Varicella-zoster virus (VZV) is an alphaherpesvirus with a genome of ∼125,000 bp encoding at least 70 unique open reading frames (ORFs) (3, 9, 10, 15). Primary VZV infection is associated with a cell-associated viremia and a diffuse cutaneous rash recognized as varicella, or chickenpox. VZV appears to cause viremia by infecting lymphocytes. VZV establishes latency in sensory ganglia and causes herpes zoster upon reactivation. VZV is sustained in the human population, which is its only natural reservoir, primarily by direct contact with infectious virus in varicella or zoster skin lesions or in respiratory secretions. Investigating the molecular mechanisms of VZV pathogenesis has been difficult because of VZV's restricted infectivity for nonhuman species in vivo and the highly cell-associated nature of VZV replication in vitro. These obstacles have been addressed by the development of the SCIDhu mouse model to examine VZV pathogenesis and immunobiology in vivo (5-7, 19, 30-34, 37, 40-42) and by the making of genetically altered VZV recombinants from VZV cosmids (11, 20, 28). Cosmid mutagenesis can be useful to define essential regions of the VZV genome if infectious virus cannot be recovered without genetic complementation. When genetic changes are not lethal, VZV mutants can be evaluated in vitro and in human T-cell and skin xenografts in SCID mice to determine how particular VZV gene products contribute to virulence in differentiated human cells within their unique tissue microenvironments in vivo. The purpose of this review is to highlight new insights about VZV pathogenesis and immunobiology that have emerged from analyses of VZV infection in the SCIDhu mouse model and from examination of the differential effects of mutations targeting ORF47, which encodes a viral kinase/tegument protein, on VZV tropism for skin and T cells.
Clinical observations indicate that primary VZV infection begins with respiratory mucosal inoculation and that the characteristic chickenpox rash develops after an incubation period of 10 to 21 days (3, 9). In the absence of experimental data, early events in VZV pathogenesis have been compared to mousepox (16). According to this model, VZV is presumed to infect mononuclear cells in regional lymph nodes, causing a primary viremia that carries the virus to reticuloendothelial organs, such as the liver, for a phase of viral amplification, which is followed by a secondary viremia in the late incubation period that results in VZV transport to skin. Instead, our recent experiments with the SCIDhu mouse model support the concept that infected T cells have the potential to mediate VZV transfer to skin immediately after entering the circulation during primary viremia and suggest that the prolonged interval between exposure and the appearance of varicella skin lesions reflects the time required for VZV to overcome previously unrecognized but potent innate immune barriers, especially alpha interferon (IFN-α) production, mounted directly by epidermal cells in vivo (27). Experiments analyzing VZV recombinants with disrupted ORF47 protein kinase activity point to differences in the minimal requirements for cell fusion and virion formation in the pathogenesis of VZV infection of skin and T cells in vivo (7). In a broader context, these observations about VZV pathogenesis and immunobiology point to accommodations between VZV and the host that have allowed the virus to persist in the human population so successfully. Virus-host interactions that ensure the gradual formation of cutaneous lesions containing infectious virus at the skin surfaces should function to avoid an otherwise incapacitating infection of the host that would limit opportunities for VZV transmission to other susceptible individuals. New information about events in VZV pathogenesis and differences in the genetic requirements for VZV replication in skin and T cells also suggest that the design of “second-generation” live attenuated varicella vaccines might focus on preserving infectivity in skin and blocking T cell tropism.
VZV T-CELL TROPISM
That infectious VZV can be recovered from peripheral blood mononuclear cells (PBMC) obtained just before and after the appearance of the varicella rash has been well established (3). VZV DNA was detected by in situ hybridization in 1 in 30,000 to 1 in 100,000 PBMC from healthy individuals with acute varicella, and infected cells appeared to be lymphocytes (25). Infection of T cells also occurred, albeit at a low frequency, when cultured PBMC were inoculated with VZV in vitro (22, 43). The tropism of VZV for T cells was supported by experiments with the SCIDhu mouse model showing that VZV targeted CD4 and CD8 T cells within thymus/liver xenografts in vivo (30). In the human host, lymphoid tissues of the upper respiratory tract include the pharyngeal, palatine, and lingual tonsils, forming Waldeyer's ring. Although the tonsils contain many B cells, CD3 T cells constitute more than 20% of the mononuclear cells in these tissues. The tonsillar surfaces are covered with respiratory epithelial cells that penetrate into the lymphoid tissue, forming “crypts” adjacent to foci of mononuclear cells containing migratory T cells. Tonsil T cells, especially activated memory subpopulations, have proved to be highly permissive for VZV infection in vitro (26). Although VZV infects transformed B cells in vitro (25), exposure to the virus triggered apoptosis of primary tonsillar B cells. Thus, our hypothesis is that, like Epstein-Barr virus, VZV may gain access to migratory cells of the immune system at these upper respiratory lymphoid sites, but it exhibits tropism for T cells rather than B cells (Fig. 1). When infected T cells were treated with phorbol ester, the frequency of VZV-positive T cells increased twofold, indicating that VZV DNA could be harbored silently by T cells and that expression of VZV genes of the putative α, β, and γ kinetic classes was inducible by T-cell activation. VZV preferentially infected the activated memory CD4 T cells common in tonsil T-cell populations. T-cell subpopulations that expressed the skin homing markers cutaneous leukocyte antigen (CLA) and chemokine receptor 4 (CCR4) were also preferentially infected without disruption of their chemotaxis functions. According to the new model of VZV pathogenesis that we propose, VZV tropism for memory T cells that are already programmed for immune surveillance may facilitate VZV transfer to skin.
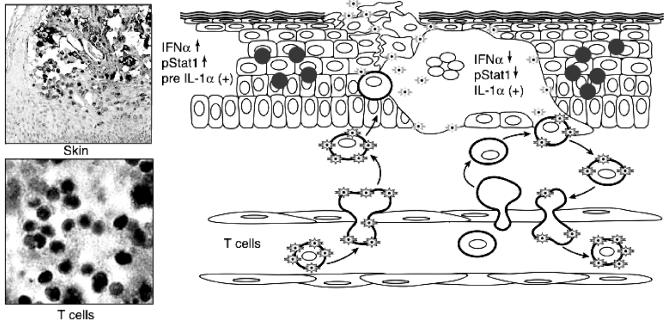
FIG. 1.
A model of the pathogenesis of primary VZV infection. This figure illustrates new concepts about VZV pathogenesis and immunobiology that have emerged from experiments with the SCIDhu mouse model. The upper left panel shows the appearance of a mature VZV lesion in a SCIDhu skin xenograft stained for VZV protein expression with a polyclonal human anti-VZV IgG antiserum. The lower left panel shows VZV infection of T cells in a SCIDhu T-cell xenograft, detected by in situ hybridization with a VZV DNA probe. The right panel is a diagram depicting proposed events in the pathogenesis of VZV infection of skin. According to this model, T cells within the tonsillar lymphoid tissues become infected by VZV transfer into these migratory cells of the immune system following the initial inoculation of respiratory epithelial cells with the virus. Infected T cells enter the circulation and transport the virus to the skin shortly thereafter, exiting through capillary endothelium by the usual mechanisms for trafficking of migratory T cells. The infected T cells then release infectious VZV at skin sites of replication. The remainder of the 10- to 21-day incubation period is the interval required for VZV to overcome the innate IFN-α response in enough epidermal cells to create the typical vesicular lesions containing VZV at the skin surface, as shown in the upper left panel. Signaling of enhanced IFN-α production in adjacent skin cells prevents a rapid, uncontrolled cell-cell spread of VZV. Additional “crops” of varicella lesions may result when T cells traffic through early-stage cutaneous lesions, become infected, and produce a secondary viremia. This process continues until host immune responses trigger the up-regulation of adhesion molecules and mediate the clearance of the virus by VZV-specific antiviral T cells (adapted from the Journal of Experimental Medicine [27] and reproduced by permission of The Rockefeller University Press).
VZV TRANSPORT TO SKIN BY T CELLS
In order to investigate our hypothesis that VZV can be transferred to skin from respiratory sites of inoculation by tonsillar T cells, VZV-infected tonsil T cells were given to SCID mice with human skin xenografts via intravenous injection (27). These skin xenografts contained capillaries composed of human CD31+ endothelial cells, permitting interaction with human T cells. Within 24 h after adoptive transfer, CD3 T cells were detected within the epidermis and dermis and around the hair follicles in skin tissues. Memory CD45RO+ T cells were the predominant population. The transfer of VZV to skin by infected T cells was associated with formation of characteristic VZV lesions that progressed over 10 to 21 days. Infectious virus was recovered from skin xenografts only after intravenous injection of VZV-infected T cells and not from control animals given VZV-infected fibroblasts. T-cell transfer of the virus resulted in lesions expressing the VZV proteins that are associated with lytic infection, such as the major immediate-early 62 (IE62) transactivator, the ORF47 kinase, and glycoprotein E (gE). Cytopathic changes caused by VZV in skin xenografts included extensive formation of multinucleated polykaryocytes, a gradual thickening of the epidermis, epidermal cell proliferation, destruction of basement membranes, and cellular degeneration. Foci of VZV-infected cells eventually extended up to surface keratinocytes. Infectious VZV was produced throughout this period of progressive cutaneous lesion formation. In contrast to assumptions based on the analogy to mousepox, the time required for VZV lesions to reach skin surfaces suggests that the virus must be delivered to cutaneous sites of replication before the late incubation period. The experiments do not exclude a role for other migratory cells of the immune system, e.g., dendritic cells, in transferring VZV to skin, but the relative proportions of these cells and T cells might be expected to favor the contribution of T-cell transport.
MODULATION OF VZV REPLICATION IN SKIN BY INNATE EPIDERMAL CELL RESPONSES
Since VZV causes rapid cytolysis of cultured cells in vitro (3, 9), the slow progression of VZV infection in skin xenografts suggested that its replication is modulated by innate immune mechanisms within the intact cutaneous tissue microenvironment in vivo. In initial experiments, we found that IFN-α and interleukin-1α (IL-1α) were expressed constitutively in the cytoplasm of epidermal cells in uninfected skin, while tumor necrosis factor alpha was not expressed. In VZV-infected skin xenografts, IL-1α was translocated to the nuclei of infected cells but remained in the cytoplasm of adjacent, uninfected cells. Tumor necrosis factor alpha expression was not induced in VZV-infected skin. Notably, IFN-α was down-regulated in VZV-infected cells but was expressed prominently in neighboring uninfected epidermal cells (Fig. 2) (27). Inhibition of the IFN-α pathway in VZV-infected cells and its activation in uninfected cells was confirmed by examining the phosphorylation of Stat1 protein. Binding of IFN-α to its receptors induces phosphorylation of Stat1 phosphorylation by JAK kinases (14). Unless Stat1 is phosphorylated, it does not translocate to the cell nucleus and IFN-α production is blocked. In VZV-infected skin, phosphorylated Stat1 was localized to nuclei in neighboring uninfected epidermal cells, but it was not detected in cells expressing VZV proteins. As expected, Stat1 was not phosphorylated and remained cytoplasmic, as did IFN-α, in uninfected skin xenografts. Thus, VZV replication appears to result in expression of a gene product(s) that interferes with Stat1 activation and thereby inhibits antiviral IFN-α production in foci of infected skin cells in vivo.
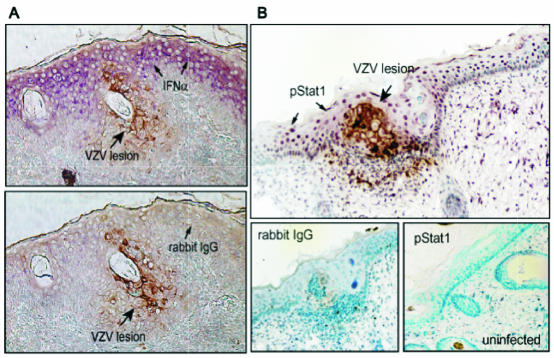
FIG. 2.
IFN-α expression and Stat1 phosphorylation in VZV-infected and uninfected epidermal cells. Formalin-fixed, paraffin-embedded sections of VZV-infected skin xenografts were pretreated and double stained with anti-VZV immunoglobulin G (IgG), detected with 1,4-diamino-2-butanone (brown), and anti-IFN-α or anti-pStat1 antibody, detected with Vector VIP (purple). Sections are shown at a magnification of ×200. (A) Infected cells were identified by VZV protein (brown) expression. In double-labeled skin sections, IFN-α (purple) expression was prominent in adjacent uninfected cells but not in VZV-infected cells (top) compared with sections stained with rabbit IgG as a control for IFN-α (bottom). (B) In double-labeled sections, phosphorylated Stat1 (purple) was up-regulated in adjacent uninfected cells but absent in VZV-infected cells (top); pStat1 was not detected in uninfected skin (bottom right) compared with sections stained with rabbit IgG as a control (bottom left) (reproduced from the Journal of Experimental Medicine [27] by permission of The Rockefeller University Press).
To prove that these observations about IFN-α expression and the phosphorylation and localization of Stat1 in VZV-infected epidermal cells are important determinants of cell-to-cell spread of the virus in vivo, skin xenografts were inoculated with VZV. Mice were then given neutralizing antibody against the human IFN-α/β receptor, which blocks IFN-α/β activity (12). The titer of infectious VZV was 10-fold higher in skin specimens from antibody-treated animals than in those from untreated animals, and the inhibition of IFN signaling by receptor blocking also resulted in substantially larger cutaneous lesions (27).
VZV IMMUNOMODULATION OF MHC-I AND MHC-II PROTEINS
VZV has mechanisms to delay clearance of virus-infected cells by interfering with the expression of major histocompatibility complex class I (MHC-I) and MHC-II proteins that are needed for CD4 and CD8 T-cell recognition (1, 2). VZV infection of cultured fibroblasts was associated with reduced MHC-I expression, as has been observed with other herpesviruses. However, SCIDhu mice with T-cell xenografts made it possible to show that MHC I expression was down-regulated on a significant percentage of VZV-infected T cells in vivo (1). This effect may help the virus to evade CD8 T-cell-mediated lysis during the critical phase of T-cell-associated viremia. Pulse-chase and immunoprecipitation experiments in the presence of endo H showed that MHC-I complexes continued to be assembled in VZV-infected cells and were not retained in the endoplasmic reticulum. VZV infection resulted in the accumulation of MHC-I molecules in the Golgi compartment. Transient expression of immediate-early viral proteins and inhibition of late viral gene expression in infected fibroblasts with phosphonoacetic acid had no effect on MHC-I. However, transient expression of the ORF66 protein kinase, homologous to the herpes simplex virus Us3 protein, was associated with a decrease in cell surface expression of MHC-I. These experiments demonstrated that VZV down-regulates MHC-I by impairing its transport from the Golgi to the plasma membranes of infected cells and suggested a role for ORF66 protein in this immunomodulatory effect.
Whereas MHC-I is produced by most cells, the constitutive expression of MHC-II proteins, required for presenting viral peptides to CD4 T cells, is restricted to antigen-presenting cells. Host immune cells that release IFN-γ stimulate MHC II expression on other cell types. During primary infection, natural killer cells have the capacity to provide IFN-γ and, as the VZV-specific T-cell response develops, CD4 T cells that make IFN-γ are induced (1). Therefore, inhibiting the up-regulation of MHC-II by IFN-γ should allow a protected interval for VZV replication to occur in skin. When fibroblasts were infected with VZV and treated with IFN-γ, only 20 to 30% of cells expressed cell surface MHC-II protein, compared to its expression on 60 to 86% of uninfected fibroblasts exposed to IFN-γ (2). Analysis of the synthesis of MHC-II DR-α RNA transcripts by in situ and Northern blot hybridization showed that DR-α mRNA accumulated in uninfected cells but not in VZV-infected cells in response to IFN-γ. The relevance of these observations to VZV immunobiology was confirmed by showing that MHC-II mRNA was not detected within VZV-infected cells in skin biopsy specimens of varicella lesions but that transcripts were detected in neighboring cells. Analysis of the IFN-γ pathway showed that VZV infection inhibited Stat1α and Jak2 proteins and transcription of interferon regulatory factor 1 and class II transactivator in fibroblasts. VZV interference with IFN-γ signal transduction via the Jak/Stat pathway, resulting in the inhibition of IFN-γ-induced MHC-II expression and thereby modulating the efficiency of VZV-specific CD4 T-cell responses, should facilitate the initial formation of VZV lesions in skin, e.g., during VZV reactivation as zoster in the immune host.
T-CELL MIGRATION TO VZV-INFECTED SKIN
As noted, the formation of VZV skin lesions that penetrate through the skin surface appears to require many days after the virus reaches cutaneous sites of replication. Under these conditions, VZV pathogenesis is likely to require the usual migration of T cells through skin sites of replication without triggering an early inflammatory response that might block the appearance of virus-filled vesicles at the skin surface. At the same time, amplification of the initial T-cell viremia may be needed to ensure that enough cutaneous lesions are formed for efficient viral transmission to other susceptible individuals. In order to examine patterns of mononuclear cell migration to VZV-infected skin, one of two bilateral skin xenografts was inoculated with VZV-infected HEL cells and the other was inoculated with uninfected fibroblasts (27). After 10 days, uninfected mononuclear cells were injected intravenously, and skin xenografts were harvested 24 to 48 h later. CD4 and CD8 T cells were distributed throughout the VZV-infected and uninfected skin xenografts. A few more T cells were recovered from VZV-infected skin than from the corresponding uninfected xenografts, but profiles of T-cell subpopulations did not differ. Thus, T-cell migration into infected skin continued in the typical pattern but was not influenced by expression of VZV proteins or up-regulation of cellular proteins IL-1α and IFN-α.
VZV replication and extensive production of viral proteins, along with altered expression of IL-1-α and IFN-α, were not sufficient to up-regulate the adhesion molecules E-selectin, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1) on endothelial cells in VZV-infected skin xenografts. Skin homing of inflammatory T cells is regulated by adhesion molecules and chemoattractant receptors (8). Therefore, the fact that these molecules were not triggered as a direct consequence of VZV skin infection suggests that the early phase of cutaneous replication could occur without immediate signaling of host immune responses. Infectious virus titers increased as the formation of cutaneous lesions progressed. Continued T-cell trafficking through these sites of active VZV replication in skin should allow the virus to infect more migrating T cells that are programmed to return to the circulation, leading to later “crops” of varicella lesions. While viral amplification may also occur in reticuloendothelial organs, this phase is not essential in our proposed model of VZV pathogenesis. Just as the model does not exclude a role for immune cells other than T cells in the process of VZV transfer to skin, viral amplification at other tissue sites, e.g., liver, might contribute to VZV cell-associated viremia.
In contrast to VZV-infected skin xenografts, biopsy specimens of cutaneous lesions obtained from patients at the onset of the varicella rash showed extensive expression of E-selectin, ICAM-1, and VCAM-1. Many infiltrating mononuclear cells, most of which expressed CD4 or CD8, were detected and included CD45RO+ memory T cells, naive CD45RA+ T cells, and skin homing CLA+ and CCR4+ T cells. These differences in mononuclear cell profiles in VZV lesion biopsies and in VZV-infected skin xenografts after adoptive transfer of PBMC suggest that recruitment and/or retention of inflammatory T cells requires host immune-mediated signals that develop during the later stages of cutaneous lesion formation. These observations are consistent with our finding that VZV-specific antiviral T cells were not detectable in healthy individuals during the late incubation period before varicella skin lesions had appeared (4).
DIFFERENCES BETWEEN REQUIREMENTS FOR VZV INFECTION OF T CELLS AND SKIN IN VIVO
The evaluation of VZV recombinants expressing mutant forms of the ORF47 protein provided an opportunity to use the SCIDhu model for further analysis of the dual tropisms of VZV for T cells and skin (Fig. 3). The ORF47 gene encodes a serine/threonine protein kinase with homology to herpes simplex virus 1 UL13 and similarities to casein kinase II (CKII) proteins (10, 35, 36). ORF47 protein is a component of the VZ virion tegument and can phosphorylate the major immediate-early transactivator, IE62 protein, which is required to induce viral gene transcription (23, 24, 38, 39). Whereas blocking ORF47 protein expression or disrupting its kinase activity had no apparent consequences for VZV infection in cell culture, we have found that these mutations have dramatic effects on VZV virulence in vivo (6, 7, 18, 34). Our initial investigation of the consequences of eliminating ORF47 protein expression showed that ORF47 protein is essential for VZV growth in differentiated skin and T cells in vivo (34). Our recent experiments with VZV mutants that express kinase-defective forms of ORF47 protein have suggested that VZV cell-to-cell spread does not depend on the efficient assembly of complete, enveloped virions in vitro or in skin xenografts in vivo (7). In contrast, the pathogenesis of VZV infection of T cells appears to be linked to virion assembly and release.
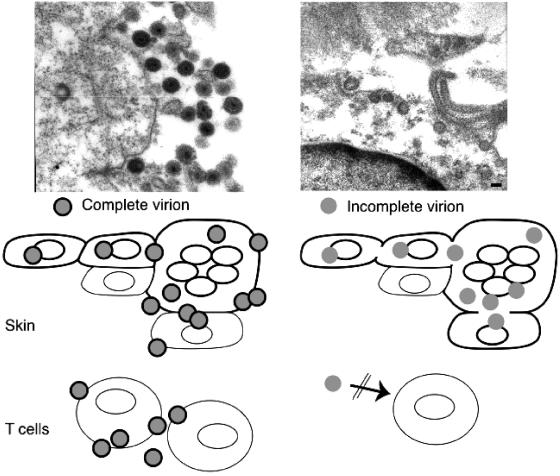
FIG. 3.
Differential requirement for cell fusion and virion formation in VZV infection of skin and T cells. The upper panels show virion formation in skin xenografts infected with VZV and kinase-defective ORF47 mutants. Transmission microscopy demonstrated many intact viral particles in rOka-infected skin cells. Viral particles were incomplete and fewer in rOka47D-N-infected skin cells (adapted from reference 7). As illustrated, virus-induced cell fusion, with the characteristic formation of polykaryocytes, supports VZV infection in skin. Cell-cell spread in skin progressed despite limited virion formation, as shown with kinase-defective ORF47 mutants. VZV infection of T cells is not associated with cell fusion. ORF47 kinase mutants do not infect T cells, suggesting that VZV T-cell tropism depends upon efficient assembly and release of complete virions.
As a kinase, the ORF47 protein autophosphorylates and phosphorylates the major immediate-early transactivator, IE62 protein, the IE63 regulatory protein, and gE, which is the predominant VZV glycoprotein, as shown by in vitro experiments and in cells infected with our ORF47 kinase-deficient mutants. ORF47 phosphorylates gE at sites different from that of CKII, which regulates trafficking to cell membranes and to the trans-Golgi network for virion assembly (21, 22). ORF47 protein must bind to its substrates in order to be a functional kinase, but these protein-protein interactions may have other functions, such as for the assembly of virion tegument structures (44). Mutations to disrupt ORF47 kinase activity were designed to delete the C terminus and to alter two conserved kinase motifs (7). The ORF47 protein kinase mutants were rOka47ΔC, rOka47D-N, and rOka47P-S, each of which has a single amino acid substitution in the putative kinase motif. The mutants did not differ from rOka in their plaque morphology or growth kinetics, as shown by infectious center assay. Kinase assays showed that ORF47-mediated autophosphorylation and IE62 phosphorylation required the C terminus and, specifically, the DYS motif that was altered in rOka47D-N. Complex formation between ORF47 and IE62 proteins and with VZV gE, the predominant VZV glycoprotein, mapped to the ORF47 N terminus and was independent of kinase activity. However, eliminating ORF47 kinase activity was associated with an aberrant nuclear retention of ORF47 and IE62 proteins. Complex formation with IE62 protein may be an essential function of ORF47 protein, a prediction which is consistent with a model of VZV tegument formation in which ORF47 protein binds to IE62 protein, and perhaps to other tegument proteins, encoded by ORFs 4, 9, 10, and 63 (44). VZV gE along with its heterodimer partner, gI, contributes to creating the characteristic VZV syncytia in melanoma cells and fibroblasts (13, 29, 33, 45). The typical pattern of gE distribution to plasma membranes and the trans-Golgi network depended upon its phosphorylation by ORF47 kinase. In cells infected with rOka47ΔC or rOka47D-N, gE was detected diffusely in the cytoplasm and in a punctate distribution within apparent intracellular vesicles. Polykaryocyte formation persisted despite decreased gE expression on plasma membranes in cells infected with rOka47ΔC or rOka47D-N, suggesting that a relatively small concentration of gE in plasma membranes is sufficient for its fusion functions or that gE is less important than the other VZV fusogens, gB and gH, in facilitating cell fusion (13).
EVALUATION OF VZV ORF47 PROTEIN KINASE-DEFECTIVE MUTANTS IN SKIN AND T CELLS IN VIVO
Analyses of the kinase-defective ORF47 mutants in SCIDhu mice showed that blocking ORF47 kinase function decreases VZV virulence in skin substantially. Nevertheless, in contrast to the null mutant, rOKA47S, some replication of rOka47ΔC and rOka47D-N occurred in epidermal cells in vivo. Infectious virus was recovered from 32% of skin xenografts inoculated with rOka47ΔC and 22% of those inoculated with rOka47D-N, compared with more than 80% of rOka or rOkaORF47P-S-infected xenografts. rOka47ΔC and rOka47D-N titers were reduced compared to that of rOka at all time points up to 28 days after inoculation. rOka47ΔC and rOka47D-N infection of the epidermis remained highly localized, without penetrating basement membranes. All mutants recovered from skin had the expected ORF47 mutation, as indicated by sequencing, and retained their kinase-defective phenotypes; thus, no gain of function changes occurred during prolonged replication in skin. ORF47/IE62 protein binding also persisted. The reduced growth of rOka47ΔC or rOka47D-N in skin xenografts suggested that any potential complementation by ORF66 or CKII kinase activity, which was abundant in skin, provided limited support for VZV replication in vivo.
Absence of ORF47 kinase function resulted in abnormal localization of gE in epidermal cells in skin xenografts, which was characterized by reduced plasma membrane expression. In rOka-infected epidermal cells, gE was localized predominantly to plasma membranes and polykaryocyte formation was extensive. Skin xenografts infected with rOka47D-N and rOka47ΔC showed reduced membrane localization of gE relative to cytoplasmic accumulation, but cell fusion with polykaryocyte formation persisted. The pattern of gE expression in skin xenografts infected with kinase-defective rOka47 mutants was similar to its distribution in melanoma cells infected with rOka47ΔC or rOka47D-N in vitro.
VZV replication in T cells in vivo is characterized by release of cell-free virus and infection of T cells scattered throughout the xenografts (30, 31). Since VZV replication is entirely cell-associated in vitro, xenografts are injected with VZV-infected fibroblasts. Infectious virions are presumed to be transferred into a few T cells at the site of injection. When wild-type VZV is injected (10 μl of a ∼105-PFU/ml inoculum), infectious virus is not detected initially but titers increase over 7 to 21 days. Since fusion of VZV-infected T cells is not observed, we suggest that the scattered foci of VZV-positive cells result from the release of cell-free virus into the T-cell xenografts. Whereas infectivity was restricted in skin, no infectious virus was recovered from any T-cell xenografts at any time point following inoculation with the ORF47 kinase-defective mutants, rOka47ΔC and rOka47D-N (7). VZV DNA was not detected in these T-cell xenografts by in situ hybridization or PCR, even at early time points, which suggests that abortive replication did not occur or was quite limited. In contrast, many infected T cells were identified in xenografts inoculated with rOka.
THE ROLE OF VIRION FORMATION IN VZV SKIN AND T-CELL TROPISM
The differential impact of blocking ORF47 kinase activity on VZV virulence in T cells and skin permitted an investigation of possible differences between the mechanisms of VZV pathogenesis in these two critical target cell types (Fig. 3). As described, replication of rOka47ΔC and rOka47D-N in skin was much reduced but infectivity for T cells was completely eliminated. The mislocalization of ORF47 and IE62 proteins and gE in cells infected with rOka47ΔC and rOka47D-N suggested that blocking ORF47 kinase function might interfere with the production of complete VZ virions. This possibility was pursued by using electron microscopy to examine VZ virion production and envelopment in melanoma cells and skin xenografts infected with rOka or the kinase-defective ORF47 mutants (7). VZ virion formation, envelopment, and transport to cell surfaces was decreased dramatically in melanoma cells infected with rOkaORF47ΔC and rOkaORF47D-N even though cell-cell spread appeared normal based on plaque sizes. Complete enveloped virions, around 200 nm in diameter, were numerous in cytoplasmic vacuoles in rOka-infected cells, as shown by transmission electron microscopy, and rOka virions emerged in typical “viral highways” on surfaces of cells, as shown by scanning electron microscopy. In contrast, melanoma cells infected with rOka47D-N or rOka47ΔC contained only a few small viral particles. Virions had no or poorly defined envelope structures, and few or no virions were detected on cell surfaces by scanning electron microscopy. Thus, electron microscopy analyses demonstrated significant differences between the replication of rOkaORF47ΔC, rOkaORF47D-N, and rOka in cultured cells, which could not be discerned by examining plaque morphology or growth kinetics by infectious center assay.
In previous work, we found that varicella-zoster virions made in skin xenografts resemble those detected in vesicle fluid from clinical VZV lesions, with most particles having the characteristic dense core, capsid, and uniform envelope of the herpesviruses (31). In contrast, virions produced in cultured cells are much more pleomorphic, with many defective particles. As expected, electron microscopy studies showed that “authentic” varicella-zoster virions were abundant in skin infected with rOka. Virions were located in clusters near the outer plasma membrane and were 150 to 200 nm in diameter. Most virions were complete, with both a capsid and an envelope. In contrast, very few cells contained virions in skin xenografts infected with rOkaORF47ΔC or rOkaORF47D-N. Infected cells harbored only a few, smaller virions which appeared to be incompletely enveloped. Virions were detected in adjacent cells, suggesting that cell-cell spread occurred despite diminished and incomplete virion assembly. These observations are consistent with the detection of fused, multinucleated epidermal cells in skin lesions produced by these kinase-defective ORF47 mutants.
The preservation of polykaryocyte formation in skin infected with the kinase-defective ORF47 mutants indicated that VZV could induce cell fusion events and produce syncytia despite minimal virion production. Nevertheless, the virulence of rOkaORF47ΔC and rOkaORF47D-N was reduced in skin, whereas plaque formation and growth kinetics were not altered in cultured cells. As shown in experiments using intact VZV, cell-cell spread of the virus in skin was tightly controlled by innate responses of epidermal cells, especially by IFN-α. These innate barriers are likely to be more effective when the blocking of ORF47 kinase activity results in aberrant intracellular localization of gE, ORF47 protein, and IE62 protein and impaired virion formation.
In contrast to cell-cell spread and polykaryocyte formation in skin, VZV infection does not trigger fusion of T cells in infected xenografts (30). Transfer of the virus between T cells, which are the only cells that are permissive for VZV replication within T-cell xenografts, appears to depend upon the release of cell-free virus. It is possible that T cells require some other ORF47 protein function that is not needed in skin cells. However, interference with the assembly of enveloped VZ virions and their release for entry into uninfected T cells in the absence of ORF47 kinase activity provides a straightforward and plausible explanation for the observation that rOka47ΔC and rOka47D-N were completely avirulent in T cells in vivo.
SUMMARY
These experiments provide evidence in support of a critical role for T-cell tropism in VZV pathogenesis and suggest that the prolonged varicella incubation period represents the time required for VZV to overcome previously unrecognized potent innate antiviral responses, especially IFN-α production, mediated directly by epidermal cells. The initial phase of VZV pathogenesis appears to be facilitated by virus-mediated modulation of MHC-I and MHC-II expression and by the failure of VZV to trigger up-regulation of inflammatory adhesion molecules on capillary endothelial cells in skin. Eliminating ORF47 kinase activity impaired virion production and envelopment, which was associated with a dramatic reduction of VZV virulence in skin and a complete block of VZV infectivity for T cells in SCIDhu xenografts. Our interpretation of these observations is that VZV T-cell tropism is much more dependent on virion assembly than is VZV replication in epidermal cells. Some VZV infectivity is retained in skin if cell fusion functions are preserved, allowing cell-cell spread even when virion production and envelopment are impaired quite significantly. In contrast, VZV T-cell tropism appears to require efficient virion formation and egress for transfer to uninfected cells. Genetically engineered mutations in the VZV genome that result in the retention of some capacity to replicate in skin, at a level sufficient to elicit adaptive immunity to VZV, while eliminating infection of T cells by interfering with virion assembly may yield improved live attenuated VZV vaccines.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID) (AI20459 and AI053846 to A.M.A.) and the VZV Research Foundation (to C.-C.K.), Stanford Developmental and Neonatal Biology Training Grant T32 HD07249 (to J.B), and NIAID grant AI22795 (to C.G.).
REFERENCES
- 1.Abendroth, A., I. Lin, B. Slobedman, H. Ploegh, and A. M. Arvin. 2001. Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells. J. Virol. 75:4878-4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abendroth, A., B. Slobedman, E. Lee, E. Mellins, M. Wallace, and A. M. Arvin. 2000. Modulation of major histocompatibility class II protein expression by varicella-zoster virus. J. Virol. 74:1900-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvin, A. M. 2001. Varicella-zoster virus, p. 2731-2768. In D. M. Knipe and P. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 4.Arvin, A. M. 1999. Varicella-zoster virus. persistent viral infections of humans, p. 183-208. In R. Ahmed and I. S. Y. Chen (ed.), Persistent viral infections. John Wiley & Sons Ltd., West Sussex, England.
- 5.Baiker, A., C. Bagowski, H. Ito, M. Sommer, L. Zerboni, K. Fabell, J. Hay, W. Ruyechan, and A. M. Arvin. 2004. The immediate-early 63 protein of varicella-zoster virus: analysis of functional domains required for replication in vitro and for T-cell and skin tropism in the SCIDhu model in vivo. J. Virol. 78:1181-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Besser, J., M. H. Sommer, L. Zerboni, C. Bagowski, H. Ito, J. Moffat, C-C. Ku, and A. M. Arvin. 2003. Differentiation of varicella-zoster virus ORF47 protein kinase and IE62 protein binding domains and their contributions to replication in human skin xenografts in the SCID-hu mouse. J. Virol. 77:5964-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besser, J., M. Ikoma, K. Fabel, M. H. Sommer, L. Zerboni, C. Grose, and A. M. Arvin. 2004. Differential requirement for cell fusion and virion formation in varicella-zoster virus infection of skin and T cells. J. Virol. 23:13293-13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell, J. J., and E. C. Butcher. 2000. Chemokines in tissue-specific and microenvironment specific lymphocyte homing. Curr. Opin. Immunol. 12:336-341. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, J., and S. Straus. 2001. Varicella-zoster virus and its replication, p. 2547-2586. In D. M. Knipe and P. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 10.Cohen, J. I. 1999. Genomic structure and organization of varicella-zoster virus. Contrib. Microbiol. 3:10-20. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, J. I., and K. E. Seidel. 1993. Generation of VZV and viral mutants from cosmid DNAs: VZV thymidine kinase is not essential for replication in vitro. Proc. Natl. Acad. Sci. USA 90:7376-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colamonici, O. R., and P. Domanski. 1993. Identification of a novel subunit of the type I interferon receptor localized to human chromosome 21. J. Biol. Chem. 268:10895-10899. [PubMed] [Google Scholar]
- 13.Cole, N. L., and C. Grose. 2003. Membrane fusion mediated by herpesvirus glycoproteins: the paradigm of varicella zoster virus. Rev. Med. Virol. 13:207-222. [DOI] [PubMed] [Google Scholar]
- 14.Darnell, J. E., Jr., I. M. Kerr, and G. R. Stark. 1994. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415-1421. [DOI] [PubMed] [Google Scholar]
- 15.Davison, A. J., and J. Scott. 1986. Complete DNA sequence of varicella-zoster virus. J. Gen.Virol. 67:1759-1816. [DOI] [PubMed] [Google Scholar]
- 16.Grose, C. 1981. Variation on a theme by Fenner: the pathogenesis of chickenpox. Pediatrics 68:735-737. [PubMed] [Google Scholar]
- 17.Hanks, S. K., and T. Hunter. 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576-596. [PubMed] [Google Scholar]
- 18.Heineman, T. C., and J. I. Cohen. 1995. The varicella-zoster virus (VZV) open reading frame 47 (ORF47) protein kinase is dispensable for viral replication and is not required for phosphorylation of ORF63 protein, the VZV homolog of herpes simplex virus ICP22. J. Virol. 69:7367-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito, H., M. H. Sommer, L. Zerboni, H. He, J. Hay, W. Ruyechan, and A. M. Arvin. 2003. Promoter sequences of varicella-zoster virus glycoprotein I targeted by cellular tranactivating factors Sp1 and USF determine virulence in skin and T cells in SCIDhu mouse in vivo. J. Virol. 77:489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemble, G. W., P. Annunziato, O. Lungu, R. E. Winter, T. A. Cha, S. J. Silverstein, and R. R. Spaete. 2000. Open reading frame S/L of varicella-zoster virus encodes a cytoplasmic protein expressed in infected cells. J. Virol. 74:11311-11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenyon, T. K., J. Lynch, J. Hay, W. Ruyechan, and C. Grose. 2001. Varicella-zoster virus ORF47 protein serine kinase: characterization of a cloned, biologically active phosphotransferase and two viral substrates, ORF62 and ORF63. J. Virol. 75:8854-8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenyon, T. K., J. I. Cohen, and C. Grose. 2002. Phosphorylation by the varicella-zoster virus ORF47 protein serine kinase determines whether endocytosed viral gE traffics to the trans-Golgi network or recycles to the cell membrane. J. Virol. 76:10980-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinchington, P. R., J. K. Hougland, A. M. Arvin, W. T. Ruyechan, and J. Hay. 1992. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J. Virol. 66:359-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinchington, P. R., K. Fite, and S. E. Turse. 2000. Nuclear accumulation of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, is inhibited by phosphorylation mediated by the VZV open reading frame 66 protein kinase. J. Virol. 74:2265-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koropchak, C. M., S. Solem, P. S. Diaz, and A. M. Arvin. 1989. Investigation of varicella-zoster virus infection of lymphocytes by in situ hybridization. J. Virol. 63:2392-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ku, C.-C., J. Padilla, C. Grose, E. C. Butcher, and A. M. Arvin. 2002. Tropism of varicella-zoster virus for human tonsillar CD4+ T lymphocytes that express activation, memory, and skin homing markers. J. Virol. 76:11425-11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ku, C.-C., L. Zerboni, H. Ito, M. Wallace, B. Graham, and A. M. Arvin. 2004. Transport of varicella-zoster virus to skin by infected CD4 T cells and modulation of viral replication by epidermal cell interferon-α. J. Exp Med. 200:917-925. [DOI] [PMC free article] [PubMed]
- 28.Mallory, S., M. Sommer, and A. M. Arvin. 1997. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein conformation and trafficking. J. Virol. 71:8279-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mo, C., J. Lee, M. Sommer, C. Grose, and A. M. Arvin. 2002. The requirement of varicella-zoster virus glycoprotein E for viral replication and the effects of glycoprotein I on gE in melanoma cells. Virology 304:176-186. [DOI] [PubMed] [Google Scholar]
- 30.Moffat, J. F., M. D. Stein, H. Kaneshima, and A. M. Arvin. 1995. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J. Virol. 69:5236-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moffat, J., L. Zerboni, M. Stein, C. Grose, H. Kaneshima, and A. Arvin. 1998. The attenuation of the vaccine Oka strain of varicella-zoster virus and the role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mouse. J. Virol. 72:965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moffat, J., M. Sommer, S. Taylor, S. Mallory, and A. M. Arvin. 2002. VZV glycoprotein I is required for viral replication in skin and T cells. J. Virol. 76:8468-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moffat, J., C. Mo, J. J. Cheng, M. Sommer, L. Zerboni, S. Stamatis, and A. M. Arvin. 2004. Functions of the C-terminal domain of varicella-zoster virus glycoprotein E in viral replication in vitro and skin and T-cell tropism in vivo. J. Virol. 78:12406-12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moffat, J. F., L. Zerboni, M. H. Sommer, T. C. Heineman, J. I., Cohen, H. Kaneshima, and A. M. Arvin. 1998. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc. Natl. Acad. Sci. USA 95:11969-11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ng, T. I., and C. Grose. 1992. Serine protein kinase associated with varicella-zoster virus ORF 47. Virology 191:9-18. [DOI] [PubMed] [Google Scholar]
- 36.Ng, T. I., L. Keenan, P. R. Kinchington, and C. Grose. 1994. Phosphorylation of varicella zoster virus open reading frame (ORF) 62 regulatory product by viral ORF 47-associated protein kinase. J. Virol. 68:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niizuma, T., M. Sommer, H. Ito, S. Hinchliffe, L. Zerboni, and A. M. Arvin. 2003. Mutational analysis of varicella-zoster virus ORF65 protein and its role in infection of human skin and T cell xenografts in the SCIDhu mouse model. J. Virol. 77:6062-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perera, L. P., J. D. Mosca, W. Ruyechan, and J. Hay. 1992. Regulation of varicella-zoster virus gene expression in human T lymphocytes. J. Virol. 66:5298-5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perera, L. P., J. D. Mosca, W. T. Ruyechan, G. S. Hayward, S. E. Straus, and J. Hay. 1993. A major transactivator of varicella-zoster virus, the immediate-early protein IE62, contains a potent N-terminal activation domain. J. Virol. 67:4474-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos, R. A., C. Hatfield, B. P. Faga, J. A. Padilla, N. L. Cole, J. F. Moffat, A. M. Arvin, W. T. Ruyechan, J. Hay, and C. Grose. 2000. Varicella-zoster virus gE escape mutant VZV-MSP exhibits an accelerated cell-to-cell spread phenotype in both infected cell cultures and SCID-hu mice. Virology 275:306-317. [DOI] [PubMed] [Google Scholar]
- 41.Sato, B., H. Ito, S. Hinchliffe, M. H. Sommer, L. Zerboni, and A. M. Arvin. 2003. Mutational analysis of open reading frames 62 and 71, encoding the varicella-zoster virus immediate-early transactivating protein, IE62, and effects on replication in vitro and in skin xenografts in the SCID-hu mouse in vivo. J. Virol. 77:5607-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sommer, M. H., C. C. Ku, C. Grose, W. Ruyechan, J. Hay, and A. M. Arvin. 2001. Mutational analysis of varicella-zoster virus ORF63/70 and ORF64/69. J. Virol. 75:8224-8239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soong, W., J. C. Schultz, A. C. Patera, M. H. Sommer, and J. I. Cohen. 2000. Infection of human T lymphocytes with varicella-zoster virus: an analysis with viral mutants and clinical isolates. J. Virol. 74:1864-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spengler, M., N. Niesen, C. Grose, W. T. Ruyechan, and J. Hay. 2001. Interactions among structural proteins of varicella zoster virus. Arch. Virol. Suppl. 17:71-79. [DOI] [PubMed] [Google Scholar]
- 45.Wang, Z. H., M. D. Gershon, O. Lungu, Z. Zhu, S. Mallory, A. M. Arvin, and A. A. Gershon. 2001. Essential role played by the C-terminal domain of glycoprotein I in envelopment of varicella-zoster virus in the trans-Golgi network: interactions of glycoproteins with tegument. J. Virol. 75:323-340. [DOI] [PMC free article] [PubMed] [Google Scholar]