque assay.
In order to determine whether the induction of MHV-68 viral lytic proteins by H/RTA resulted in viral DNA replication and processing, a terminal repeat assay was performed as previously described (23, 37). This assay allows linear replicated and processed viral genomes (a 1.2-kb DNA ladder) to be distinguished from latent, circular genomes (∼50 kb). Total cellular DNA was extracted from 106 S11E cells transfected as for Fig. 2A. The DNA was digested with HindIII and subjected to Southern analysis. Equal loading of DNA was determined by ethidium bromide staining of the agarose gel (data not shown). A probe corresponding to nucleotides 118314 to 117560 of the MHV-68 genome (a unique region of the MHV-68 genome next to the terminal repeats) was labeled with [α-32P]dCTP by random priming. After hybridization, radioactivity was detected with a phosphor-imaging system. Figure 2B shows that transfection of S11E cells with M/RTA or H/RTA resulted in viral DNA replication and processing. Consistent with the Western blot analysis, M/RTA transfection resulted in higher levels of viral DNA replication than with H/RTA, especially at the 24-h time point. E/RTA and pFLAG-CMV transfection failed to induce viral DNA replication.
To determine whether H/RTA was able to drive viral replication of MHV-68 to completion, infectious virions in supernatants from S11E transfections were quantitated by plaque assay as previously described (37). Figure 2C shows that transfection of either M/RTA or H/RTA results in the production of infectious MHV-68 virions. Consistent with the previous two assays, the production of virions by H/RTA is similar to that by M/RTA, varying only by approximately 1.8-fold. This result was not necessarily expected, since HHV-8 RTA and EBV RTA share only 16.4 and 13.1% amino acid identity with MHV-68 RTA, respectively (35), predominantly in the N-terminal DNA binding domain (DBD) (30). In contrast to results with H/RTA, transfection of E/RTA produced extremely low levels of infectious MHV-68, comparable to the level of pFLAG-CMV vector alone.
Determination of the functional domains of RTA.
Our data show that transactivation of MHV-68 promoters in reporter assays and reactivation of MHV-68 from latency occur with various efficiencies with the homologous RTA proteins (efficiency of M/RTA is greater than or equal to that of H/RTA, which is very much greater than that of E/RTA). In order to determine which domains of M/RTA, H/RTA, and E/RTA are necessary for reactivation, we made chimeric RTA constructs in pFLAG-CMV by fusing the DBD of each RTA protein with the C-terminal activation domain from the others (Fig. 3A). The correct sequence, size, and expression of each protein were verified by Western blotting, using a monoclonal antibody against FLAG (Sigma) (Fig. 3B). We then assayed the abilities of the chimeric proteins to transactivate three gammaherpesvirus lytic promoters in reporter assays. Increasing amounts of each construct along with 50 ng of the indicated promoter were transfected into 293T cells by the calcium phosphate method (Fig. 3C, D, and E). Figure 3C shows that both the M-H/RTA and H-M/RTA chimeric proteins were able to activate the MHV-68 ORF57 promoter, which is consistent with the fact that both M/RTA and H/RTA are able to significantly activate MHV-68 p57luc. In Fig. 3D and E, only the chimeric proteins that had the DBD domain of the autologous RTA protein were able to significantly activate transcription.
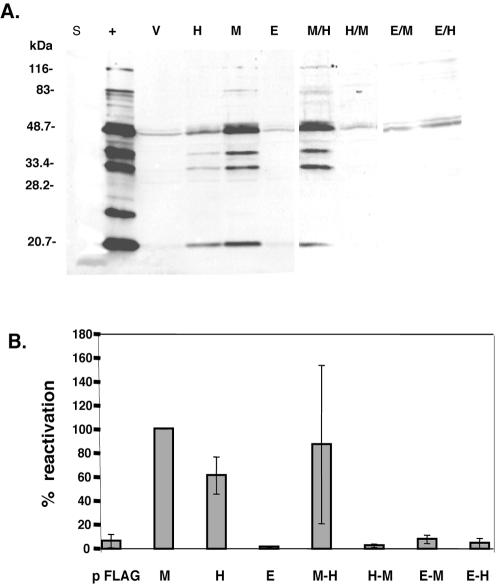
We next determined which domain of RTA is the determinant for the functional differences in reactivation efficiency. S11-E cells (107) were electroporated with 10 μg of each RTA expression vector or pFLAG-CMV alone, and reactivation was assayed by Western blotting as before (Fig. 4A). M-H/RTA was able to induce MHV-68 lytic viral protein expression. M-H/RTA was also the only chimeric construct able to induce lytic replication and processing of viral DNA (data not shown) and the production of infectious virions (Fig. 4B). Thus, the domain-swapping results suggest that the specificity of M/RTA to transactivate MHV-68 promoters and reactivate MHV-68 resides in the DBD domain.
FIG. 4.
Transfection of chimeric RTA proteins into MHV-68-latently infected cells. (A) MHV-68-latently infected S11-E cells were electroporated with pFLAG (V), pFLAG-H/RTA (H), pFLAG-M/RTA (M), pFLAG-E/RTA (E), pFLAG-M-H/RTA (M/H), pFLAG-H-M/RTA (H/M), pFLAG-E-M/RTA (E/M), or pFLAG-E-H/RTA (E/H). Protein from 104 MHV-68-infected BHK-21 cells (+) was included as a positive control, and lane S is the protein marker standard (Bio-Rad). Western analysis was performed with rabbit hyperimmune serum against MHV-68-infected rabbit cell lysates. (B) Supernatants from the transfected S11E cells were collected at the time points indicated, and the viral titer was measured by plaque assay. The percentage of reactivation was determined by comparing the amount of virions produced by each construct to the amount of virions produced by electroporation of pFLAG-M/RTA, which was set at 100%.
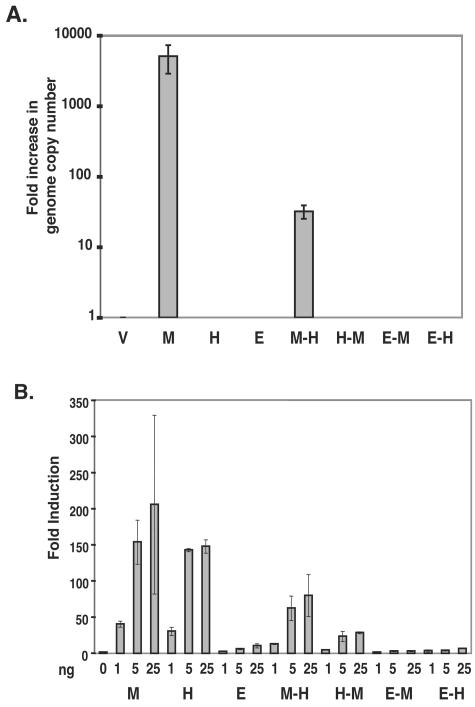
Our data indicate that the RTA protein of HHV-8 has the ability to activate the entire lytic cycle of MHV-68 in S11-E cells. This suggests either that H/RTA is able to directly bind and transactivate MHV-68 lytic promoters or that H/RTA is upregulating the expression of MHV-68 RTA, leading to reactivation of MHV-68 from latency. However, the ability of H/RTA to transactivate MHV-68 lytic promoters in a reporter assay in the absence of M/RTA suggests that H/RTA may be able to drive lytic gene expression of MHV-68 independently of MHV-68 RTA. In order to address this issue, we utilized a BAC clone that contains an MHV-68 RTA-deficient virus that was obtained from a transposon-mediated signature tag mutagenesis study performed in our lab (M. J. Song, S. Hwang, W. H. Wong, T.-T. Wu, S. Lee, H. Liao, and R. Sun, submitted for publication). We cotransfected 100 ng of the BAC clone (STM 8-42) with 300 ng of each of the RTA constructs or pFLAG-CMV alone into BHK-21 and 293T cells. Six days posttransfection, DNA was extracted from the cells and digested with DpnI to remove the transfected BAC DNA. Real-time PCR, using QIAGEN Quantitect SYBR Green PCR master mix and primers against MHV-68 M1, was performed on 40 ng of DNA per sample. The samples were quantitated as previously described (26). Only the M/RTA and M-H/RTA constructs were able to produce an increase in viral genome copy number in comparison to results with the BAC transfected with pFLAG-CMV alone (Fig. 5A). Similar results were seen with BHK-21 cells (data not shown).
FIG. 5.
Cotransfection of a recombinant MHV-68 RTA-deficient virus with each of the RTA constructs. (A) A BAC clone containing a recombinant MHV-68 RTA-deficient virus was cotransfected with pFLAG (V), pFLAG-H/RTA (H), pFLAG-M/RTA (M), pFLAG-E/RTA (E), pFLAG-M-H/RTA (M-H), pFLAG-H-M/RTA (H-M), pFLAG-E-M/RTA (E-M), or pFLAG-E-H/RTA (E-H) into BHK-21 (shown) or 293T cells. Six days posttransfection, DNA was extracted from these cells and real-time PCR was performed with M9 primers and probe. (B) An MHV-68 ORF50 promoter construct (pMRP1.2kb) was cotransfected with each construct or pFLAG-CMV in the amounts indicated. All transfections were done with 293T cells. Cell lysates were harvested at 36 h posttransfection and assayed for luciferase activity.
These data suggest that the mechanism behind MHV-68 reactivation by H/RTA is that H/RTA activates the MHV-68 RTA promoter, producing MHV-68 RTA, and it is MHV-68 RTA that is activating downstream genes to drive the lytic cycle. The H-M/RTA construct was able to activate the MHV-68 p57 promoter but was unable to reactivate MHV-68 or complement the MHV-68 RTA-deficient virus. Therefore, we tested the abilities of all of the chimeric constructs to activate the MHV-68 RTA promoter (pMRP1.2kb). The transfections and luciferase assays were carried out as described previously. In Fig. 5B, the constructs that have the ability to activate the MHV-68 RTA promoter to the highest level are M/RTA, H/RTA, and M-H/RTA, which correlates with the reactivation data (Fig. 4). The H-M/RTA construct is able to activate the RTA promoter but at a much lower level. Thus, its ability to activate the MHV-68 RTA promoter may be below the threshold amount needed to activate the RTA promoter of the viral genome, produce MHV-68 RTA, and drive the lytic cycle. A chimeric HHV-8 RTA construct consisting of the DNA binding domain of HHV-8 RTA fused with the activation domain of VP16 has the ability to activate the lytic cycle of HHV-8 (4). Therefore, the specificity of H/RTA for reactivation of MHV-68 may also lie in the DNA binding domain. The rest of the constructs were not able to significantly activate the promoter, which is consistent with the previous data.
These results suggest that the mechanism of reactivation is conserved between HHV-8 and MHV-68. The homologous RTA protein of EBV was not able to reactivate MHV-68 or significantly activate MHV-68 promoters in reporter assays, which is consistent with the current phylogenic grouping of HHV-8 and MHV-68 in the gamma-2 subfamily and that of EBV in the gamma-1 subfamily of herpesviruses, although the difference in sequence homology between the RTA proteins is not significant. The results obtained from a comparison of these three proteins by domain swapping suggest that the DBD domain of MHV-68 RTA contributes more to viral specificity than the activation domain for transactivation of viral lytic promoters and reactivation of MHV-68. These results also indicate that MHV-68 will be an invaluable model for studying common mechanisms of viral replication and pathogenesis for HHV-8.
Acknowledgments
We acknowledge and thank Samuel Speck and Herbert Virgin for generously providing the MHV-68 ORF57 promoter construct and Michael Carey for generously providing the EBV BHLF1 promoter construct. Preliminary data were presented at the 2001 Fourth International Workshop on Kaposi's Sarcoma Herpesvirus and Related Agents.
This work was supported by NIH grants CA91791 and DE14153, the Stop Cancer Foundation (R.S.), the UCLA AIDS Institute, and USPHS National Research Service Award GM07185 (T.M.R.).
REFERENCES
- 1.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 2.Cesarman, E., and D. M. Knowles. 1997. Kaposi's sarcoma-associated herpesvirus: a lymphotropic human herpesvirus associated with Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Semin. Diagn. Pathol. 14:54-66. [PubMed] [Google Scholar]
- 3.Cesarman, E., R. G. Nador, K. Aozasa, G. Delsol, J. W. Said, and D. M. Knowles. 1996. Kaposi's sarcoma-associated herpesvirus in non-AIDS related lymphomas occurring in body cavities. Am. J. Pathol. 149:53-57. [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, P. J., D. Shedd, L. Gradoville, M. S. Cho, L. W. Chen, J. Chang, and G. Miller. 2002. Open reading frame 50 protein of Kaposi's sarcoma-associated herpesvirus directly activates the viral PAN and K12 genes by binding to related response elements. J. Virol. 76:3168-3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 6.Chevallier-Greco, A., E. Manet, P. Chavrier, C. Mosnier, J. Daillie, and A. Sergeant. 1986. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 5:3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Countryman, J., and G. Miller. 1985. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA 82:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox, M. A., J. Leahy, and J. M. Hardwick. 1990. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J. Virol. 64:313-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng, H., M. J. Song, J. T. Chu, and R. Sun. 2002. Transcriptional regulation of the interleukin-6 gene of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 76:8252-8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng, H., A. Young, and R. Sun. 2000. Auto-activation of the rta gene of human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus. J. Gen. Virol. 81:3043-3048. [DOI] [PubMed] [Google Scholar]
- 11.Efstathiou, S., Y. M. Ho, and A. C. Minson. 1990. Cloning and molecular characterization of the murine herpesvirus 68 genome. J. Gen. Virol. 71:1355-1364. [DOI] [PubMed] [Google Scholar]
- 12.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardwick, J. M., P. M. Lieberman, and S. D. Hayward. 1988. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J. Virol. 62:2274-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenney, S., E. Holley-Guthrie, E. C. Mar, and M. Smith. 1989. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J. Virol. 63:3878-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu, S., I. V. Pavlova, H. W. T. Virgin, and S. H. Speck. 2000. Characterization of gammaherpesvirus 68 gene 50 transcription. J. Virol. 74:2029-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukac, D. M., L. Garibyan, J. R. Kirshner, D. Palmeri, and D. Ganem. 2001. DNA binding by Kaposi's sarcoma-associated herpesvirus lytic switch protein is necessary for transcriptional activation of two viral delayed early promoters. J. Virol. 75:6786-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukac, D. M., R. Renne, J. R. Kirshner, and D. Ganem. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF 50 transactivator, a homolog of the EBV R protein. Virology 252:304-312. [DOI] [PubMed] [Google Scholar]
- 19.Mitsouras, K., B. Wong, C. Arayata, R. C. Johnson, and M. Carey. 2002. The DNA architectural protein HMGB1 displays two distinct modes of action that promote enhanceosome assembly. Mol. Cell. Biol. 22:4390-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore, P. S., and Y. Chang. 1995. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N. Engl. J. Med. 332:1181-1185. [DOI] [PubMed] [Google Scholar]
- 21.Nash, A. A., and N. P. Sunil-Chandra. 1994. Interactions of the murine gammaherpesvirus with the immune system. Curr. Opin. Immunol. 6:560-563. [DOI] [PubMed] [Google Scholar]
- 22.O'Neill, E., T. H. Henson, A. J. Ghorbani, M. A. Land, B. L. Webber, and J. V. Garcia. 1996. Herpesvirus-like sequences are specifically found in Kaposi sarcoma lesions. J. Clin. Pathol. 49:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raab-Traub, N., and K. Flynn. 1986. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 47:883-889. [DOI] [PubMed] [Google Scholar]
- 24.Rady, P. L., A. Yen, R. W. R. Martin, I. Nedelcu, T. K. Hughes, and S. K. Tyring. 1995. Herpesvirus-like DNA sequences in classic Kaposi's sarcomas. J. Med. Virol. 47:179-183. [DOI] [PubMed] [Google Scholar]
- 25.Ragoczy, T., L. Heston, and G. Miller. 1998. The Epstein-Barr virus Rta protein activates lytic cycle genes and can disrupt latency in B lymphocytes. J. Virol. 72:7978-7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rickabaugh, T. M., H. J. Brown, D. Martinez-Guzman, T.-T. Wu, L. Tong, F. Yu, S. Cole, and R. Sun. 2004. Generation of a latency-deficient gammaherpesvirus that is protective against secondary infection. J. Virol. 78:9215-9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simas, J. P., and S. Efstathiou. 1998. Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 6:276-282. [DOI] [PubMed] [Google Scholar]
- 28.Song, M. J., H. J. Brown, T.-T. Wu, and R. Sun. 2001. Transcription activation of polyadenylated nuclear RNA by Rta in human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:3129-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Speck, S. H., and H. W. Virgin. 1999. Host and viral genetics of chronic infection: a mouse model of gamma-herpesvirus pathogenesis. Curr. Opin. Microbiol. 2:403-409. [DOI] [PubMed] [Google Scholar]
- 30.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sunil-Chandra, N. P., J. Arno, J. Fazakerley, and A. A. Nash. 1994. Lymphoproliferative disease in mice infected with murine gammaherpesvirus 68. Am. J. Pathol. 145:818-826. [PMC free article] [PubMed] [Google Scholar]
- 32.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 33.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275-3279. [DOI] [PubMed] [Google Scholar]
- 34.Usherwood, E. J., J. P. Stewart, and A. A. Nash. 1996. Characterization of tumor cell lines derived from murine gammaherpesvirus-68-infected mice. J. Virol. 70:6516-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virgin, H. W. T., P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu, T.-T., L. Tong, T. Rickabaugh, S. Speck, and R. Sun. 2001. Function of Rta is essential for lytic replication of murine gammaherpesvirus 68. J. Virol. 75:9262-9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, T.-T., E. J. Usherwood, J. P. Stewart, A. A. Nash, and R. Sun. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J. Virol. 74:3659-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zalani, S., E. Holley-Guthrie, and S. Kenney. 1996. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc. Natl. Acad. Sci. USA 93:9194-9199. [DOI] [PMC free article] [PubMed] [Google Scholar]